Performance and Establishment of a Commercial Mycorrhizal Inoculant in Viticulture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rootstock Treatments
2.2. AM Fungal Inoculant
2.3. Biomass Production
2.4. Quantification of Inoculant Establishment Via Digital PCR Assay
2.4.1. DNA Extraction
2.4.2. Digital PCR Assay
2.5. Statistical Analyses
2.5.1. Establishment
2.5.2. Software
3. Results
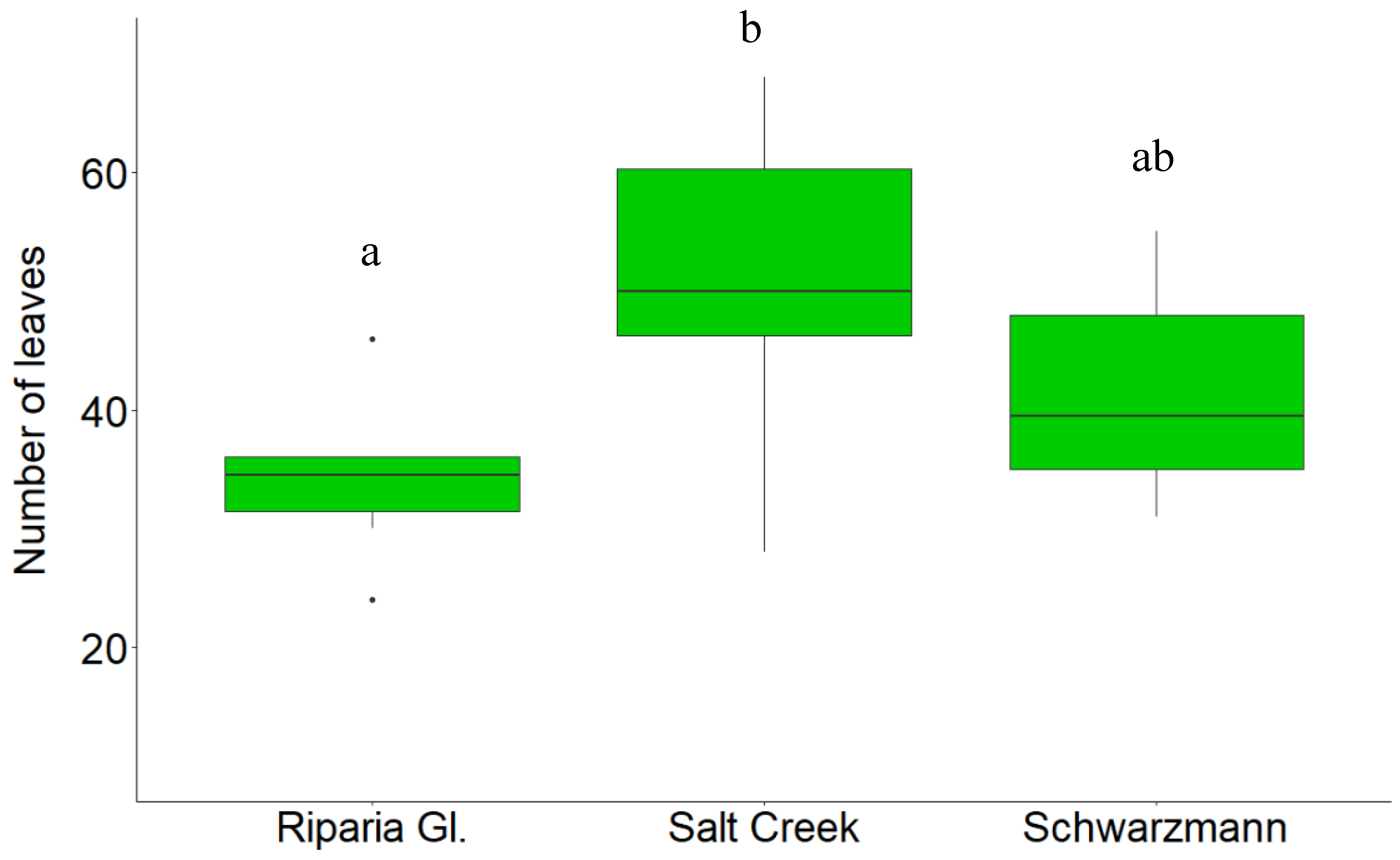
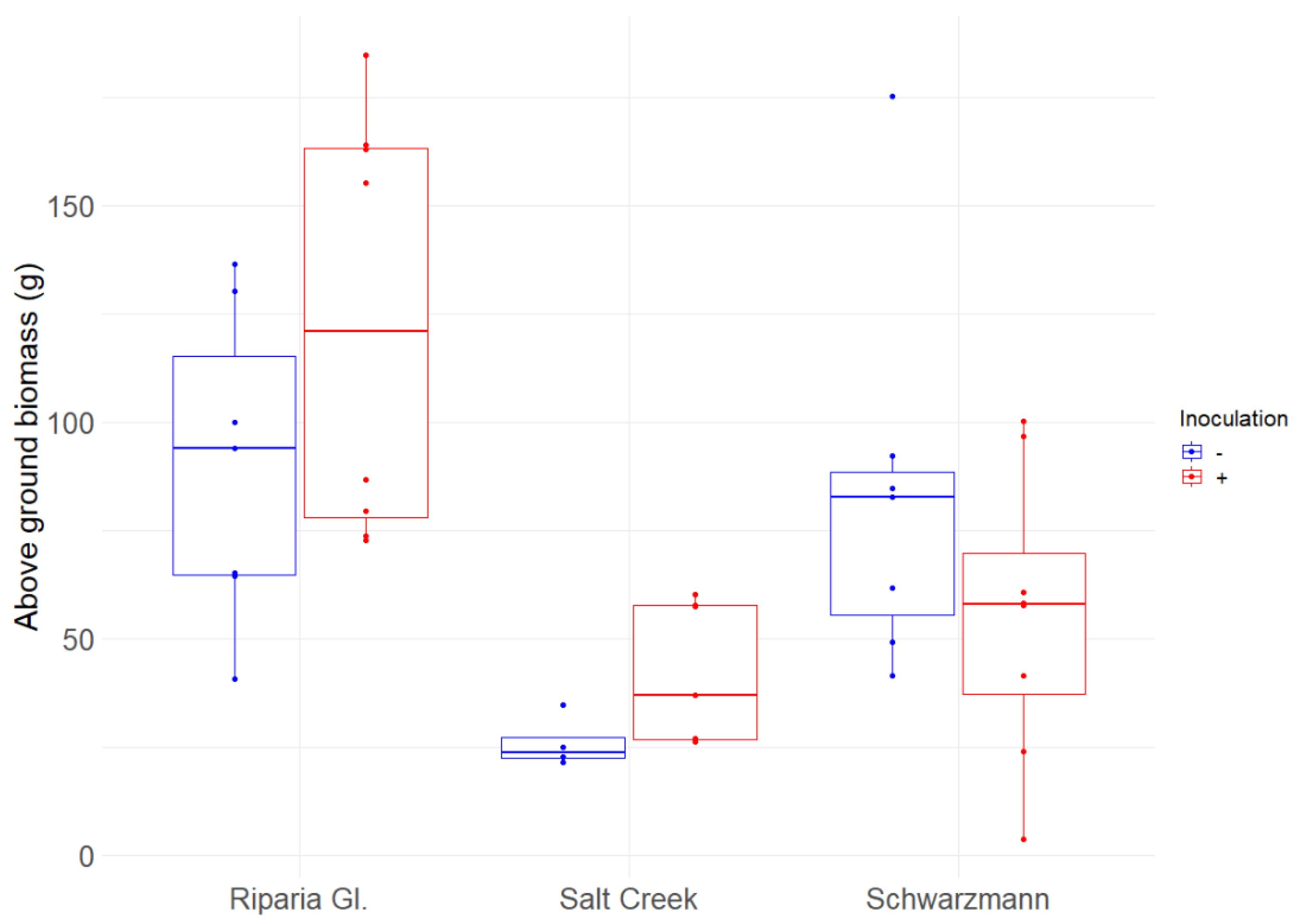
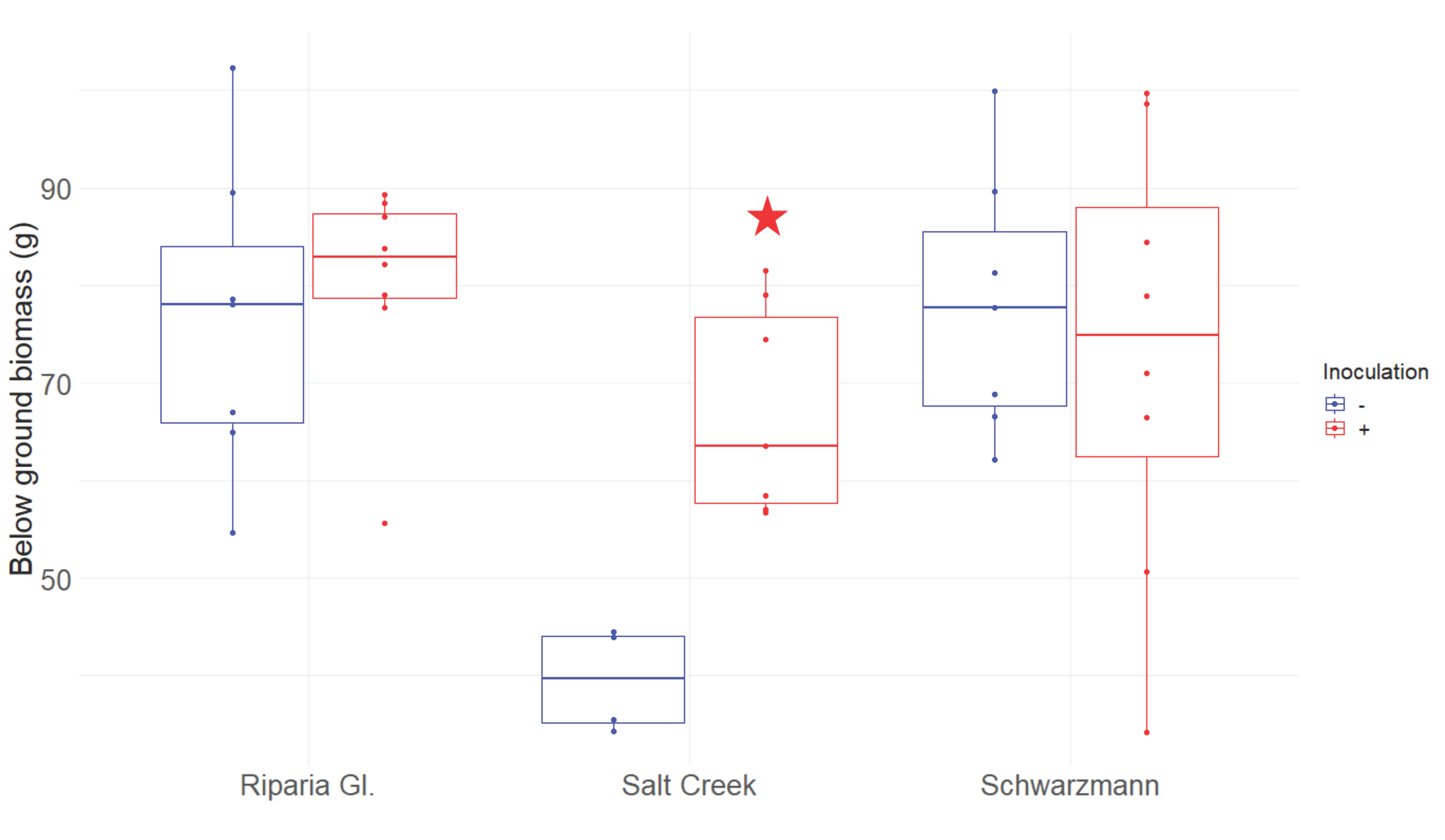
3.1. Growth Response
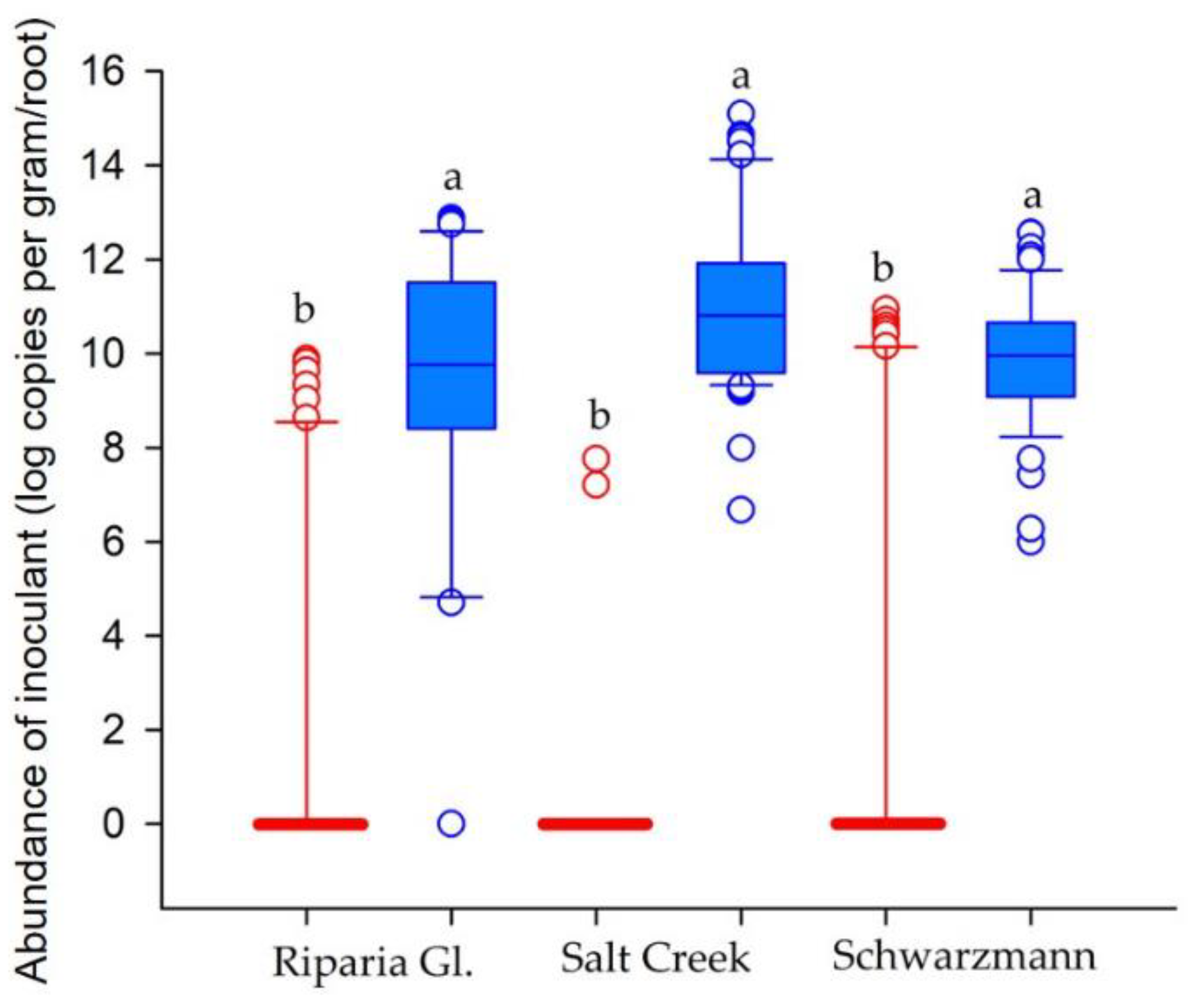
3.2. Establishment of Commercial Inoculant
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Site Information | |
|---|---|
| Soil texture | Loamy sand |
| pH | 6.68 |
| Organic matter (%) | 2.27 |
| Nitrogen (Kjeldahl) | 0.03 |
| Phosphorous (Mehlich 3) | 57.86 |
| Irrigation type | Drip |



References
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; Rosa, D.J.; Ambrosini, V.G.; Heinzen, J.; Ferreira, P.A.A.; Ceretta, C.A.; Soares, C.R.F.S.; Melo, G.W.B.; Soriani, H.H.; Nicoloso, F.T.; et al. Use of phosphorus fertilization and mycorrhization as strategies for reducing copper toxicity in young grapevines. Sci. Hortic. 2019, 248, 176–183. [Google Scholar] [CrossRef]
- Holland, T.; Bowen, P.; Kokkoris, V.; Urbez-Torres, J.R.; Hart, M. Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks? Horticulturae 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Bruisson, S.; Maillot, P.; Schellenbaum, P.; Walter, B.; Gindro, K.; Deglène-Benbrahim, L. Arbuscular mycorrhizal symbiosis stimulates key genes of the phenylpropanoid biosynthesis and stilbenoid production in grapevine leaves in response to downy mildew and grey mould infection. Phytochemistry 2016, 131, 92–99. [Google Scholar] [CrossRef]
- Ferrer, R.L.; Přikryl, Z.; Gryndler, M.; Vančaar;cura, V. Natural Occurrence of Vesicular-Arbuscular Fungi in Grape Vine and Apple Trees. Dev. Soil Sci. 1989, 18, 141–147. [Google Scholar] [CrossRef]
- Biricolti, S.; Ferrini, F.; Rinaldelli, E.; Tamantini, I.; Vignozzi, N. VAM Fungi and Soil Lime Content Influence Rootstock Growth and Nutrient Content. Am. J. Enol. Vitic. 1997, 48, 93–99. [Google Scholar]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef] [Green Version]
- Azcón, R.; Medina, A.; Roldán, A.; Biró, B.; Vivas, A. Significance of treated agrowaste residue and autochthonous inoculates (Arbuscular mycorrhizal fungi and Bacillus cereus) on bacterial community structure and phytoextraction to remediate soils contaminated with heavy metals. Chemosphere 2009, 75, 327–334. [Google Scholar] [CrossRef]
- Ames, R.N.; Reid, C.P.P.; Ingham, E.R. Rhizosphere bacterial population responses to root colonization by a vesicular-arbuscular mycorrh1zal fungus. New Phytol. 1984, 96, 555–563. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Zhang, S.; Wang, C. Effect of earthworms and arbuscular mycorrhizal fungi on the microbial community and maize growth under salt stress. Appl. Soil Ecol. 2016, 107, 214–223. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Antolín, M.C. Antioxidant properties of leaves from different accessions of grapevine (Vitis vinifera L.) cv. Tempranillo after applying biotic and/or environmental modulator factors. Ind. Crops Prod. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Camprubí, A.; Estaún, V.; Nogales, A.; García-Figueres, F.; Pitet, M.; Calvet, C. Response of the grapevine rootstock Richter 110 to inoculation with native and selected arbuscular mycorrhizal fungi and growth performance in a replant vineyard. Mycorrhiza 2008, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lovato, P.; Guillemin, J.; Gianinazzi, S. Application of commercial arbuscular endomycorrhizal fungal inoculants to the establishment of micropropagated grapevine rootstock and pineapple plants. Agronomie 1992, 12, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.C.; Hart, M.M.; Bogdanoff, C.; Bowen, P. Response of grapevine rootstocks to soil inocula from different sources. Am. J. Enol. Vitic. 2018, 69, 94–100. [Google Scholar] [CrossRef]
- Antolín, M.C.; Izurdiaga, D.; Urmeneta, L.; Pascual, I.; Irigoyen, J.J.; Goicoechea, N. Dissimilar responses of ancient grapevines recovered in Navarra (Spain) to arbuscular mycorrhizal symbiosis in terms of berry quality. Agronomy 2020, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Antunes, P.M.; Koch, A.M.; Dunfield, K.E.; Hart, M.M.; Downing, A.; Rillig, M.C.; Klironomos, J.N. Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 2009, 317, 257–266. [Google Scholar] [CrossRef]
- Nogales, A.; Luque, J.; Estaún, V.; Camprubí, A.; Garcia-Figueres, F.; Calvet, C. Differential Growth of Mycorrhizal Field-Inoculated Grapevine Rootstocks in Two Replant Soils. Am. J. Enol. Vitic. 2009, 60, 484–489. [Google Scholar]
- Berdeni, D.; Cotton, T.E.A.; Daniell, T.J.; Bidartondo, M.I.; Cameron, D.D.; Evans, K.L. The effects of arbuscular mycorrhizal fungal colonisation on nutrient status, growth, productivity, and canker resistance of apple (Malus pumila). Front. Microbiol. 2018, 9, 1461. [Google Scholar] [CrossRef]
- Estaún, V.; Camprubí, A.; Calvet, C.; Pinochet, J. Nursery and field response of olive trees inoculated with two arbuscular mycorrhizal fungi, Glomus intraradices and Glomus mosseae. J. Am. Soc. Hortic. Sci. 2003, 128, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.L.; Richardson, S.; Koziol, L.; Palmer, C.E.; Yermakov, Z.; Henning, J.A.; Schultz, P.A.; Bever, J.D. Locally adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere 2015, 6, art276. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Köhl, L.; Lukasiewicz, C.E.; van der Heijden, M.G.A. Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Environ. 2016, 39, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyard management on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.H.; Kirkegaard, J.A. The agronomic relevance of arbuscular mycorrhizas in the fertility of Australian extensive cropping systems. Agric. Ecosyst. Environ. 2012, 163, 37–53. [Google Scholar] [CrossRef]
- Klironomos, J.N.J. Host-specificity and functional diversity among arbuscular mycorrhizal fungi. Microb. Biosyst. New Front. 2000, 1, 845–851. [Google Scholar]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Herrera-Peraza, R.A.; Hamel, C.; Fernández, F.; Ferrer, R.L.; Furrazola, E. Soil-strain compatibility: The key to effective use of arbuscular mycorrhizal inoculants? Mycorrhiza 2011, 21, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Rúa, M.A.; Antoninka, A.; Antunes, P.M.; Chaudhary, V.B.; Gehring, C.; Lamit, L.J.; Piculell, B.J.; Bever, J.D.; Zabinski, C.; Meadow, J.F.; et al. Home-field advantage? evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol. Biol. 2016, 16, 122. [Google Scholar] [CrossRef] [Green Version]
- Gianinazzi, S.; Vosátka, M. Inoculum of arbuscular mycorrhizal fungi for production systems: Science meets business. Can. J. Bot. 2004, 82, 1264–1271. [Google Scholar] [CrossRef]
- Holland, T.; Bowen, P.; Kokkoris, V.; Richards, A.; Rosa, D.; Hart, M. The effect of root pruning on the arbuscular mycorrhizal symbiosis in grapevine rootstocks. Chem. Biol. Technol. Agric. 2019, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Pogiatzis, A. Comparative Responses of Six Grapevine Rootstocks to Inoculation with Arbuscular Mycorrhizal Fungi Based on Root Traits. Master’s Thesis, University of British Columbia, Kelowna, BC, Canada, 2017. Available online: https://open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0347985 (accessed on 2 November 2020).
- Holland, T.C.; Bowen, P.; Bogdanoff, C.; Hart, M.M. How distinct are arbuscular mycorrhizal fungal communities associating with grapevines? Biol. Fertil. Soils 2014, 50, 667–674. [Google Scholar] [CrossRef]
- BC Wine Grape Council. 2010 Best Practices Guide for Grapes; British Columbia Ministry of Agriculture and Lands: Peachland, BC, Canada, 2010.
- Lowe, K.M.; Walker, M.A. Genetic linkage map of the interspecific grape rootstock cross Ramsey (Vitis champinii) × Riparia Gloire (Vitis riparia). Theor. Appl. Genet. 2006, 112, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci. Total Environ. 2019, 660, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S.; Avio, L.; Bonari, E.; Giovannetti, M. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol. Biochem. 2011, 43, 367–376. [Google Scholar] [CrossRef]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Hernádi, I.; Sasvári, Z.; Albrechtová, J.; Vosátka, M.; Posta, K. Arbuscular Mycorrhizal Inoculant Increases Yield of Spice Pepper and Affects the Indigenous Fungal Community in the Field. HortScience 2012, 47, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Ortas, I.; Sari, N.; Akpinar, Ç.; Yetisir, H. Screening mycorrhiza species for plant growth, P and Zn uptake in pepper seedling grown under greenhouse conditions. Sci. Hortic. 2011, 128, 92–98. [Google Scholar] [CrossRef]
- Janoušková, M.; Krak, K.; Wagg, C.; Štorchová, H.; Caklová, P.; Vosátka, M. Effects of Inoculum Additions in the Presence of a Preestablished Arbuscular Mycorrhizal Fungal Community. Appl. Environ. Microbiol. 2013, 79, 6507–6515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 2016, 24, 35–44. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhong, H.; Zhu, L.; Yuan, Y.; Xu, L.; Wang, G.G.; Zhai, L.; Yang, L.; Zhang, J. Arbuscular mycorrhizal fungi effectively enhances the growth of Gleditsia sinensis Lam. Seedlings under greenhouse conditions. Forests 2019, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Urcelay, C.; Vaieretti, M.V.; Pérez, M.; Díaz, S. Effects of arbuscular mycorrhizal colonisation on shoot and root decomposition of different plant species and species mixtures. Soil Biol. Biochem. 2011, 43, 466–468. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Schnoor, T.K.; Mårtensson, L.-M.; Olsson, P.A. Soil disturbance alters plant community composition and decreases mycorrhizal carbon allocation in a sandy grassland. Oecologia 2011, 167, 809–819. [Google Scholar] [CrossRef]
- Kokkoris, V.; Hart, M.M. In vitro propagation of arbuscular mycorrhizal fungi may drive fungal evolution. Front. Microbiol. 2019, 10, 22. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.W.; Hartnett, D.C. Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am. J. Bot. 1998, 85, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Abbott, L.K. Unknown risks to soil biodiversity from commercial fungal inoculants. Nat. Ecol. Evol. 2017, 1, 0115. [Google Scholar] [CrossRef]
- Ricciardi, A.; Blackburn, T.M.; Carlton, J.T.; Dick, J.T.A.; Hulme, P.E.; Iacarella, J.C.; Jeschke, J.M.; Liebhold, A.M.; Lockwood, J.L.; MacIsaac, H.J.; et al. Invasion Science: A Horizon Scan of Emerging Challenges and Opportunities. Trends Ecol. Evol. 2017, 32, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Mummey, D.L.; Antunes, P.M.; Rillig, M.C. Arbuscular mycorrhizal fungi pre-inoculant identity determines community composition in roots. Soil Biol. Biochem. 2009, 41, 1173–1179. [Google Scholar] [CrossRef]
- Thomsen, C.N.; Hart, M.M. Using invasion theory to predict the fate of arbuscular mycorrhizal fungal inoculants. Biol. Invasions 2018, 20, 2695–2706. [Google Scholar] [CrossRef]
- Camargo-Ricalde, S.L. Dispersal, distribution and establishment of arbuscular mycorrhizal fungi: A review. Bot. Sci. 2017, 33. [Google Scholar] [CrossRef] [Green Version]
- Werner, G.D.A.; Kiers, E.T. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol. 2015, 205, 1515–1524. [Google Scholar] [CrossRef]
- Toljander, J.F.; Artursson, V.; Paul, L.R.; Jansson, J.K.; Finlay, R.D. Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol. Lett. 2006, 254, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, G.; Mihara, K.L.; Linderman, R.G.; Bethlenfalvay, G.J. Bacteria from rhizosphere and hyphosphere soils of different arbuscular-mycorrhizal fungi. Plant Soil 1997, 192, 71–79. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, D.; Pogiatzis, A.; Bowen, P.; Kokkoris, V.; Richards, A.; Holland, T.; Hart, M. Performance and Establishment of a Commercial Mycorrhizal Inoculant in Viticulture. Agriculture 2020, 10, 539. https://doi.org/10.3390/agriculture10110539
Rosa D, Pogiatzis A, Bowen P, Kokkoris V, Richards A, Holland T, Hart M. Performance and Establishment of a Commercial Mycorrhizal Inoculant in Viticulture. Agriculture. 2020; 10(11):539. https://doi.org/10.3390/agriculture10110539
Chicago/Turabian StyleRosa, Daniel, Antreas Pogiatzis, Pat Bowen, Vasilis Kokkoris, Andrew Richards, Taylor Holland, and Miranda Hart. 2020. "Performance and Establishment of a Commercial Mycorrhizal Inoculant in Viticulture" Agriculture 10, no. 11: 539. https://doi.org/10.3390/agriculture10110539






