Electrical Impedance Tomography for Cardio-Pulmonary Monitoring
Abstract
:1. Introduction
2. Basics of Bioimpedance
3. EIT Measurements and Image Reconstruction
4. Functional Imaging and EIT Waveform Analysis
5. Ventilation Monitoring
5.1. Validation of EIT Measurements
5.1.1. Global Ventilation
5.1.2. Global Changes in End-Expiratory Lung Volume (EELV) and Impedance (EELI)
5.1.3. Regional Changes in Lung Ventilation or Volume
5.2. Analyzing Spatial Distribution of Ventilation
5.2.1. Subtracting fEIT Images
5.2.2. Impedance Ratio
5.2.3. Regional Respiratory System Compliance (CRS)
5.2.4. Regional Pressure–Volume (P/V) Curves
5.2.5. Alveolar Overdistension and Collapse (ODCL)
5.2.6. Center of Ventilation (CoV)
5.2.7. Global Inhomogeneity Index (GI Index)
5.2.8. Dependent (DSS) and Non-Dependent Silent Spaces (NSS)
5.3. Analyzing Temporal Distribution of Ventilation
5.3.1. Regional Ventilation Delay (RVD)-Regional Ventilation Delay Inhomogeneity (RVDI)
5.3.2. Intratidal Gas Distribution-Intratidal Ventilation Index (ITVI)
5.3.3. Regional Expiratory Time Constants
5.4. Clinical Application
5.4.1. Estimation of Lung Volume, Collapse and Overdistension
5.4.2. PEEP Titration in Obese Patients
5.4.3. Pneumothorax Detection
5.4.4. Detection of Pleural Effusion
5.4.5. Prediction of Weaning Success
5.4.6. Monitoring Lung Volumes during Endotracheal Suctioning
5.4.7. Monitoring Positioning of Endotracheal Tubes
5.4.8. Monitoring Ventilatory Dyssynchrony
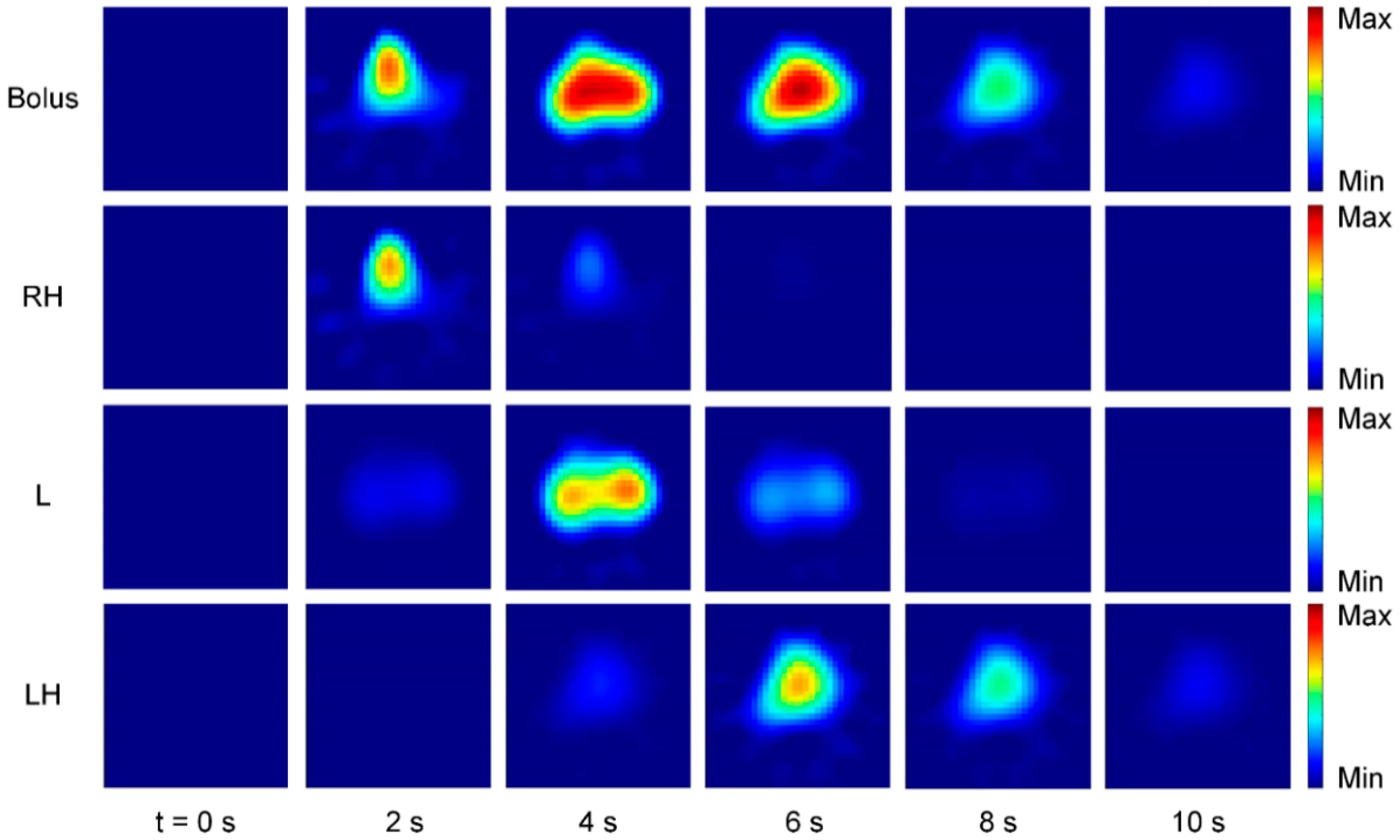
6. Perfusion Monitoring
6.1. Perfusion Monitoring Using Contrast Agents
6.1.1. Measurement Principle
6.1.2. Validation of Regional Perfusion
6.1.3. Regional Ventilation to Perfusion Ratio (V/Q)
6.2. Perfusion Monitoring Using Cardiac Activity
6.2.1. Measurement Principle
6.2.2. Separating Ventilation and Cardiac-Related Signals
6.2.3. Validation Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leonhardt, S.; Lachmann, B. Electrical impedance tomography: The holy grail of ventilation and perfusion monitoring? Intensive Care Med. 2012, 38, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Reljin, N.; Eaton-Robb, C.; Noh, Y.; Riistama, J.; Chon, K.H. Analysis of Consistency of Transthoracic Bioimpedance Measurements Acquired with Dry Carbon Black PDMS Electrodes, Adhesive Electrodes, and Wet Textile Electrodes. Sensors 2018, 18, 1719. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Karsten, J.; Stueber, T.; Voigt, N.; Teschner, E.; Heinze, H. Influence of different electrode belt positions on electrical impedance tomography imaging of regional ventilation: A prospective observational study. Crit. Care 2016, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Bodenstein, M.; David, M.; Markstaller, K. Principles of electrical impedance tomography and its clinical application. Crit. Care Med. 2009, 37, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Gaggero, P.O.; Maimaitijiang, Y. Adjacent stimulation and measurement patterns considered harmful. Physiol. Meas. 2011, 32, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.H.; Barber, D.C. Recent developments in applied potential tomography-APT. In Information Processing in Medical Imaging; Bacharach, S.L., Nijhoff, M., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 106–121. [Google Scholar] [CrossRef]
- Yorkey, T.J.; Webster, J.G.; Tompkins, W.J. Comparing reconstruction algorithms for electrical impedance tomography. IEEE Trans. Biomed. Eng. 1987, 34, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Arnold, J.H.; Bayford, R.; Borsic, A.; Brown, B.; Dixon, P.; Faes, T.J.C.; Frerichs, I.; Gagnon, H.; Gärber, Y.; et al. GREIT: A unified approach to 2D linear EIT reconstruction of lung images. Physiol. Meas. 2009, 30, S35–S55. [Google Scholar] [CrossRef]
- Rabbani, K.S.; Kabir, A.M. Studies on the effect of the third dimension on a two-dimensional electrical impedance tomography system. Clin. Phys. Physiol. Meas. 1991, 12, 393–402. [Google Scholar] [CrossRef]
- Grychtol, B.; Müller, B.; Adler, A. 3D EIT image reconstruction with GREIT. Physiol. Meas. 2016, 37, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Proenca, M.; Braun, F.; Brunner, J.; Sola, J. Origins of Cardiosynchronous Signals in EIT. In Proceedings of the 18th International Conference on Biomedical Applications of Electrical Impedance Tomography, Dartmouth College, Hanover, NH, USA, 21–24 June 2017; p. 73. [Google Scholar]
- Hahn, G.; Sipinková, I.; Baisch, F.; Hellige, G. Changes in the thoracic impedance distribution under different ventilatory conditions. Physiol. Meas. 1995, 16, A161–A173. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Amyot, R.; Guardo, R.; Bates, J.H.; Berthiaume, Y. Monitoring changes in lung air and liquid volumes with electrical impedance tomography. J. Appl. Physiol. 1997, 83, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Marquis, F.; Coulombe, N.; Costa, R.; Gagnon, H.; Guardo, R.; Skrobik, Y. Electrical impedance tomography’s correlation to lung volume is not influenced by anthropometric parameters. J. Clin. Monit. Comput. 2006, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Hahn, G.; Neumann, P.; Sydow, M.; Mohrenweiser, P.; Hellige, G.; Burchardi, H. End-expiratory lung impedance change enables bedside monitoring of end-expiratory lung volume change. Intensive Care Med. 2003, 29, 37–43. [Google Scholar] [CrossRef]
- Grivans, C.; Lundin, S.; Stenqvist, O.; Lindgren, S. Positive end-expiratory pressure-induced changes in end-expiratory lung volume measured by spirometry and electric impedance tomography. Acta Anaesthesiol. Scand. 2011, 55, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Bikker, I.G.; Leonhardt, S.; Bakker, J.; Gommers, D. Lung volume calculated from electrical impedance tomography in ICU patients at different PEEP levels. Intensive Care Med. 2009, 35, 1362–1367. [Google Scholar] [CrossRef] [Green Version]
- Wrigge, H.; Zinserling, J.; Muders, T.; Varelmann, D.; Günther, U.; von der Groeben, C.; Magnusson, A.; Hedenstierna, G.; Putensen, C. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit. Care Med. 2008, 36, 903–909. [Google Scholar] [CrossRef]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Dudykevych, T.; Quintel, M.; Hellige, G. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J. Appl. Physiol. 2002, 93, 660–666. [Google Scholar] [CrossRef]
- Hinz, J.; Neumann, P.; Dudykevych, T.; Andersson, L.G.; Wrigge, H.; Burchardi, H.; Hedenstierna, G. Regional ventilation by electrical impedance tomography: A comparison with ventilation scintigraphy in pigs. Chest 2003, 124, 314–322. [Google Scholar] [CrossRef]
- Kunst, P.W.; Vazquez de Anda, G.; Böhm, S.H.; Faes, T.J.; Lachmann, B.; Postmus, P.E.; de Vries, P.M. Monitoring of recruitment and derecruitment by electrical impedance tomography in a model of acute lung injury. Crit. Care Med. 2000, 28, 3891–3895. [Google Scholar] [CrossRef] [PubMed]
- Bikker, I.G.; Preis, C.; Egal, M.; Bakker, J.; Gommers, D. Electrical impedance tomography measured at two thoracic levels can visualize the ventilation distribution changes at the bedside during a decremental positive end-expiratory lung pressure trial. Crit. Care 2011, 15, R193. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, P.A.; Rimensberger, P.C.; Frerichs, I. Regional tidal ventilation and compliance during a stepwise vital capacity manoeuvre. Intensive Care Med. 2010, 36, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Spadaro, S.; Waldmann, A.D.; Böhm, S.H.; Ragazzi, R.; Marangoni, E.; Alvisi, V.; Spinelli, E.; Mauri, T.; Volta, C.A. Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit. Care 2019, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Kunst, P.W.; Böhm, S.H.; Vazquez de Anda, G.; Amato, M.B.; Lachmann, B.; Postmus, P.E.; de Vries, P.M. Regional pressure volume curves by electrical impedance tomography in a model of acute lung injury. Crit. Care Med. 2000, 28, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Moerer, O.; Neumann, P.; Dudykevych, T.; Frerichs, I.; Hellige, G.; Quintel, M. Regional pulmonary pressure volume curves in mechanically ventilated patients with acute respiratory failure measured by electrical impedance tomography. Acta Anaesthesiol. Scand. 2006, 50, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Beda, A.; Carvalho, A.R.; Carvalho, N.C.; Hammermüller, S.; Amato, M.B.P.; Muders, T.; Gittel, C.; Noreikat, K.; Wrigge, H.; Reske, A.W. Mapping Regional Differences of Local Pressure-Volume Curves With Electrical Impedance Tomography. Crit. Care Med. 2017, 45, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.L.V.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B.P. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, J.; Santiago, R.R.S.; Teggia Droghi, M.; Zhang, C.; Fintelmann, F.J.; Troschel, F.M.; Morais, C.C.A.; Amato, M.B.P.; Kacmarek, R.M.; Berra, L.; et al. Lung Recruitment in Obese Patients with Acute Respiratory Distress Syndrome. Anesthesiology 2019, 130, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Hahn, G.; Golisch, W.; Kurpitz, M.; Burchardi, H.; Hellige, G. Monitoring perioperative changes in distribution of pulmonary ventilation by functional electrical impedance tomography. Acta Anaesthesiol. Scand. 1998, 42, 721–726. [Google Scholar] [CrossRef]
- Luepschen, H.; Meier, T.; Grossherr, M.; Leibecke, T.; Karsten, J.; Leonhardt, S. Protective ventilation using electrical impedance tomography. Physiol. Meas. 2007, 28, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.Y.; Ayoub, G.; Kim, Y.E.; Oh, T.I.; Chung, C.R.; Suh, G.Y.; Woo, E.J. Integrated EIT system for functional lung ventilation imaging. BioMed Eng. OnLine 2019, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Sobota, V.; Roubik, K. Center of Ventilation—Methods of Calculation Using Electrical Impedance Tomography and the Influence of Image Segmentation. In XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016; Kyriacou, E., Christofides, S., Pattichis, C.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 57, pp. 1264–1269. ISBN 978-3-319-32701-3. [Google Scholar]
- Bauer, M.; Opitz, A.; Filser, J.; Jansen, H.; Meffert, R.H.; Germer, C.T.; Roewer, N.; Muellenbach, R.M.; Kredel, M. Perioperative redistribution of regional ventilation and pulmonary function: A prospective observational study in two cohorts of patients at risk for postoperative pulmonary complications. BMC Anesthesiol. 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Möller, K.; Steinmann, D.; Frerichs, I.; Guttmann, J. Evaluation of an electrical impedance tomography-based Global Inhomogeneity Index for pulmonary ventilation distribution. Intensive Care Med. 2009, 35, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Pulletz, S.; Frerichs, I.; Müller-Lisse, U.; Möller, K. The EIT-based global inhomogeneity index is highly correlated with regional lung opening in patients with acute respiratory distress syndrome. BMC Res. Notes 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Ukere, A.; März, A.; Wodack, K.H.; Trepte, C.J.; Haese, A.; Waldmann, A.D.; Böhm, S.H.; Reuter, D.A. Perioperative assessment of regional ventilation during changing body positions and ventilation conditions by electrical impedance tomography. Br. J. Anaesth. 2016, 117, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, S.; Mauri, T.; Böhm, S.H.; Scaramuzzo, G.; Turrini, C.; Waldmann, A.D.; Ragazzi, R.; Pesenti, A.; Volta, C.A. Variation of poorly ventilated lung units (silent spaces) measured by electrical impedance tomography to dynamically assess recruitment. Crit. Care 2018, 22, 26. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Zinserling, J.; Greschus, S.; Fimmers, R.; Guenther, U.; Buchwald, M.; Grigutsch, D.; Leonhardt, S.; Putensen, C.; et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury*. Crit. Care Med. 2012, 40, 903–911. [Google Scholar] [CrossRef]
- Muders, T.; Hentze, B.; Simon, P.; Girrbach, F.; Doebler, M.R.G.; Leonhardt, S.; Wrigge, H.; Putensen, C. A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography. JCM 2019, 8, 1161. [Google Scholar] [CrossRef]
- Lowhagen, K.; Lundin, S.; Stenqvist, O. Regional intratidal gas distribution in acute lung injury and acute respiratory distress syndrome assessed by electric impedance tomography. Minerva Anestesiol. 2010, 76, 1024–1035. [Google Scholar]
- Karagiannidis, C.; Waldmann, A.D.; Róka, P.L.; Schreiber, T.; Strassmann, S.; Windisch, W.; Böhm, S.H. Regional expiratory time constants in severe respiratory failure estimated by electrical impedance tomography: A feasibility study. Crit. Care 2018, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Victorino, J.A.; Borges, J.B.; Okamoto, V.N.; Matos, G.F.J.; Tucci, M.R.; Caramez, M.P.R.; Tanaka, H.; Sipmann, F.S.; Santos, D.C.B.; Barbas, C.S.V.; et al. Imbalances in regional lung ventilation: A validation study on electrical impedance tomography. Am. J. Respir. Crit. Care Med. 2004, 169, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.; Zhao, Z.; Zabel, P.; Weiler, N.; Frerichs, I. Regional lung response to bronchodilator reversibility testing determined by electrical impedance tomography in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L8–L19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikker, I.G.; Leonhardt, S.; Reis Miranda, D.; Bakker, J.; Gommers, D. Bedside measurement of changes in lung impedance to monitor alveolar ventilation in dependent and non-dependent parts by electrical impedance tomography during a positive end-expiratory pressure trial in mechanically ventilated intensive care unit patients. Crit. Care 2010, 14, R100. [Google Scholar] [CrossRef] [PubMed]
- Zick, G.; Elke, G.; Becher, T.; Schädler, D.; Pulletz, S.; Freitag-Wolf, S.; Weiler, N.; Frerichs, I. Effect of PEEP and tidal volume on ventilation distribution and end-expiratory lung volume: A prospective experimental animal and pilot clinical study. PLoS ONE 2013, 8, e72675. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; He, H.-W.; Möller, K.; Frerichs, I.; Liu, D.; Zhao, Z. Assessment of Lung Recruitment by Electrical Impedance Tomography and Oxygenation in ARDS Patients. Medicine (Baltimore) 2016, 95, e3820. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Bellani, G.; Confalonieri, A.; Tagliabue, P.; Turella, M.; Coppadoro, A.; Citerio, G.; Patroniti, N.; Pesenti, A. Topographic distribution of tidal ventilation in acute respiratory distress syndrome: Effects of positive end-expiratory pressure and pressure support. Crit. Care Med. 2013, 41, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chang, M.-Y.; Chang, M.-Y.; Gow, C.-H.; Zhang, J.-H.; Hsu, Y.-L.; Frerichs, I.; Chang, H.-T.; Möller, K. Positive end-expiratory pressure titration with electrical impedance tomography and pressure–volume curve in severe acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Franchineau, G.; Bréchot, N.; Lebreton, G.; Hekimian, G.; Nieszkowska, A.; Trouillet, J.-L.; Leprince, P.; Chastre, J.; Luyt, C.-E.; Combes, A.; et al. Bedside Contribution of Electrical Impedance Tomography to Setting Positive End-Expiratory Pressure for Extracorporeal Membrane Oxygenation-treated Patients with Severe Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 447–457. [Google Scholar] [CrossRef]
- Mauri, T.; Alban, L.; Turrini, C.; Cambiaghi, B.; Carlesso, E.; Taccone, P.; Bottino, N.; Lissoni, A.; Spadaro, S.; Volta, C.A.; et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: Effects of increasing flow rates. Intensive Care Med. 2017, 43, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Hahn, G.; Just, A.; Dudykevych, T.; Frerichs, I.; Hinz, J.; Quintel, M.; Hellige, G. Imaging pathologic pulmonary air and fluid accumulation by functional and absolute EIT. Physiol. Meas. 2006, 27, S187–S198. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.L.V.; Chaves, C.N.; Gomes, S.; Beraldo, M.A.; Volpe, M.S.; Tucci, M.R.; Schettino, I.A.L.; Bohm, S.H.; Carvalho, C.R.R.; Tanaka, H.; et al. Real-time detection of pneumothorax using electrical impedance tomography. Crit. Care Med. 2008, 36, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.C.A.; De Santis Santiago, R.R.; Filho, J.R.B.d.O.; Hirota, A.S.; Pacce, P.H.D.; Ferreira, J.C.; Camargo, E.D.L.B.; Amato, M.B.P.; Costa, E.L.V. Monitoring of Pneumothorax Appearance with Electrical Impedance Tomography during Recruitment Maneuvers. Am. J. Respir. Crit. Care Med. 2017, 195, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Bußmeyer, M.; Lautenschläger, I.; Schädler, D.; Weiler, N.; Frerichs, I. Characteristic pattern of pleural effusion in electrical impedance tomography images of critically ill patients. Br. J. Anaesth. 2018, 120, 1219–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Peng, S.-Y.; Chang, M.-Y.; Hsu, Y.-L.; Frerichs, I.; Chang, H.-T.; Möller, K. Spontaneous breathing trials after prolonged mechanical ventilation monitored by electrical impedance tomography: An observational study. Acta Anaesthesiol. Scand. 2017, 61, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Odenstedt, H.; Olegård, C.; Söndergaard, S.; Lundin, S.; Stenqvist, O. Regional lung derecruitment after endotracheal suction during volume- or pressure-controlled ventilation: A study using electric impedance tomography. Intensive Care Med. 2007, 33, 172–180. [Google Scholar] [CrossRef]
- Steinmann, D.; Stahl, C.A.; Minner, J.; Schumann, S.; Loop, T.; Kirschbaum, A.; Priebe, H.J.; Guttmann, J. Electrical impedance tomography to confirm correct placement of double-lumen tube: A feasibility study. Br. J. Anaesth. 2008, 101, 411–418. [Google Scholar] [CrossRef]
- Pohlman, M.C.; McCallister, K.E.; Schweickert, W.D.; Pohlman, A.S.; Nigos, C.P.; Krishnan, J.A.; Charbeneau, J.T.; Gehlbach, B.K.; Kress, J.P.; Hall, J.B. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit. Care Med. 2008, 36, 3019–3023. [Google Scholar] [CrossRef]
- Yoshida, T.; Torsani, V.; Gomes, S.; De Santis, R.R.; Beraldo, M.A.; Costa, E.L.V.; Tucci, M.R.; Zin, W.A.; Kavanagh, B.P.; Amato, M.B.P. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2013, 188, 1420–1427. [Google Scholar] [CrossRef]
- Borges, J.B.; Suarez-Sipmann, F.; Bohm, S.H.; Tusman, G.; Melo, A.; Maripuu, E.; Sandström, M.; Park, M.; Costa, E.L.V.; Hedenstierna, G.; et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J. Appl. Physiol. 2012, 112, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Hentze, B.; Muders, T.; Luepschen, H.; Maripuu, E.; Hedenstierna, G.; Putensen, C.; Walter, M.; Leonhardt, S. Regional lung ventilation and perfusion by electrical impedance tomography compared to single-photon emission computed tomography. Physiol. Meas. 2018, 39, 065004. [Google Scholar] [CrossRef]
- Hellige, N.C.; Hahn, G.; Hellige, G. Comment on Borges et al. “regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse”. J. Appl. Physiol. 2012, 112, 2127, author reply 2128. [Google Scholar] [CrossRef]
- Bluth, T.; Kiss, T.; Kircher, M.; Braune, A.; Bozsak, C.; Huhle, R.; Scharffenberg, M.; Herzog, M.; Roegner, J.; Herzog, P.; et al. Measurement of relative lung perfusion with electrical impedance and positron emission tomography: An experimental comparative study. Br. J. Anaesth. 2019. [Google Scholar] [CrossRef]
- Brown, B.H.; Leathard, A.; Sinton, A.; McArdle, F.J.; Smith, R.W.; Barber, D.C. Blood flow imaging using electrical impedance tomography. Clin. Phys. Physiol. Meas. 1992, 13, 175–179. [Google Scholar] [CrossRef]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Quintel, M.; Hellige, G. Regional lung perfusion as determined by electrical impedance tomography in comparison with electron beam CT imaging. IEEE Trans. Med. Imaging 2002, 21, 646–652. [Google Scholar] [CrossRef]
- Reinius, H.; Borges, J.B.; Fredén, F.; Jideus, L.; Camargo, E.D.L.B.; Amato, M.B.P.; Hedenstierna, G.; Larsson, A.; Lennmyr, F. Real-time ventilation and perfusion distributions by electrical impedance tomography during one-lung ventilation with capnothorax. Acta Anaesthesiol. Scand. 2015, 59, 354–368. [Google Scholar] [CrossRef]
- Hentze, B.; Muders, T.; Luepschen, H.; Leonhardt, S.; Putensen, C.; Walter, M. Gamma-variate modeling of indicator dilution curves in electrical impedance tomography. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 3596–3599. [Google Scholar]
- Vonk Noordegraaf, A.; Kunst, P.W.; Janse, A.; Marcus, J.T.; Postmus, P.E.; Faes, T.J.; de Vries, P.M. Pulmonary perfusion measured by means of electrical impedance tomography. Physiol. Meas. 1998, 19, 263–273. [Google Scholar] [CrossRef]
- Grant, C.A.; Pham, T.; Hough, J.; Riedel, T.; Stocker, C.; Schibler, A. Measurement of ventilation and cardiac related impedance changes with electrical impedance tomography. Crit. Care 2011, 15, R37. [Google Scholar] [CrossRef]
- Leonhardt, S.; Pikkemaat, R.; Stenqvist, O.; Lundin, S. Electrical Impedance Tomography for hemodynamic monitoring. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 122–125. [Google Scholar]
- Eyüboglu, B.M.; Brown, B.H. Methods of cardiac gating applied potential tomography. Clin. Phys. Physiol. Meas. 1988, 9, 43–48. [Google Scholar] [CrossRef]
- Braun, F.; Proença, M.; Adler, A.; Riedel, T.; Thiran, J.-P.; Solà, J. Accuracy and reliability of noninvasive stroke volume monitoring via ECG-gated 3D electrical impedance tomography in healthy volunteers. PLoS ONE 2018, 13, e0191870. [Google Scholar] [CrossRef]
- Frerichs, I.; Pulletz, S.; Elke, G.; Reifferscheid, F.; Schadler, D.; Scholz, J.; Weiler, N. Assessment of changes in distribution of lung perfusion by electrical impedance tomography. Respiration 2009, 77, 282–291. [Google Scholar] [CrossRef]
- Zadehkoochak, M.; Blott, B.H.; Hames, T.K.; George, R.F. Pulmonary perfusion and ventricular ejection imaging by frequency domain filtering of EIT (electrical impedance tomography) images. Clin. Phys. Physiol. Meas. 1992, 13, 191–196. [Google Scholar] [CrossRef]
- Deibele, J.M.; Luepschen, H.; Leonhardt, S. Dynamic separation of pulmonary and cardiac changes in electrical impedance tomography. Physiol. Meas. 2008, 29, S1–S14. [Google Scholar] [CrossRef]
- Kunst, P.W.; Vonk Noordegraaf, A.; Hoekstra, O.S.; Postmus, P.E.; de Vries, P.M. Ventilation and perfusion imaging by electrical impedance tomography: A comparison with radionuclide scanning. Physiol. Meas. 1998, 19, 481–490. [Google Scholar] [CrossRef]
- Fagerberg, A.; Stenqvist, O.; Aneman, A. Monitoring pulmonary perfusion by electrical impedance tomography: An evaluation in a pig model. Acta Anaesthesiol. Scand. 2009, 53, 152–158. [Google Scholar] [CrossRef]
- Pikkemaat, R.; Lundin, S.; Stenqvist, O.; Hilgers, R.-D.; Leonhardt, S. Recent advances in and limitations of cardiac output monitoring by means of electrical impedance tomography. Anesth. Analg. 2014, 119, 76–83. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Janse, A.; Marcus, J.T.; Bronzwaer, J.G.; Postmust, P.E.; Faes, T.J.; De Vries, P.M. Determination of stroke volume by means of electrical impedance tomography. Physiol. Meas. 2000, 21, 285–293. [Google Scholar] [CrossRef]
- Braun, F.; Proença, M.; Lemay, M.; Bertschi, M.; Adler, A.; Thiran, J.-P.; Solà, J. Limitations and challenges of EIT-based monitoring of stroke volume and pulmonary artery pressure. Physiol. Meas. 2018, 39, 014003. [Google Scholar] [CrossRef] [Green Version]
- Proença, M.; Braun, F.; Rapin, M.; Solà, J.; Adler, A.; Grychtol, B.; Bohm, S.H.; Lemay, M.; Thiran, J.-P. Influence of heart motion on cardiac output estimation by means of electrical impedance tomography: A case study. Physiol. Meas. 2015, 36, 1075–1091. [Google Scholar] [CrossRef]





| Tissue | Resistivity (Ω·cm) |
|---|---|
| Blood | 150 |
| Lungs, inspiration | 2400 |
| Lungs, expiration | 700 |
| Heart muscle, longitudinal | 125 |
| Heart muscle, transversal | 1800 |
| Skeletal muscle, longitudinal | 160–575 |
| Skeletal muscle, transversal | 420–5200 |
| Fat | 2000–2700 |
| Bone | 16,600 |
| Manufacturer | EIT System | Electrodes | Image Reconstruction Algorithm | Measurement and Data Acquisition | |
|---|---|---|---|---|---|
| Number | Configuration | ||||
| Swisstom AG | BB2 | 32 | electrode belt | Graz consensus reconstruction algorithm for EIT (GREIT) | pair drive (adjustable skip) |
| algorithm for EIT (GREIT) | serial measurement | ||||
| Timpel SA | Enlight | 32 | electrode stripes | Finite Element Method-based Newton-Raphson method | pair drive (3-electrode skip) |
| parallel measurement | |||||
| CareFusion | Goe-MF II | 16 | individual electrodes | Sheffield back-projection | pair drive (adjacent) |
| serial measurement | |||||
| Dräger Medical | PulmoVista 500 | 16 | electrode belt | Finite Element Method-based Newton-Raphson method | pair drive (adjacent) |
| serial measurement | |||||
| Maltron Inc | Mark 1 | 16 | individual electrodes | Sheffield back-projection | pair drive (adjacent) |
| Mark 3.5 | 8 | individual electrodes | serial measurement | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putensen, C.; Hentze, B.; Muenster, S.; Muders, T. Electrical Impedance Tomography for Cardio-Pulmonary Monitoring. J. Clin. Med. 2019, 8, 1176. https://doi.org/10.3390/jcm8081176
Putensen C, Hentze B, Muenster S, Muders T. Electrical Impedance Tomography for Cardio-Pulmonary Monitoring. Journal of Clinical Medicine. 2019; 8(8):1176. https://doi.org/10.3390/jcm8081176
Chicago/Turabian StylePutensen, Christian, Benjamin Hentze, Stefan Muenster, and Thomas Muders. 2019. "Electrical Impedance Tomography for Cardio-Pulmonary Monitoring" Journal of Clinical Medicine 8, no. 8: 1176. https://doi.org/10.3390/jcm8081176
APA StylePutensen, C., Hentze, B., Muenster, S., & Muders, T. (2019). Electrical Impedance Tomography for Cardio-Pulmonary Monitoring. Journal of Clinical Medicine, 8(8), 1176. https://doi.org/10.3390/jcm8081176






