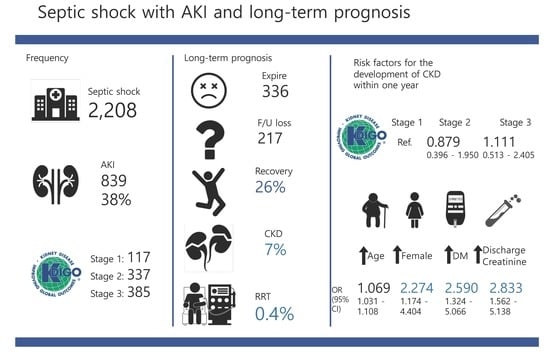
One-Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Study Population
2.2. Data Collection and Definition
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. KDIGO Stages and Outcomes
3.3. Risk Factors for the Development of CKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knoop, S.T.; Skrede, S.; Langeland, N.; Flaatten, H.K. Epidemiology and impact on all-cause mortality of sepsis in Norwegian hospitals: A national retrospective study. PLoS ONE 2017, 12, e0187990. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.-M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1309. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Harhay, M.O.; Small, D.S.; Prescott, H.C.; Bowles, K.H.; Gaieski, D.F.; Mikkelsen, M.E. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit. Care Med. 2018, 46, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, S. Organ dysfunction as a new standard for defining sepsis. Inflamm. Regen. 2016. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, R.; Basu, R.K.; Goldstein, S.L.; Bagshaw, S.M. Sepsis-associated acute kidney injury. Semin. Nephrol. 2015, 35, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Yearly, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar]
- Kim, W.Y.; Huh, J.W.; Lim, C.M.; Koh, Y.S.; Hong, S.B. Analysis of progression in risk, injury, failure, loss, and end-stage renal disease classification on outcome in patients with severe sepsis and septic shock. J. Crit. Care 2012, 27, 104.e1–104.e7. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.M.; Shafer, L.A.; Ho, J.; Reslerova, M.; Martinka, G.; Keenan, S.; Dial, S.; Wood, G.; Rigatto, C.; Kumar, A. Early reversible acute kidney injury is associated with improved survival in septic shock. J. Crit. Care 2014, 29, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-R.; Zhu, J.-M.; Jiang, J.; Ding, X.-Q.; Fang, Y.; Shen, B.; Liu, Z.-H.; Zou, J.-Z.; Liu, L.; Wang, C.-S.; et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine 2015, 94, e2025. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Available online: https://kdigo.org/guidelines/ (accessed on 15 December 2018).
- KDIGO. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Available online: https://kdigo.org/guidelines/ (accessed on 15 December 2018).
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.J.; Barreto, S.; Gentil, T.; Assis, L.S.; Soeiro, E.M.; de Castro, I.; Laranja, S.M. Risk factors for the progression of chronic kidney disease after acute kidney injury. J. Bras. Nefrol. 2017, 39, 1–7. [Google Scholar] [CrossRef]
- Fujii, T.; Uchino, S.; Doi, K.; Sato, T.; Kawamura, T. Diagnosis, management, and prognosis of patients with acute kidney injury in Japanese intensive care units: The JAKID study. J. Crit. Care 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- Heung, M.; Chawla, L.S. Acute kidney injury: Gateway to chronic kidney disease. Nephron Clin. Pract. 2014, 127, 30–34. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Shaw, A.D.; Faselis, C.; Palant, C.E.; Kimmel, P.L. Association between acute kidney injury and long-term renal and cardiovascular outcomes in United States Veterans. Clin. J. Am. Soc. Nephrol. 2014, 9, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Holmen, J.; De Graauw, J.; Jovanovich, A.; Thornton, S.; Chonchol, M. Association of complete recovery from acute kidney injury with incident CKD Stage 3 and all-cause mortality. Am. J. Kidney Dis. 2012, 60, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Wen, X.; Shah, N.; Lee, M.; Kong, L.; Pike, F.; Keener, C.; Unruh, M.; Finkel, K.; Vijayan, A.; et al. Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol. Dial. Transplant. 2014, 29, 1854–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mårtensson, J.; Vaara, S.T.; Pettilä, V.; Ala-Kokko, T.; Karlsson, S.; Inkinen, O.; Uusaro, A.; Larsson, A.; Bell, M. Assessment of plasma endostatin to predict acute kidney injury in critically ill patients. Acta Anaesthesiol. Scand. 2017, 61, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Prowle, J.R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Straaten, H.M.O.-V.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal recovery after acute kidney injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Dumoulin, A.; Janssen, A.; Picavet, S.; Vanthuyne, S.; Van Eynde, R.; Vanholder, R.; Delanghe, J.; De Schoenmakere, G.; De Waele, J.J.; et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015, 41, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kaddourah, A.; Basu, R.K.; Bagshaw, S.M.; Goldstein, S.L. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 2017, 376, 11–20. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total n = 286 | Non-CKD after 1 Year n = 229 | CKD after 1 Year n = 57 | p-Value |
|---|---|---|---|---|
| Age | 63.7 (56.0–72.0) | 64.0 (55.0–70.0) | 71.0 (61.3–77.8) | 0.001 |
| Male | 181 (63.3) | 152 (66.4) | 29 (50.9) | 0.033 |
| Underlying disease | ||||
| HTN | 102 (35.7) | 75 (32.8) | 27 (47.4) | 0.045 |
| Stroke | 24 (8.4) | 18 (7.9) | 6 (10.5) | 0.592 |
| DM | 84 (29.4) | 54 (23.6) | 30 (52.6) | <0.001 |
| Coronary artery disease | 22 (7.7) | 14 (6.1) | 8 (14.0) | 0.054 |
| Chronic pulmonary disease | 29 (10.1) | 22 (9.6) | 7 (12.3) | 0.623 |
| Liver cirrhosis | 43 (15.0) | 37 (16.2) | 6 (10.5) | 0.312 |
| Malignancy | 79 (27.6) | 56 (25.8) | 20 (35.1) | 0.186 |
| Infection site | ||||
| Unknown | 4 (4.1) | 2 (8.8) | 2 (2.7) | 0.788 |
| Pulmonary | 21 (21.6) | 15 (20.5) | 6 (25.0) | 0.776 |
| Urinary | 23 (23.7) | 18 (24.7) | 5 (20.8) | 0.788 |
| Gastrointestine | 15 (15.5) | 13 (17.8) | 2 (8.3) | 0.345 |
| Hepatobiliary | 30 (30.9) | 21 (28.8) | 9 (37.5) | 0.452 |
| Others | 11 (11.3) | 9 (12.3) | 2 (8.3) | 0.572 |
| Laboratory | ||||
| WBC (×103/uL) | 10.5 (5.1–17.5) | 10.5 (5.7–18.0) | 10.6 (4.0–14.9) | 0.491 |
| Hb (g/dL) | 11.5 (9.3–13.2) | 11.9 (9.9–13.6) | 14.93 (10.6–22.6) | <0.001 |
| PLT (×103/uL) | 137.0 (72.5–207.0) | 138.0 (75.5–207.0) | 130.0 (66.25–210.5) | 0.921 |
| BUN (mg/dL) | 32.0 (24.8–45.0) | 31.0 (23.0–40.5) | 36.0 (29.0–53.0) | 0.017 |
| Baseline Cr (mg/dL) | 0.72 (0.63–0.86) | 0.70 (0.61–0.82) | 0.80 (0.72–0.98) | <0.001 |
| Initial Cr (mg/dL) | 1.8 (1.4–2.5) | 1.8 (1.4–2.4) | 2.1 (1.5–3.0) | 0.037 |
| Peak Cr (mg/dL) | 2.0 (1.5–2.7) | 1.9 (1.5–2.6) | 2.4 (1.6–3.5) | 0.002 |
| Discharge Cr (mg/dL) | 0.9 (0.7–1.1) | 0.8 (0.6–1.0) | 1.1 (0.9–1.8) | <0.001 |
| Lactate (mmol/L) | 3.3 (2.0–5.5) | 3.3 (2.0–5.6) | 3.0 (1.8–4.5) | 0.389 |
| CRP (mg/dL) | 15.3 (5.9–22.2) | 16.1 (6.7–22.3) | 11.9 (5.13–22.0) | 0.278 |
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| CKD Development | |||
| Initial Cr | |||
| KDIGO stage 1 | Reference | ||
| KDIGO stage 2 | 0.783 | 0.375–1.635 | 0.515 |
| KDIGO stage 3 | 0.924 | 0.444–1.923 | 0.832 |
| Maximum Cr | |||
| KDIGO stage 1 | Reference | ||
| KDIGO stage 2 | 0.879 | 0.396–1.950 | 0.751 |
| KDIGO stage 3 | 1.111 | 0.513–2.405 | 0.789 |
| All-Cause Mortality | |||
| Initial Cr | |||
| KDIGO stage 1 | Reference | ||
| KDIGO stage 2 | 2.637 | 1.719–4.046 | <0.001 |
| KDIGO stage 3 | 2.933 | 1.913–4.499 | <0.001 |
| Maximum Cr | |||
| KDIGO stage 1 | Reference | ||
| KDIGO stage 2 | 4.832 | 2.696–8.668 | <0.001 |
| KDIGO stage 3 | 5.909 | 3.316–10.530 | <0.001 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 1.066 | 1.027–1.107 | <0.001 | 1.070 | 1.033–1.108 | <0.001 |
| HTN | 0.996 | 0.487–2.039 | 0.991 | |||
| DM | 2.656 | 1.341–5.257 | 0.005 | 2.620 | 1.352–5.078 | 0.004 |
| CAD | 1.914 | 0.638–5.745 | 0.247 | |||
| LC | 0.992 | 0.360–2.730 | 0.987 | |||
| Malignancy | 1.250 | 0.601–2.600 | 0.551 | |||
| Hb | 0.833 | 0.734–0.946 | 0.005 | 0.840 | 0.744–0.949 | 0.005 |
| Discharge Cr | 2.503 | 1.371–4.569 | 0.003 | 2.686 | 1.499–4.812 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-s.; Kim, Y.-J.; Ryoo, S.M.; Sohn, C.H.; Seo, D.W.; Ahn, S.; Lim, K.S.; Kim, W.Y. One-Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study. J. Clin. Med. 2018, 7, 554. https://doi.org/10.3390/jcm7120554
Kim J-s, Kim Y-J, Ryoo SM, Sohn CH, Seo DW, Ahn S, Lim KS, Kim WY. One-Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study. Journal of Clinical Medicine. 2018; 7(12):554. https://doi.org/10.3390/jcm7120554
Chicago/Turabian StyleKim, June-sung, Youn-Jung Kim, Seung Mok Ryoo, Chang Hwan Sohn, Dong Woo Seo, Shin Ahn, Kyoung Soo Lim, and Won Young Kim. 2018. "One-Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study" Journal of Clinical Medicine 7, no. 12: 554. https://doi.org/10.3390/jcm7120554