Chemical Variation and Implications on Repellency Activity of Tephrosia vogelii (Hook f.) Essential Oils Against Sitophilus zeamais Motschulsky
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Sites
2.2. Plant Materials and Botanical Identification
2.3. Extraction and Analysis of Essential Oils
2.3.1. Extraction
2.3.2. Identification and Quantification of Compounds
Synthetic Chemicals
Identification of Compounds with Gas Chromatography (GC)
Identification and Quantification of Volatile Constituents by Gas Chromatography-Mass Spectrometry (GC/MS)
2.4. Principal Component Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC) of Major Chemical Components from Oils of T. vogelii Species
2.5. Repellency Evaluation
2.5.1. Rearing of Weevils
2.5.2. Repellence Bioassay against S. zeamais
- C = insect number found on untreated half,
- T = insect found on treated half.
- A = percentage of insects in treated halves,
- B = percentage of insects in untreated halves.
3. Results and Discussion
3.1. Chemical Constituents and Composition of Essential Oils
3.2. Chemotypes in T. vogelii Essential Oil
3.3. Effect of Season Variation on the Percentage Yield and Major Composition of the Oils
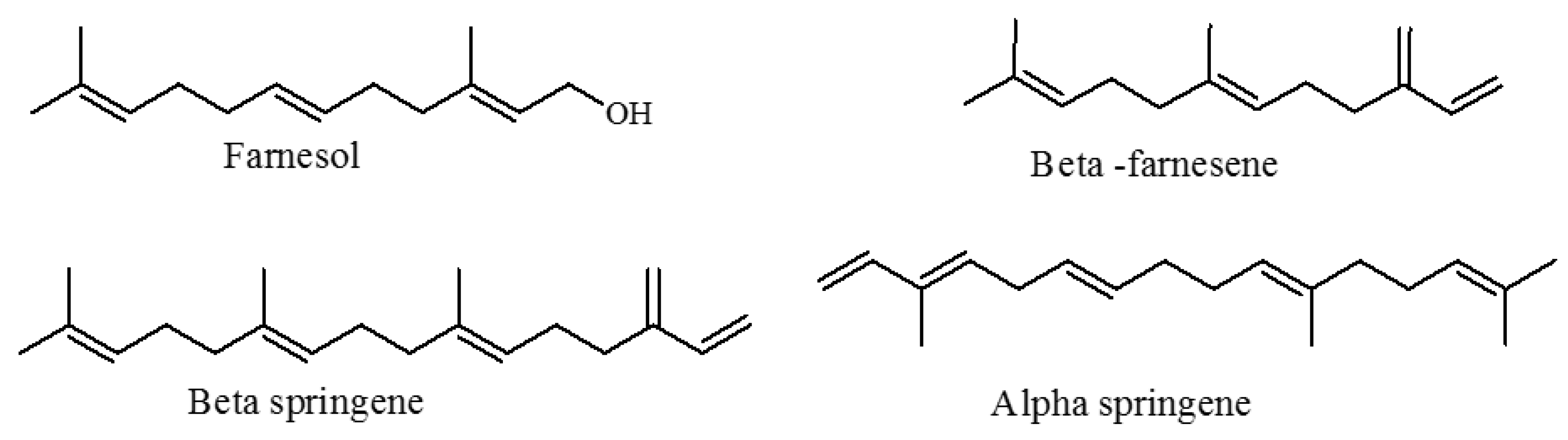
3.4. Chemotaxonomic Significance of These Chemical Varieties
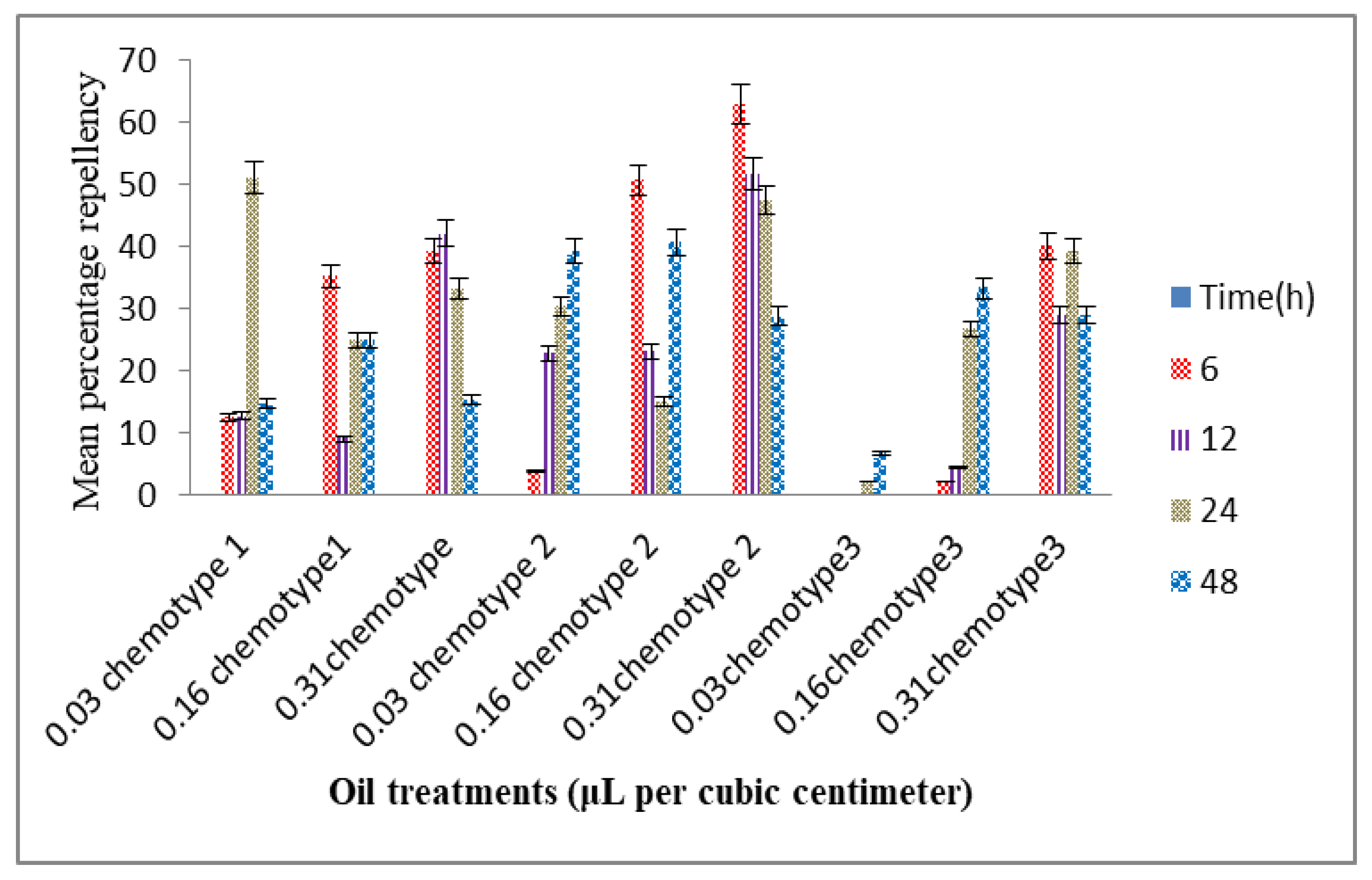
3.5. Evaluation of the Repellency Potential of the Chemotypes of the Volatile Constituents of T. vogelii
3.6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matovu, H.; Olila, D. Acaricidal activity of Tephrosia vogelii extracts on nymph and adult ticks. Int. J. Trop Dis. 2007, 2, 83–88. [Google Scholar]
- Neuwinger, H.D. Plants used for poison fishing in tropical Africa. Toxicon 2004, 44, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Mwaura, L.; Anjarwalla, P.; Ofori, D.A.; Stevenson, P.C.; Smith, P.; Jamnadass, R. PesticidaL Plant. Leaflet. The Agroforestree Database—World Agroforestry. 2015. Available online: www.worldagroforestry.org (accessed on 18 February 2020).
- Kamanula, J.F.; Sileshi, G.; Belmain, S.R.; Sola, P.; Mvumi, B.; Nyirenda, G.K.C.; Nyirenda, S.P.; Stevenson, P.C. Farmers’ insect pest management practices and pesticidal plant use in the protection of stored maize and beans in southern Africa. Int. J. Pest. Manag. 2011, 57, 41–49. [Google Scholar]
- Nyirenda, S.P.; Sileshi, G.; Belmain, S.R.; Kamanula, J.F.; Mvumi, B.; Sola, P.; Nyirenda, G.K.C.; Stevenson, P.C. Farmers’ ethno-ecological knowledge of vegetable pests and their management using pesticidal plants in northern Malawi and eastern Zambia. Afr. J. Agric. Res. 2011, 6, 1525–1537. [Google Scholar]
- Ogendo, J.O.; Belmain, S.R.; Deng, A.L.; Walker, D.J. Efficacy of Lantana camara L. and Tephrosia vogelii Hook against Sitophilus zeamais (Coleoptera: Curculionidae) in stored maize grains. In Proceedings of the III WOCMAP Congress on Medicinal and Aromatic Plants, Chiang Mai, Thailand, 3 February 2003; pp. 137–143. [Google Scholar]
- Koona, P.; Malaa, D.; Koona, O.E. Hexane extracts from Tephrosia vogelii Hook. f. protect stored maize against the weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Entomol. Sci. 2007, 10, 107–111. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kite, G.C.; Lewis, G.P. Distinct Chemotypes of Tephrosia Vogelii and Implications for Their Use in Pest Control and Soil Enrichment. Phytochemistry 2012, 78, 135–146. [Google Scholar] [CrossRef]
- Kamanula, J.F.; Belmain, S.R.; Hall, D.R.; Farman, D.I.; Goyder, D.J.; Mvumi, B.M.; Masumbu, F.F.; Stevenson, P.C. Chemical variation and insecticidal activity of Lippia javanica (Burm. f.) Spreng essential oil against Sitophilus zeamais Motschulsky. Ind. Crops. Prod. 2017. [Google Scholar] [CrossRef]
- Mkindi, A.G.; Tembo, Y.; Mbega, E.R.; Medvecky, B.; Kendal-Smith, A.; Farrell, I.W.; Ndakidemi, P.A.; Belmain, S.R.; Stevenson, P.C. Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant. Plants 2019, 8, 597. [Google Scholar] [CrossRef]
- Bendera, M.M. Isolation and Characterization of Essential Oils from Ocimum americanum, Lantana camara, Lantana trifolia and Tephrosia vogelii. Ph.D. Thesis, Egerton University, Njoro, Kenya, 2007. [Google Scholar]
- Noudogbessi, J.P.; Sessou, P.; Wotto, V.D.; Figueredo, G.; Chalard, P.; Chalchat, J.C.; Sohounhloué, D.C.K. Chemical compositions and preventive activity of essential oils extracted from the leaves of two varieties of Tephrosia(Leguminosae-Papilionoideae) collected in Benin on Callosobruchus maculatus (Fabricius). AJRC 2012, 5, 1431–1436. [Google Scholar]
- Lee, B.H.; Choi, W.S.; Lee, S.E.; Park, B.S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop. Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Chaubey, M.K. Fumigant toxicity of essential oils and pure compounds against Sitophilus oryzae L. (Coleoptera: Curculionidae). Biol. Agric. Hortic. 2012, 28, 111–119. [Google Scholar] [CrossRef]
- Government of Uganda. Atlas of Uganda; Government Printers: Entebbe, Uganda, 1967.
- Oonyu, J. Upland rice growing: A potential solution to declining crop yields and the degradation of the Doho wetlands, Butaleja district-Uganda. Afr. J. Agric. Res. 2011, 6, 2774–2783. [Google Scholar]
- Wannes, W.A.; Mhamdi, B.; Marzouk, B. Variations in essential oil and fatty acid composition during Myrtus communis var. italica fruit maturation. Food Chem. 2009, 112, 621–626. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Johnson, G.W.; Ehrlich, R.; Full, W.; Ramos, S. Chapter 7—Principal Components Analysis and Receptor Models in Environmental Forensics. In Introduction to Environmental Forensics, 2nd ed.; Murphy, B.L., Morrison, R.D., Eds.; Academic Press: Burlington, ON, Canada, 2007; pp. 207–208. [Google Scholar]
- Tapondjou, A.; Adler, C.; Fontem, D.; Bouda, H.; Reichmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Mojab, F.; Rustaiyan, A.; Jasbi, A.R. Essential oils of Heracleum persicum Desf. ex Fischer leaves. Daru 2002, 10, 6–8. [Google Scholar]
- Heinrich, G.; Pfeifhofer, H.W.; Stabentheiner, E.; Sawidis, T. Glandular Hairs of Sigesbeckia jorullensis Kunth (Asteraceae): Morphology, Histochemistry and Composition of Essential Oil. Ann. Bot. 2002, 89, 459–469. [Google Scholar] [CrossRef]
- Jeppesen, A.S.; Soelberg, J.; Jager, A.K. Chemical composition of the essential oil from nine medicinal plants of the Wakhan Corridor, Afghanistan. J. Essent. Oil Bear. Plants 2012, 15, 204–212. [Google Scholar] [CrossRef]
- Laville, R.; Castel, C.; Filippi, J.J.; Delbecque, C.; Audran, A.; Garry, P.P.; Legendre, L.; Fernandez, X. Amphilectane diterpenes from Salvia sclarea: Biosynthetic considerations. J. Nat. Prod. 2012, 75, 121–126. [Google Scholar] [CrossRef]
- Karamian, R.; Asadbegy, M.; Pakzad, R. Essential oil compositions and in vitro antioxidant and antibacterial activities of the methanol extracts of two Salvia species (Lamiaceae) from Iran. Int. J. Agric. Crop Sci. 2013, 5, 1171–1182. [Google Scholar]
- Raina, V.K.; Verma, S.C.; Dhawan, S.; Khan, M.; Ramesh, S.; Singh, S.C.; Yadav, A.; Srivastava, S.K. Essential oil composition of Murraya exotica from the plains of northern India. Flavour Fragr. J. 2006, 21, 140–142. [Google Scholar] [CrossRef]
- Djabou, N.; Andreani, S.; Varesi, L.; Tomi, F.; Costa, J.; Muselli, A. Analysis of the volatile fraction of Teucrium marum L. Flavour Fragr. J. 2013, 28, 14–24. [Google Scholar] [CrossRef]
- Micha, S.G.; Wyss, U. Aphid alarm pheromone (E)-β-farnesene: A host finding kairomone for the aphid primary parasitoid Aphidius uzbekistanicus (Hymenoptera: Aphidiinae). Chemoecology 1996, 7, 132–139. [Google Scholar] [CrossRef]
- Joachim, C.; Weisser, W.W. Does the Aphid Alarm Pheromone (E)-β-farnesene Act as a Kairomone under Field Conditions. J. Chem. Ecol. 2015, 41, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Cardoso, P.; Freitas, R.; Figueira, E. Protective effects of farnesol on a Rhizobium strain exposed to cadmium. Ecotox. Environ. Safe 2018, 165, 622–629. [Google Scholar] [CrossRef]





| Village (Sample) | Flower Color | Location | Sampling Date | Altitude (m) | |
|---|---|---|---|---|---|
| Latitude North | Longitude East | ||||
| Muyago (TV1 muya) | White | 0°84′20″ | 34°03′15″ | 14 May 2017 | 1080 |
| KyadongoB (TV1 kya) | White | 0°90′14″ | 34°08′30″ | 1 June 2017 | 1098 |
| Kyadongo B (TV1 kyb) | White | 0°90′30″ | 34°09′45″ | 1 June 2017 | 1098 |
| Kyadongo B (TV1 kyc) | White | 0°80′10″ | 34°08′40″ | 1 June 2017 | 1098 |
| Muyago (TV2 muya) | White | 0°84′14.9″ | 34°03′10″ | 15 August 2017 | 1080 |
| Kyadongo B (TV2 kya) | White | 0°70′30″ | 34°07′35″ | 15 August 2017 | 1098 |
| Kyadongo B (TV2 kyb) | White | 0°90′14″ | 34°08′30″ | 15 August 2017 | 1098 |
| Kyadongo B (TV2 kyc) | White | 0°90′20″ | 34°09′40″ | 15 August 2017 | 1098 |
| Kyadongo B (TV2 kyd) | White | 0°90′35″ | 34°08′45″ | 15 August 2017 | 1098 |
| Muyago (TV3 muya) | White | 0°84′00″ | 34°02′45″ | 1 March 2018 | 1090 |
| Muyago (TV3 muyb) | White | 0°84′14″ | 34°03′09″ | 1 March 2018 | 1090 |
| Muyago (TV3 muyc) | White | 0°84′14.9″ | 34°03′10″ | 1 March 2018 | 1090 |
| Kyadongo B (TV3 kya) | White | 0°90′14″ | 34°08′30″ | 1 March 2018 | 1098 |
| Kyadongo B (TV4 kya) | White | 0°70′30″ | 34°07′35″ | 10 January 2019 | 1098 |
| Kyadongo B (TV4 kyb) | White | 0°90′14″ | 34°08′30″ | 10 January 2019 | 1098 |
| Kyadongo B (TV4 kyc) | White | 0°90′20″ | 34°09′40″ | 10 January 2019 | 1098 |
| Muyago (TV4 muya) | White | 0°84′00″ | 34°02′45″ | 10 January 2019 | 1090 |
| Muyago (TV4 muyb) | White | 0°84′14″ | 34°03′09″ | 10 January 2019 | 1090 |
| Muyago (TV4 muyc) | White | 0°84′14.9″ | 34°03′10″ | 10 January 2019 | 1090 |
| Compounds (RT, KI) 1 | TV1 kya | TV1 kyb | TV1 kyc | TV2 kya | TV2 kyb | TV2 kyc | TV2 kyd | TV3 kya | TV4 kya | TV4 kyb | TV4 kyc | Identification Method 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylbenzene (4.753, 878) | 0.2 (0.0) | 4.0 (0.4) | 0.6 (0.3) | 0.4 (0.1) | 1.4 (0.4) | 0.2 (0.0) | 0.5 (0.0) | 0.7 (0.1) | nd | nd | nd | MS/KI/ CI |
| o-Xylene (4.962, 867) | 1.1 (0.0) | 29.4 (1.6) | 3.2 (2.1) | 2.1 (0.3) | 9.0 (1.7) | 1.1 (0.1) | 3.4 (0.6) | 6.7 (2.3) | nd | nd | nd | MS/KI/ CI |
| p-Xylene (5.448, 887) | 0.9 (0.0) | nd | 1.6 (0.6) | nd | nd | nd | 1.5 (0.1) | nd | nd | nd | nd | MS/KI |
| m-Xylene (5.539, 896) | 0.3 (0.0) | 25.0 (1.2) | 3.6 (1.6) | 1.0 (0.0) | 5.1 (0.8) | 0.3 (0.0) | 2.1 (0.1) | 3.3 (0.6) | nd | nd | nd | MS/KI/ CI |
| 2-Butoxyethanol (5.776, 895) | 0.3 (0.0) | 2.7 (0.1) | 3.4 (0.9) | 0.6 (0.2) | 1.0 (0.2) | 0.2 (0.0) | 2.9 (0.3) | 1.1 (0.2) | nd | nd | nd | MS/KI |
| D-(+)-Alpha-pinene (6.811, 931) | 0.7 (0.1) | 1.7 (0.1) | 0.7 (0.1) | 0.5 (0.1) | 1.0 (0.2) | nd | 0.5 (0.1) | nd | 0.4 (0.1) | 0.9 (0.2) | nd | MS/KI/ CI |
| D-Limonene (10.419, 1031) | nd | 0.2 (0.0) | 0.2 (0.0) | 0.8 (0.4) | 0.2 (0.0) | nd | 0.1 (0.0) | nd | nd | 0.1 (0.0) | nd | MS/KI/ CI |
| Linalool (13.514, 1102) | nd | 1.8 (0.0) | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS/KI/ CI |
| (E,E)-Cosmene (15.875, 1132) | nd | nd | nd | nd | nd | nd | nd | nd | 0.2 (0.1) | 0.6 (0.1) | 0.5 (0.0) | MS/KI |
| 6,10-Dimethyl-5,9-undecadien-2-one (28.883, 1453) | nd | 0.8 (0.2) | 0.9 (0.2) | 1.2 (0.4) | 1.3 (0.0) | 0.3 (0.0) | nd | nd | 0.2 (0.0) | 0.5 (0.0) | 1.1 (0.1) | MS/KI |
| Isocaryophyllene (30.511, 1409) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.2 (0.0) | MS/KI |
| (E)-Nerolidol (33.432, 1564) | nd | 0.2 (0.0) | nd | nd | 0.2 (0.0) | 0.2 (0.0) | nd | nd | nd | MS/KI | ||
| (-)-Spathulenol (33.959, 1566) | nd | 0.2 (0.0) | 0.2 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.2 (0.0) | nd | nd | 0.2 (0.0) | nd | 0.2 (0.0) | MS/KI |
| 3,4-Dimethyl-3-cyclohexen-1-carboxaldehyde (34.520, 1492) | nd | nd | nd | nd | 2.9 (0.6) | nd | nd | nd | nd | nd | nd | MS/KI |
| Cis-p-metha-1(7)-8-dien-2-ol (35.208, 1233) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.2 (0.0) | MS/KI |
| Isoaromadendrene epoxide (37.465, 1577) | nd | 0.2 (0.0) | nd | nd | nd | nd | nd | 0.2 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.7 (0.3) | MS/KI |
| 1,4-Dihydroxy-p-menth-2-ene (37.767, 1243) | nd | 0.2 (0.0) | 0.4 (0.0) | nd | 1.4 (0.2) | nd | nd | nd | nd | 1.1 (0.2) | 0.9 (0.1) | MS/KI |
| β-Farnesene (39.188, 1456) | 0.3 (0.0) | nd | nd | nd | 0.3 (0.0) | 0.8 (0.3) | nd | nd | nd | nd | nd | MS/KI |
| Farnesol(E)-methylether (39.186, 1682) | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.0 (0.0) | nd | MS/KI |
| Z, Nerolidol (39.204, 1558) | nd | nd | nd | nd | nd | nd | nd | nd | 0.9 (0.1) | nd | nd | MS/KI |
| Farnesol | nd | 1.8 (0.2) | 2.2 (0.7) | nd | nd | nd | 0.3 (0.0) | 0.4 (0.1) | nd | nd | 2.9 (0.2) | MS/KI/ CI |
| β-Springene (39.410, 1918) | nd | nd | nd | 0.6 (0.1) | 5.7 (2.9) | nd | nd | 0.3 (0.1) | nd | nd | MS/KI | |
| α-Springene (40.793, 1731) | nd | 0.2 (0.0) | 0.2 (0.0) | nd | nd | nd | nd | nd | nd | nd | 0.2 (0.0) | MS/KI |
| Hexadecane (42.274, 1818) | 0.2 (0.0) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS/KI |
| Density (g/mL) | 1.014 (0.014) | 1.001 (0.024) | 1.026 (0.003) | 0.986 (0.014) | 0.964 (0.036) | 0.979 (0.043) | 0.959 (0.013) | 0.987 (0.013) | 1.087 (0.016) | 0.974 (0.002) | 1.076 (0.049) | |
| Yield (% w/w) | 0.19 (0.01) | 0.21 (0.00) | 0.20 (0.01) | 0.18 (0.01) | 0.20 (0.01) | 0.19 (0.04) | 0.18 (0.00) | 0.19 (0.01) | 0.22 (0.01) | 0.19 (0.02) | 0.21 (0.02) |
| Compounds (RT, KI) 1 | TV1 muya | TV2 muya | TV3 muya | TV3 muyb | TV3 muyc | TV4 muya | TV4 muyb | Tv4 muyc | Identification Method 2 |
|---|---|---|---|---|---|---|---|---|---|
| Ethylbenzene (4.753, 878) | 2.7 (0.2) | 2.4 (0.4) | nd | 1.7 (0.3) | 2.3 (1.6) | nd | nd | nd | MS/KI/CI |
| o-Xylene (4.962, 867) | 17.6 (0.6) | 22.1 (1.2) | 0.9 (0.0) | 8.6 (0.8) | 23.4 (17.3) | nd | nd | nd | MS/KI/CI |
| p-Xylene (5.448, 887) | nd | nd | 0.9 (0.0) | 3.1 (2.0) | 5.2 (3.4) | nd | nd | nd | MS/KI |
| m-Xylene (5.539, 896) | 14.4 (0.2) | 18.1 (2.7) | nd | 5.3 (0.6) | 6.7 (5.1) | nd | nd | nd | MS/KI/CI |
| 2-Butoxyethanol (5.776, 895) | nd | 2.2 (0.7) | 0.3 (0.1) | 3.7 (0.9) | 2.7 (0.1) | nd | nd | nd | MS/KI |
| D-(+)-Alpha-pinene (6.811, 931) | 1.2 (0.0) | 1.6 (0.3) | nd | 1.5 (0.2) | 1.8 (1.2) | 0.4 (0.0) | 0.6 (0.0) | nd | MS/KI/CI |
| D-Limonene (10.419, 1031) | 0.2 (0.0) | 0.3 (0.0) | nd | 0.2 (0.0) | 0.1 (0.0) | nd | nd | nd | MS/KI/CI |
| Linalool (13.514, 1102) | nd | 1.5 (0.3) | nd | nd | nd | 1.0 (0.2) | nd | nd | MS/KI/CI |
| (E,E)-Cosmene (15.875, 1132) | nd | nd | nd | nd | nd | 0.4 (0.2) | 0.6 (0.2) | nd | MS/KI |
| 6,10-Dimethyl-5,9-undecadien-2-one (28.883, 1453) | 0.7 (0.0) | 0.6 (0.4) | nd | 0.7 (0.0) | nd | 0.7 (0.4) | 1.0 (0.2) | 0.6 (0.2) | MS/KI |
| Isocaryophyllene (30.511, 1409) | nd | nd | nd | nd | nd | 0.3 (0.0) | 0.2 (0.0) | 0.4 (0.0) | MS/KI |
| (E)-Nerolidol (33.432, 1564) | nd | 0.3 (0.0) | nd | 0.2 (0.0) | nd | nd | nd | 0.2 (0.0) | MS/KI |
| (-)-Spathulenol (33.959, 1566) | 0.2 (0.0) | 0.3 (0.0) | nd | 0.2 (0.0) | 0.3 (0.0) | nd | 0.2 (0.0) | nd | MS/KI |
| 3,4-Dimethyl-3-cyclohexen-1-carboxaldehyde (34.520, 1492) | nd | nd | nd | nd | 3.4 (1.6) | nd | nd | nd | |
| Cis-p-metha-1(7)-8-dien-2-ol (35.208, 1233) | nd | nd | nd | nd | nd | nd | nd | 1.1 (0.0) | MS/KI |
| Isoaromadendrene epoxide (37.465, 1577) | nd | nd | nd | 0.8 (0.2) | nd | 0.3 (0.0) | nd | nd | MS/KI |
| 1,4-Dihydroxy-p-menth-2-ene (37.767, 1243) | 1.1 (0.0) | 2.3 (2.1) | nd | nd | nd | nd | 1.3 (0.4) | nd | MS/KI |
| β-Farnesene (39.188, 1456) | nd | nd | nd | nd | nd | nd | nd | 0.8 (0.5) | MS/KI |
| Farnesol(E)-methylether (39.186, 1682) | 0.8 (0.0) | nd | nd | nd | nd | nd | nd | 0.2 (0.0) | MS/KI |
| Farnesol | nd | 6.3 (1.3) | nd | 4.5 (2.1) | 5.9 (1.7) | nd | 1.2 (0.8) | 0.2 (0.0) | MS/KI/CI |
| β-Springene (39.410, 1918) | nd | 0.2 (0.0) | 0.2 (0.1) | 0.2 (0.0) | 2.0 (0.3) | 0.9 (0.1) | 2.0 (0.1) | MS/KI | |
| α-Springene (40.793, 1731) | nd | nd | nd | nd | 0.2 (0.0) | nd | nd | 0.2 (0.0) | MS/KI |
| Hexadecane (42.274, 1818) | 0.2 (0.0) | 0.5 (0.1) | nd | 0.3 (0.1) | nd | nd | nd | nd | |
| Density | 0.988 (0.038) | 0.988 (0.012) | 0.942 (0.001) | 0.973 (0.000) | 0.973 (0.027) | 1.030 (0.030) | 1.000 (0.000) | 1.017 (0.017) | |
| Yield (% w/w) | 0.20 (0.01) | 0.22 (0.01) | 0.16 (0.00) | 0.18 (0.00) | 0.18 (0.00) | 0.18 (0.00) | 0.17 (0.01) | 0.18 (0.01) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerebba, N.; Oyedeji, A.O.; Byamukama, R.; Kuria, S.K.; Oyedeji, O.O. Chemical Variation and Implications on Repellency Activity of Tephrosia vogelii (Hook f.) Essential Oils Against Sitophilus zeamais Motschulsky. Agriculture 2020, 10, 164. https://doi.org/10.3390/agriculture10050164
Kerebba N, Oyedeji AO, Byamukama R, Kuria SK, Oyedeji OO. Chemical Variation and Implications on Repellency Activity of Tephrosia vogelii (Hook f.) Essential Oils Against Sitophilus zeamais Motschulsky. Agriculture. 2020; 10(5):164. https://doi.org/10.3390/agriculture10050164
Chicago/Turabian StyleKerebba, Nasifu, Adebola O. Oyedeji, Robert Byamukama, Simon K. Kuria, and Opeoluwa O. Oyedeji. 2020. "Chemical Variation and Implications on Repellency Activity of Tephrosia vogelii (Hook f.) Essential Oils Against Sitophilus zeamais Motschulsky" Agriculture 10, no. 5: 164. https://doi.org/10.3390/agriculture10050164
APA StyleKerebba, N., Oyedeji, A. O., Byamukama, R., Kuria, S. K., & Oyedeji, O. O. (2020). Chemical Variation and Implications on Repellency Activity of Tephrosia vogelii (Hook f.) Essential Oils Against Sitophilus zeamais Motschulsky. Agriculture, 10(5), 164. https://doi.org/10.3390/agriculture10050164





