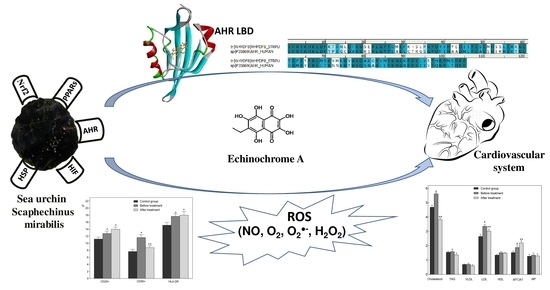
Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets
Abstract
:1. Introduction
2. Experimental Section
2.1. Research Program
2.2. Studies on the Clinical Effect of the Histochrome
2.3. Clinical Effect of Thymarin Dietary Supplement Studies
2.4. Hematological Studies
2.4.1. Biochemical Parameters of the Blood Studies
2.4.2. Method for the Determination of MDA in Red Blood Cells
2.4.3. Method for the Determination of TOA and TAA in Blood Serum
2.4.4. Nitric Oxide (NO) Determination
2.4.5. Determination of the Complex MMP-9/TIMP-1
2.4.6. Immunological Parameters Studies
2.4.7. Indicators Characterizing Lipid Peroxidation (LPO) and the Mechanisms of Antioxidant Protection (AOP) Studies
2.4.8. Data Analysis and Statistics
2.5. Exercise Stress
2.6. Molecular Modeling
2.6.1. The HuAhR Homology Modeling
2.6.2. Protein-Ligand Docking
2.6.3. Molecular Dynamics Simulation
3. Results
3.1. Effects of EchA from Its Use in the Forms of Histochrome and Thymarin on Correction of Lipid Metabolism Disorders and Antioxidant Status in Patients with CVD
3.1.1. The Drug Histochrome
3.1.2. Thymarin Dietary Supplement Effects
3.2. Effect EchA on Hemostasis in CVD Patients
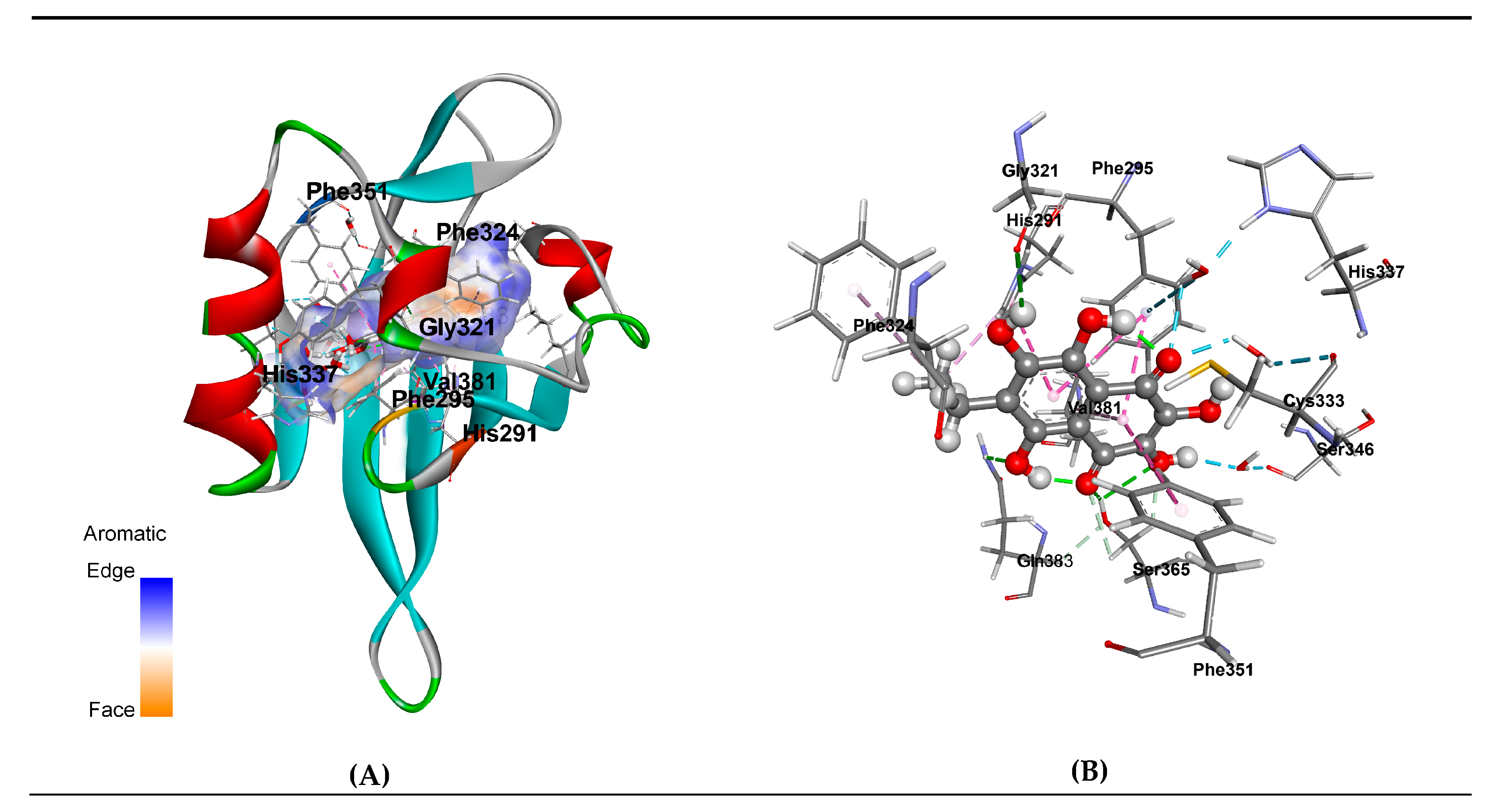
3.3. Molecular Modeling of EchA-huAhR Interactions
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Orr, A.W.; Yurdagul, A., Jr.; Patel, B.M. Pathogenesis of atherosclerosis: from cell biology to therapeutics. In Colloquium Series on Integrated Systems Physiology: From Molecule to Function to Disease; Granger, D.N., Granger, J.P., Eds.; Morgan & Claypool Life Sciences: Williston, VT, USA, 2014; pp. 1–125. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Hansson, G.K.; Robertson, A.K.; Söderberg-Naucler, C. Inflammation and atherosclerosis. Annu. Rev. Pathol.: Mech. Dis. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallas, Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, C.J.; Shaw, P.X.; Chang, M.K.; Bouller, A.; Hartvigsen, K.; Hörkkö, S.; Miller, Y.I.; Woelkers, D.A.; Corr, M.; Witztum, J.L. The role of natural antibodies in atherogenesis. J. Lipid Res. 2005, 46, 1357–1363. [Google Scholar] [CrossRef] [Green Version]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessner, A.; Weyand, C.M. Dendritic cells in atherosclerotic disease. Clin. Immunol. 2010, 134, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitriu, I.E.; Kaski, J.C. The role of T and B cells in atherosclerosis: potential clinical implications. Curr. Pharm. Des. 2011, 17, 4159–4171. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Perry, H.M.; Bender, T.P.; McNamara, C.A. B cells subsets in atherosclerosis. Front. Immunol. 2012, 3, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobryshev, Y.V.; Karagodin, V.P.; Orekhov, A.N. Dendritic cells and their role in immune reaction of atherosclerosis. Cell Tissue Biol. 2013, 7, 113–125. [Google Scholar] [CrossRef]
- Subramanian, M.; Thorp, E.; Hansson, G.K.; Tobas, I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Investig. 2013, 123, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [Green Version]
- Granik, V.G. Some chemical and biochemical aspects of the problem of atherosclerosis. Pharm. Chem. J. 2012, 46, 139–153. [Google Scholar] [CrossRef]
- Bandeali, S.; Farmer, J. High-density lipoprotein and atherosclerosis: the role of antioxidant activity. Curr. Atheroscler. Rep. 2012, 14, 101–107. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Gale, C.R.; Ashurst, H.E.; Powers, H.J.; Martyn, C. Antioxidant vitamin status and carotid atherosclerosis in the elderly. Am. J. Clin. Nutr. 2001, 74, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Salonen, R.M.; Nyyssönen, K.; Kaikkonen, J.; Porkkala-Sarataho, E.; Voutilainen, S.; Rissanen, T.H.; Tuomainen, T.P.; Valkonen, V.P.; Ristonmaa, U.; Lakka, H.M.; et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the antioxidant supplementation in atherosclerosis prevention (ASAP) study. Circulation 2003, 107, 947–953. [Google Scholar] [CrossRef]
- Cherubini, A.; Vigna, G.B.; Zuliani, G.; Ruggiero, C.; Senin, U.; Fellin, R. Role of antioxidants in atherosclerosis: epidemiological and clinical update. Curr. Pharm. Des. 2005, 11, 2017–2032. [Google Scholar] [CrossRef]
- Siekmeier, R.; Steffen, C.; März, W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J. Cardiovasc. Pharmacol. Ther. 2007, 12, 265–282. [Google Scholar] [CrossRef]
- Niki, E. Antioxidants and atherosclerosis. Biochem. Soc. Trans. 2004, 32, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Kehrle, B.; Kohlhammer, K.; Grüb, M.; Koenig, W.; Hombach, V.; Plutzky, J. PPAR Activators as Antiinflammatory Mediators in Human T Lymphocytes. Circ. Res. 2002, 90, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, G.; Duez, H.; Blanquart, C.; Berezowski, V.; Poulain, P.; Fruchart, J.C.; Najib-Fruchart, J.; Glineur, C.; Staels, B. Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I. J Clin. Invest. 2001, 107, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkkilä, A.T.; Booth, S.L. Vitamin K intake and atherosclerosis. Cur. Opin. Lipidol. 2008, 19, 39–42. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Bots, M.L.; Atsmaa, F.; Bartelinka, M.-L.; Prokopb, M.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; van der Schouw, Y.T. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 20, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef]
- Chatrou, M.L.L.; Winckers, K.; Hackeng, T.M.; Reutelingsperger, C.P.; Schurgers, L. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012, 26, 155–166. [Google Scholar] [CrossRef]
- Yi, T.; Wang, J.; Zhu, K.; Tang, Y.L.; Huang, S.; Shui, X.; Chen, Y.; Lei, W. Aryl Hydrocarbon Receptor: A New Player of Pathogenesis and Therapy in Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Maksimov, O.B.; Mishchenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A.A. Histochrome and Its Therapeutic Use in Acute Myocardial Infarction and Ischemic Heart Disease. US Patent 6,410,601, 25 June 2001. [Google Scholar]
- Potapov, V.N.; Lupach, N.M.; Veselkina, E.J.; Khludeeva, E.A.; Artjukov, A.A.; Kurika, A.V.; Kozlovskaja, E.P.; Rasskazov, V.A.; Dolgikh, S.N. Method of Lipid Metabolic Disorder Correction. Russian Patent 2,337,696, 10 November 2008. [Google Scholar]
- Potapov, V.N.; Lupach, N.M.; Veselkina, E.J.; Khludeeva, E.A.; Artjukov, A.A.; Kurika, A.V.; Kozlovskaja, E.P.; Rasskazov, V.A.; Dolgikh, S.N.; Luk’janov, P.A. Way of Correction of Endothelial Dysfunction. Russian Patent 2,359,686, 27 June 2009. [Google Scholar]
- Artyukov, A.A.; Popov, A.M.; Tsybulsky, A.V.; Krivoshapko, O.N.; Polyakova, N.V. Pharmacological activity of echinochrome A alone and in the biologically active additive Timarin. Biochem. (Moscow) Suppl. Ser. B: Biomed. Chem. 2013, 7, 237–242. [Google Scholar] [CrossRef]
- Tsybulsky, A.V.; Popov, A.M.; Artyukov, A.A.; Kostetsky, E.Y.; Krivoshapko, O.N.; Maseyka, A.N.; Kozlovskaya, E.P. The comparative study of the medical action of lyuteolin, rosmarinic acid and echinochrom A at experimental stress-induced cardiopathology. Biomeditsinskaia Khimiia 2011, 57, 314–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agafonova, I.G.; Bogdanovich, R.N.; Kolosova, N.G. Assessment of Nephroprotective Potential of Histochrome during Induced Arterial Hypertension. Bull. Exp. Biol. Med. 2015, 160, 223–227. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Noh, S.J.; Marquez, J.; Ko, K.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome A increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef] [PubMed]
- Talalaeva, O.S.; Momot, A.P.; Bryukhanov, V.M.; Zverev, Y.F.; Zamyatina, S.V.; Mishenko, N.P.; Lycheva, N.A. Effect of prolonged histochrome introduction on haemostasis in rats. Trombos Hemostas Reol. (Mosc.) 2014, 2, 33–36. [Google Scholar]
- Hasanov, B.B.; Ryzhov, G.L.; Maltseva, E.B. Methods of research antioxidants. Khimiya Rastit. Syrya 2004, 3, 63–75. [Google Scholar]
- Key, J.; Scheuermann, T.H.; Anderson, P.C.; Daggett, V.; Gardner, K.H. Principles of ligand binding within a completely buried cavity in HIF2α PAS-B. J. Am. Chem. Soc. 2009, 131, 17647–17654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using modeller. Curr. Protoc. Protein Sci. 2007, 50, 2.9.1–2.9.31. [Google Scholar] [CrossRef] [Green Version]
- Molecular Operating Environment (MOE), 2013.08; H3A 2R7; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016.
- Case, D.A.; Darden, T.A.; Iii, T.E.C.; Simmerling, C.L.; Wang, J.; Duke, E.H.M.; Luo, R.; Walker, R.C. Amber 12; University of California: San Francisco, CA, USA, 2012. [Google Scholar]
- Gerber, P.R.; Müller, K. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J. Comput. Aided Mol. Des. 1995, 9, 251–268. [Google Scholar] [CrossRef]
- MOPAC; James, J.P. Stewart Computational Chemistry: Colorado Springs, CO, USA. 2009. Available online: http://OpenMOPAC.net (accessed on 10 February 2020).
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminf. 2009, 1, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afanasiev, S.A.; Vecherskiy, Y.Y.; Maksimov, I.V.; Markov, V.A.; Rebrova, T.Y. Cardioprotective Effect of Antioxidant Histochrome in Cardiology and Cardiac Surgery Practice; STT: Tomsk, Russia, 2012; pp. 1–150. ISBN 978-5-93629-463-1. [Google Scholar]
- Tsybul’skii, A.V.; Popov, A.M.; Artiukov, A.A.; Mazeika, A.N.; Kostetskii, E.A.; Sanina, N.M.; Krivoshapko, O.N. Enhancing the immunogenic activity of influvac vaccine in the use of adjuvant TI complexes modified by echinochrome A. Vopr. Virusol. 2012, 57, 23–27. [Google Scholar] [PubMed]
- Seneviratne, A.N.; Sivagurunathan, B.; Monaco, C. Toll-like receptors and macrophage activation in atherosclerosis. Clin. Chim. Acta 2012, 413, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fenyo, I.M.; Gafencu, A.V. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology 2013, 218, 1376–1384. [Google Scholar] [CrossRef]
- Hanieh, H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends. BioMed Res. Int. 2014, 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Barouki, R.; Aggerbeck, M.; Aggerbeck, L.; Coumoul, X. The aryl hydrocarbon receptor system. Drug Metabol. Drug Interact. 2012, 27, 3–8. [Google Scholar] [CrossRef]
- Hirano, M.; Hwang, J.H.; Park, H.J.; Bak, S.M.; Iwata, H.; Kim, E.Y. In silico analysis of the interaction of avian aryl hydrocarbon receptors and dioxins to decipher isoform-, ligand-, and species-specific activations. Environ. Sci. Technol. 2015, 49, 3795–3804. [Google Scholar] [CrossRef]
- Fukunaga, B.N.; Probst, M.R.; Reisz-Porszasz, S.; Hankinson, O. Identification of functional domains of the aryl hydrocarbon receptor. J. Biol. Chem. 1995, 270, 29270–29278. [Google Scholar] [CrossRef] [Green Version]
- McGuire, J.; Okamoto, K.; Whitelaw, M.L.; Tanaka, H.; Poellinger, L. Definition of a dioxin receptor mutant that is a constitutive activator of transcription: delineation of overlapping repression and ligand binding functions within the PAS domain. J. Biol. Chem. 2001, 276, 41841–41849. [Google Scholar] [CrossRef] [Green Version]
- Seok, S.-H.; Lee, W.; Jiang, L.; Molugu, K.; Zheng, A.; Li, Y.; Park, S.; Bradfield, C.A.; Xing, Y. Structural hierarchy controlling dimerization and target DNA recognition in the AHR transcriptional complex. Proc. Natl. Acad. Sci. USA 2017, 114, 5431–5436. [Google Scholar] [CrossRef] [Green Version]
- Bonati, L.; Corrada, D.; Tagliabue, S.G.; Motta, S. Molecular modeling of the AhR structure and interactions can shed light on ligand-dependent activation and transformation mechanisms. Curr. Opin. Pharmacol. 2017, 2, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motto, I.A.; Bordogna, A.A.; Soshilov, M.l.; Denison, S.; Bonati, L.A. New Aryl Hydrocarbon Receptor Homology Model Targeted to Improve Docking Reliability. J. Chem. Inf. Model. 2011, 51, 2868–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernomian L, da Silva CH Current basis for discovery and development of aryl hydrocarbon receptor antagonists for experimental and therapeutic use in atherosclerosis. Eur. J. Pharmacol. 2015, 764, 118–123. [CrossRef] [PubMed]
- Pandini, A.; Soshilov, A.A.; Song, Y.; Zhao, J.; Bonati, L.; Denison, M.S. Detection of the TCDD binding-fingerprint within the Ah receptor ligand binding domain by structurally driven mutagenesis and functional analysis. Biochemistry 2009, 48, 5972–5983. [Google Scholar] [CrossRef] [Green Version]
- Bisson, W.H.; Koch, D.; O’Donnell, E.F.; Kerkvliet, N.I.; Tanguay, R.L.; Abagyan, R.; Kolluri, S.K. Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J. Med. Chem. 2009, 52, 5635–5641. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.; Phillips, J.L.; Kerkvliet, N.I.; Tanguay, R.L.; Perdew, G.H.; Kolluri, S.K.; Bisson, W.H. A structural switch between agonist and antagonist bound conformations for a ligand-optimized model of the human aryl hydrocarbon receptor ligand binding domain. Biology 2014, 3, 645–669. [Google Scholar] [CrossRef]
- Goryo, K.; Suzuki, A.; Carpio, C.A.D.; Siizaki, K.; Kuriyama, E.; Mikami, Y.; Kinoshita, K.; Yasumoto, K.-I.; Rannug, A.; Miyamoto, A.; et al. Identification of amino acid residues in the Ah receptor involved in ligand binding. Biochem. Biophys. Res. Commun. 2007, 354, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Backlund, M.; Ingelman-Sundberg, M. Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high- and low-affinity ligand binding and receptor activation. Mol. Pharmacol. 2004, 65, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura-Alves, P.; Fae, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Krasnovid, N.L.; Koltzova, E.A. Weak acid properties of hydroxylated naphthazarins and their reaction with superoxide anion-radical. Vopr. Med. Khim. 1999, 45, 123–130. [Google Scholar] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Krasnovid, N.L. Interaction of natural polyhydroxy-1,4-naphthoquinones with superoxide anion-radical. Biochemistry (Biokhimiia) 1999, 64, 1273–1278. [Google Scholar] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Ruuge, E.K. How do calcium ions induce free radical oxidation of hydroxy-1,4-naphthoquinone? Ca2+ stabilizes the naphthosemiquinone anion-radical of echinochrome A. Arch. Biochem. Biophys. 2003, 413, 191–198. [Google Scholar] [CrossRef]
- Novikov, V.L.; Shestak, O.P.; Mishchenko, N.P.; Fedoreev, S.A.; Vasileva, E.A.; Glazunov, V.P.; Artyukov, A.A. Oxidation of 7-ethyl-2,3,5,6,8-pentahydroxy-1,4-naphthoquinone (echinochrome A) by atmospheric oxygen 1. Structure of dehydroechinochrome. Russ. Chem. Bull. 2018, 67, 282–290. [Google Scholar] [CrossRef]
- Kruger-Zeitzer, E.; Sullivan, S.G.; Stern, A.; Munday, R. Effects of 1,4-naphthoquinone derivatives on red blood cell metabolism. J. Appl. Toxicol. 1990, 10, 129–133. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. Role of Oxidant Stress in Lawsone-Induced Hemolytic Anemia. Toxicol. Sci. 2004, 82, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [Green Version]
- Perry, G.; Epel, D. Ca2+-stimulated production of H2O2 from naphthoquinone oxidation in Arbacia eggs. Exp. Cell Res. 1981, 134, 65–72. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-α transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009, 296, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Busquets-Cortes, C.; Capo, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of Millimolar Steady-State Hydrogen Peroxide Exposure on Inflammatory and Redox Gene Expression in Immune Cells from Humans with Metabolic Syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef] [Green Version]
- Suhara, T.; Fukuo, K.; Sugimoto, T.; Morimoto, S.; Nakahashi, T.; Hata, S.; Shimizu, M.; Ogihara, T. Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. J. Immunol. 1998, 160, 4042–4047. [Google Scholar] [PubMed]
- Afanas’ev, S.A.; Lasukova, T.V.; Chernyavskii, A.M. ATP-sparing effect of histochrome in acute myocardial ischemia in patients with coronary heart disease. B Exp. Biol. Med. 1997, 124, 1217–1219. [Google Scholar] [CrossRef]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.A.; Vlisidou, I.; Blunt, M.D.; Wood, W.; Ward, S.G. Hydrogen Peroxide Triggers a Dual Signaling Axis to Selectively Suppress Activated Human T Lymphocyte Migration. J. Immunol. 2017, 198, 3679–3689. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, V.K.; Kozlov, M.V.; Lebedko, О.А.; Yephimenko, M.V.; Guseva, O.E.; Morozova, N.V. Influence of echinochrome A on some parameters of systemic free-radical status and T-cell immunity under chronic inflammatory lung diseases in children at the period of remission. Far East Med. J. 2010, 1, 55–58. [Google Scholar]
- Sung, D.J.; So, W.Y.; Ryu, H.Y.; An, H.S.; Cha, K.S. Induction of vasodilation by hydrogen peroxide and its application in exercise science. Biol. Sport 2012, 29, 87–92. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y. NAD(P)H: quinone oxidoreductase 1 and its potential protective role in cardiovascular diseases and related conditions. Cardiovasc. Toxicol. 2012, 12, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Glazunov, V.P.; Berdyshev, D.V.; Novikov, V.L. DFT study of mechanisms of the antioxidant effect of natural polyhydroxy-1,4-naphthoquinones. Reactions of echinamines A and B, metabolites of sea urchin Scaphechinus mirabilis, with hydroperoxyl radical. Russ. Chem. B 2014, 63, 1993–1999. [Google Scholar] [CrossRef]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Burke, R.D.; et al. The Genome of the Sea Urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, J.V.; Hamdoun, A.; Cole, B.J.; Howard-Ashby, M.; Nebert, D.W.; Scally, M.; Dean, M.; Epel, D.; Hahn, M.E.; Stegeman, J.J. The chemical defensome: Environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev. Biol. 2006, 300, 366–384. [Google Scholar] [CrossRef] [Green Version]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef] [Green Version]
- Jaronen, M.; Quintana, F.J. Immunological Relevance of the Coevolution of IDO1 and AHR. Front. Immunol. 2014, 5, 521. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Jobin, C.; Chen, Z.E.; Zhou, L. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, C.; Tischkau, S.A. Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction. Environ. Health Insights 2016, 10, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaal, A.Q.; Jaeger, C.D.; Bottum, K.M.; Tischkau, S.A. Environmental factors act through aryl hydrocarbon receptor activation and circadian rhythm disruption to regulate energy metabolism. J. Recept. Ligand Channel Res. 2018, 10, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Horke, S.; Förstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]



| Total Surveyed | Anthropometric Characteristics | Risk Factors | Clinical Characteristics | |||||
|---|---|---|---|---|---|---|---|---|
| Age | Men | Women | Smoking | Arterial Hypertension | A Burdened History of Cardiovascular Disease | Persons with Hyperchole- Sterolemia | Patients with Stable Angina 3 Functional Class | |
| n (%) | year | n (%) | n (%) | n (%) | ||||
| 140.0 (100) | 53.63 ± 0.16 | 53 (38.86) | 87 (62.14) | 44 (31.42) | 105 (95.24) | 105 (95.24) | 105 (95.24) | 100 (71.14) |
| Group (n) | TPO (Units) | FR (Units) | NO (µmole/L) | MDA Erythro- Cytes (nmole/gHb) | MDA Plasma (µmole/L) | TAA (%) | TOA (%) |
|---|---|---|---|---|---|---|---|
| Control (n = 15) | 449.0 ± 54.6 | 73.4 ± 3.4 | 13.9 ± 0.3 | 9.84 ± 0.31 | 3.54 ± 0.21 | 115.77 ± 1.85 | 13.49 ± 1.45 |
| Before treatment (n = 15) | 445.0 ± 60.0 | 72.8 ± 3.1 | 14.1 ± 0.4 | 10.50 ± 0.40 | 3.61 ± 0.22 | 110.0 ± 2.1 | 13.60 ± 0.87 |
| After treatment (n = 15) | 334.0 ± 26.0 * | 67.2 ± 2.6 * | 14.5 ± 0.7 | 10.17 ± 0.24 * | 2.88 ± 0.23 * | 110.0 ± 2.9 | 12.0 ± 0.58 * |
| Indicators | Placebo Group | Main Group | Comparison Group | |||
|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Before Treatment with Dietary Supplements | After Treatment with Dietary Supplements | Before Treatment with Atorvastatin | After Treatment with Atorvastatin | |
| n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | |
| Total Cholesterol, mmol/L | 5.46 ± 0.28 | 5.72 ± 0.47 | 6.21 ± 0.02 | 5.40 ± 0.13 * | 6.49 ± 0.05 | 4.00 ± 0.24 |
| Cholesterol of LDL, mmol/L | 3.40 ± 0.32 | 3.90 ± 0.46 | 3.87 ± 0.16 | 3.20 ± 0.10 * | 3.88 ± 0.21 | 2.43 ± 0.13 |
| Cholesterol of HDL, mmol/L | 1.71 ± 0.33 | 1.38 ± 0.12 | 1.30 ± 0.09 | 1.36 ± 0.06 | 0.99 ± 0.15 | 1.00 ± 0.10 |
| Cholesterol of VLDL, mmol/L | 0.78 ± 0.16 | 0.90 ± 0.12 | 1.10 ± 0.13 | 0.90 ± 0.07 | 1.12 ± 0.14 | 0.98 ± 0.08 |
| Triglycerides, TAG, mmol/L | 1.88 ± 0.29 | 1.73 ± 0.25 | 1.98 ± 0.15 | 1.61 ± 0.18 | 2.12 ± 0.20 | 1.88 ± 0.13 |
| Total Antioxidant Activity, TAA, % | 115.77 ± 1.85 | 115.82 ± 1.90 | 114.20 ± 1.05 | 121.10 ± 0.85 * | 109.80 ± 2.50 | 109.70 ± 2.30 |
| Total Oxidant Activity, TOA, % | 13.49 ± 1.45 | 12.74 ± 1.34 | 14.50 ± 0.29 | 11.00 ± 0.32 * | 14.60 ± 0.90 | 14.00 ± 0.30 |
| Indicators | Before Treatment with Dietary Supplements | After Treatment with Dietary Supplements |
|---|---|---|
| Total cholesterol, mol/L | 7.17 | 5.44 |
| Cholesterol of LDL, mol/L | 5.25 | 2.96 |
| Cholesterol of HDL, mmol/L | 1.19 | 1.15 |
| Cholesterol of VLDL, mmol/L | 0.73 | 1.33 |
| Triglycerides, mmol/L | 8.43 | 5.75 |
| Atherogenic Index | 5.03 | 3.07 |
| Total Antioxidant Activity, TAA, % | 119.00 | 124.00 |
| Total Oxidant Activity, TOA, % | 20.00 | 14.00 |
| Aspartate Aminotransferase, AAT, U/L | 87.00 | 43.00 |
| Alanine Aminotransferase, ALT, U/L | 68.00 | 27.00 |
| Indicators | Control Group | Main Group | Comparison Group | ||
|---|---|---|---|---|---|
| Before Treatment with Dietary Supplements | After Treatment with Dietary Supplements | Before Treatment with Atorvastatin | After Treatment with Atorvastatin | ||
| n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | |
| Level of NO Metabolites, µmole/L | 47.00 ± 0.43 | 39.94 ± 0.78 * | 43.70 ± 0.82 *, ** | 35.50 ± 0.65 * | 40.10 ± 0.65 *, ** |
| Level of MMP-9/TIMP-1 Complex, ng/mL | 2.77 ± 0.12 | 5.64 ± 0.16 * | 4.10 ± 0.24 *, ** | 5.12 ± 0.16 * | 4.77 ± 0.14 *, ** |
| Group (n) | Total Protein (g/L) | Alb (%) | BR (µmole/L) | AAT (Units/L) | ALT (Units/L) | Creat (mmol/L) | Urea (mmol/L) | LDH (Units/L) | CPK (Units/L) | CPK MV (Units/L) | C-RP (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 15) | 72.8 ± 0.8 | 51.4 ± 0.7 | 9.6 ± 0.2 * | 28.2 ± 2.4 | 26.9 ± 3.6 | 76.2 ± 4.9 | 5.8 ± 0.5 | 214.3 ± 9.3 | 98.4 ± 8.7 | 20.5 ± 0.9 | 73.4 ± 0.6 |
| Before Treatment (n = 15) | 73.9 ± 0.7 | 52.1 ± 0.6 | 9.7 ± 0.1 | 28.6 ± 2.9 | 27.6 ± 4.6 | 75.0 ± 5.6 | 6.0 ± 0.3 | 230.2 ± 11.6 | 102.0 ± 9.2 | 20.8 ± 1.0 | 73.9 ± 0.7 |
| After Treatment (n = 15) | 75.2 ± 0.9 | 55.1 ± 0.8 | 8.6 ± 0.2 ** | 26.0 ± 1.5 | 23.5 ± 2.2 | 77.2 ± 3.1 | 6.3 ± 0.3 | 216.0 ± 12.7 | 102.0 ± 7.7 | 21.4 ± 1.2 | 75.2 ± 0.9 |
| Group (n) | Total protein (g/L) | Alb (%) | BR (µmole/L) | AAT (Units/L) | ALT (Units/L) | Creatinine (mmol/L) | Urea (mmol/L) | Glucose (mmol/L) |
|---|---|---|---|---|---|---|---|---|
| Control (n = 15) | 71.2 ± 0.7 | 43.7 ± 1.4 | 9.6 ± 0.20 | 28.2 ± 2.4 | 26.9 ± 3.6 | 76.2 ± 4.9 | 5.8 ± 0.5 | 5.9 ± 0.3 |
| Before Treatment (n = 15) | 72.6 ± 0.5 | 41.0 ± 1.4 | 8.90 ± 0.70 | 24.3 ± 1.2 | 24.0 ± 1.2 | 94.9 ± 3.3 | 5.7 ± 0.2 | 6.2 ± 0.4 |
| After Treatment (n = 15) | 73.1 ± 0.5 | 37.9 ± 0.7 | 8.40 ± 0.10 | 24.0 ± 2.0 | 24.0 ± 1.2 | 80.6 ± 3.0 | 5.6 ± 0.2 | 5.2 ± 0.2 |
| Glucose(mmol/L) Before Treatment | Glucose(mmol/L) After Treatment | Lactate (mmol/L) Before Treatment | Lactate (mmol/L) After Treatment | C-peptide (ng/mL) Before Treatment | C-peptide (ng/mL) After Treatment |
|---|---|---|---|---|---|
| 5.20 ± 0.08 | 5.06 ± 0.13 | 2.02 ± 0.18 | 1.79 ± 0.12 | 2.14 ± 0.27 | 2.43 ± 0.34 |
| Group (n) | Leucocytes (109 cells/L) | Thrombocytes (109 cells/L) | MCV (fl) | MCHC (g/L) | E % | CN % | S % | L % | M % |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 15) | 7.1 ± 0.2 | 231.8 ± 14.8 | 82.4 ± 1.1 | 365.0 ± 2.6 | 2.4 ± 0.2 | 2.9 ± 0.2 | 53.6 ± 1.9 | 37.0 ± 1.8 | 5.0 ± 0.2 |
| Before Treatment (n = 15) | 7.2 ± 0.6 | 228.5 ± 12.9 | 81.8 ± 0.8 | 369.0 ± 2.7 | 2.0 ± 0.1 | 2.6 ± 0.1 | 52.3 ± 2.2 | 37.8 ± 1.9 | 4.8 ± 0.1 |
| After Treatment (n = 15) | 6.2 ± 0.3 | 299.0 ± 23.6 * | 81.2 ± 0.8 | 370.8 ± 1.8 | 2.8 ± 0.1 | 3.3 ± 0.1 | 53.8 ± 1.6 | 35.0 ± 1.6 | 4.8 ± 0.1 |
| Group (n) | Leucocytes (109 cells/L) | CD3+, CD4+ | CD3+, CD8+ | CD19+ | IRI | |||
|---|---|---|---|---|---|---|---|---|
| % | 109 cells/L | % | 109 cells/L | % | 109 cells/L | |||
| Control (n = 15) | 6.8 ± 0.4 | 47.10 ± 4.40 | 0.99 ± 0.01 | 27.00 ± 1.00 | 0.65 ± 0.04 | 11.50 ± 0.70 | 0.28 ± 0.02 | 1.7 ± 0.1 |
| Before Treatment (n = 15) | 7.2 ± 0.6 | 47.00 ± 4.11 | 1.00 ± 0.01 | 27.00 ± 1.70 | 0.70 ± 0.05 | 11.00 ± 0.60 | 0.30 ± 0.01 | 1.7 ± 0.1 |
| After Treatment (n = 15) | 6.7 ± 0.8 | 46.20 ± 0.02 | 0.90 ± 0.01 | 24.00 ± 1.00 | 0.50 ± 0.04 * | 12.50 ± 0.60 | 0.20 ± 0.01 | 2.0 ± 0.1 |
| Group (n) | IL-1β (pg/mL) | IL-4 (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | IL-10 (pg/mL) | TNFα (pg/mL) | IFNγ (pg/mL) | sTNF-RII (ng/mL) | sIL-1RII (ng/mL) | sIL-6R (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 15) | 25.6 ± 1.8 | 37.9 ± 2.7 | 35.0 ± 1.9 | 44.9 ± 3.8 | 42.8 ± 4.7 | 26.4 ± 2.4 | 60.2 ± 4.5 | 3.4 ± 0.3 | 7.9 ± 0.2 | 61.4 ± 3.4 |
| Before Treatment (n = 15) | 27.3 ± 1.3 | 38.9 ± 2.9 | 39.0 ± 3.9 | 45.7 ± 4.9 | 47.7 ± 9.3 | 26.6 ± 4.3 | 63.3 ± 14.1 | 3.5 ± 0.1 | 8.1 ± 1.2 | 60.2 ± 3.6 |
| After Treatment (n = 15) | 13.7 ± 2.9 * | 27.6 ± 2.1 | 18.1 ± 2.9 * | 39.4 ± 4.0 | 24.5 ± 4.5 | 22.1 ± 4.5 | 50.4 ± 4.3 | 3.6 ± 0.4 | 6.3 ± 1.3 | 55.0 ± 4.8 |
| Type | Non-Covalent EchA Interaction with the Ligand Binding Domain of Human Aryl Hydrocarbon Receptor (huAhR LBD) | Agonist Binding Involved [64] | Antagonist Binding [64] |
|---|---|---|---|
| Hydrogen Bond | huAhR:Ser365:HG - EchA:O1 | + | − |
| huAhR:Ser365:HG - EchA:O2 | + | − | |
| huAhR:Gln383:HE21 - EchA:O8 | + | + | |
| EchA:H6 - huAhR:Gly321:O | − | + | |
| huAhR:Ser365:HB2 - EchA:O1 | + | − | |
| huAhR:Ser365:HB3 - EchA:O2 | + | - | |
| Pi-Pi Stacked - Pi-Pi T-shaped | huAhR:Phe295 - EchA - huAhR:Phe351 | up to 5% | + |
| huAhR:Phe295 - EchA - huAhR:His291 | |||
| Pi-Alkyl | huAhR:His291 - EchA:C10 | + | + |
| huAhR:Phe324 - EchA:C10 | − | + | |
| EchA - huAhR:Val381 | + | up to 30% | |
| Water-Mediated Hydrogen Bond | huAhR:His 337:HD - HOH - EchA:O4 | + | +/− |
| huAhR:Ser346:O - HOH - EchA:H2 | + | - | |
| huAhR:Phe295: Pi-Orbitals - HOH - EchA:O4 | up to 3% | + | |
| huAhR:Cys33:O - HOH - EchA:O4 | up to 70% | up to 30% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. https://doi.org/10.3390/jcm9051494
Artyukov AA, Zelepuga EA, Bogdanovich LN, Lupach NM, Novikov VL, Rutckova TA, Kozlovskaya EP. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. Journal of Clinical Medicine. 2020; 9(5):1494. https://doi.org/10.3390/jcm9051494
Chicago/Turabian StyleArtyukov, Aleksandr A., Elena A. Zelepuga, Larisa N. Bogdanovich, Natalia M. Lupach, Vyacheslav L. Novikov, Tatyana A. Rutckova, and Emma P. Kozlovskaya. 2020. "Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets" Journal of Clinical Medicine 9, no. 5: 1494. https://doi.org/10.3390/jcm9051494