Monitoring and Modelling Analysis of Maize (Zea mays L.) Yield Gap in Smallholder Farming in Ghana
Abstract
:1. Introduction
2. Materials and Methods
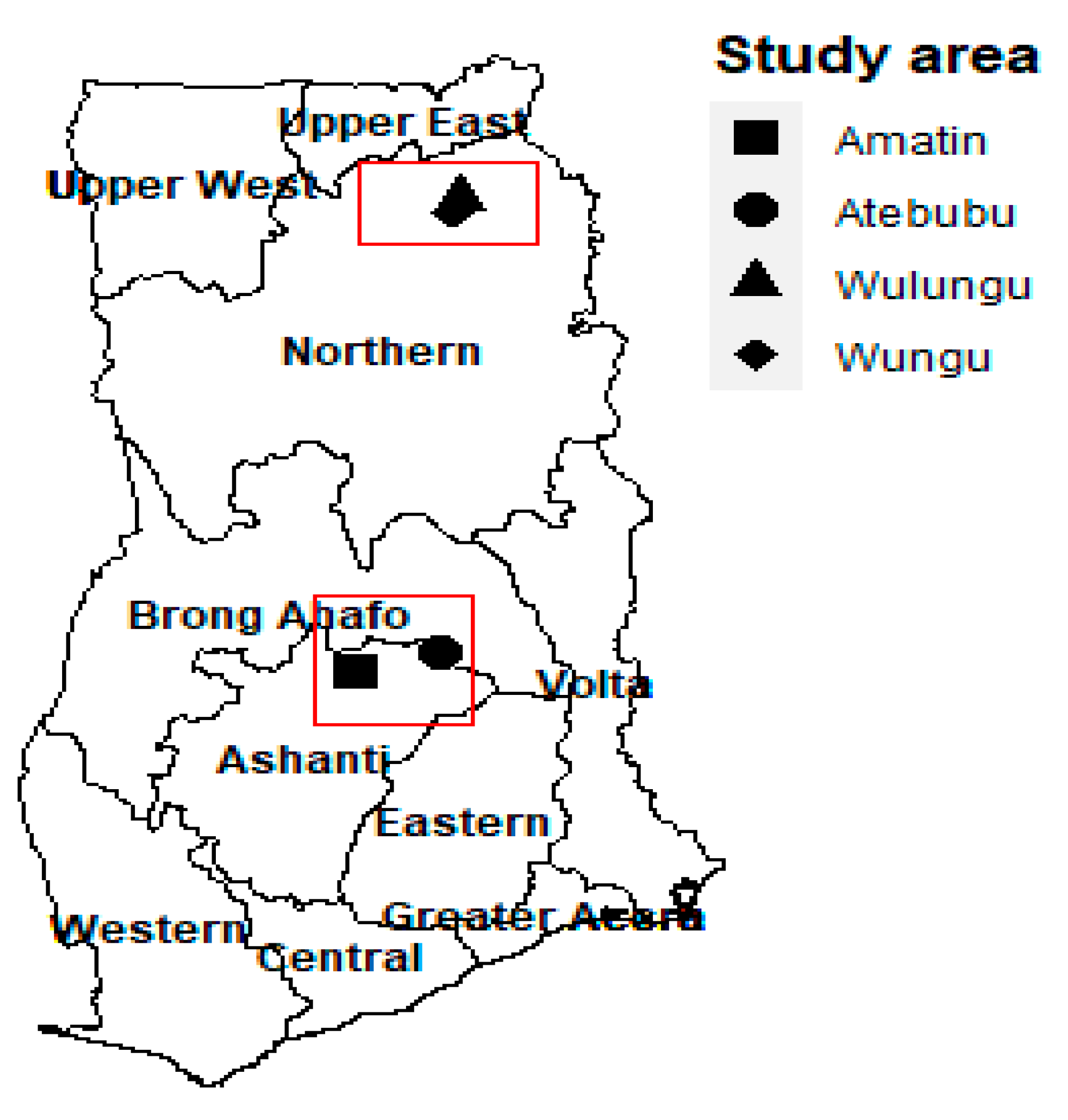
2.1. Site Description and Climate
2.2. Field Measurements
2.2.1. Crop Data
2.2.2. Soil Data
2.3. Modelling Using APSIM
Modelling Scenarios
3. Results
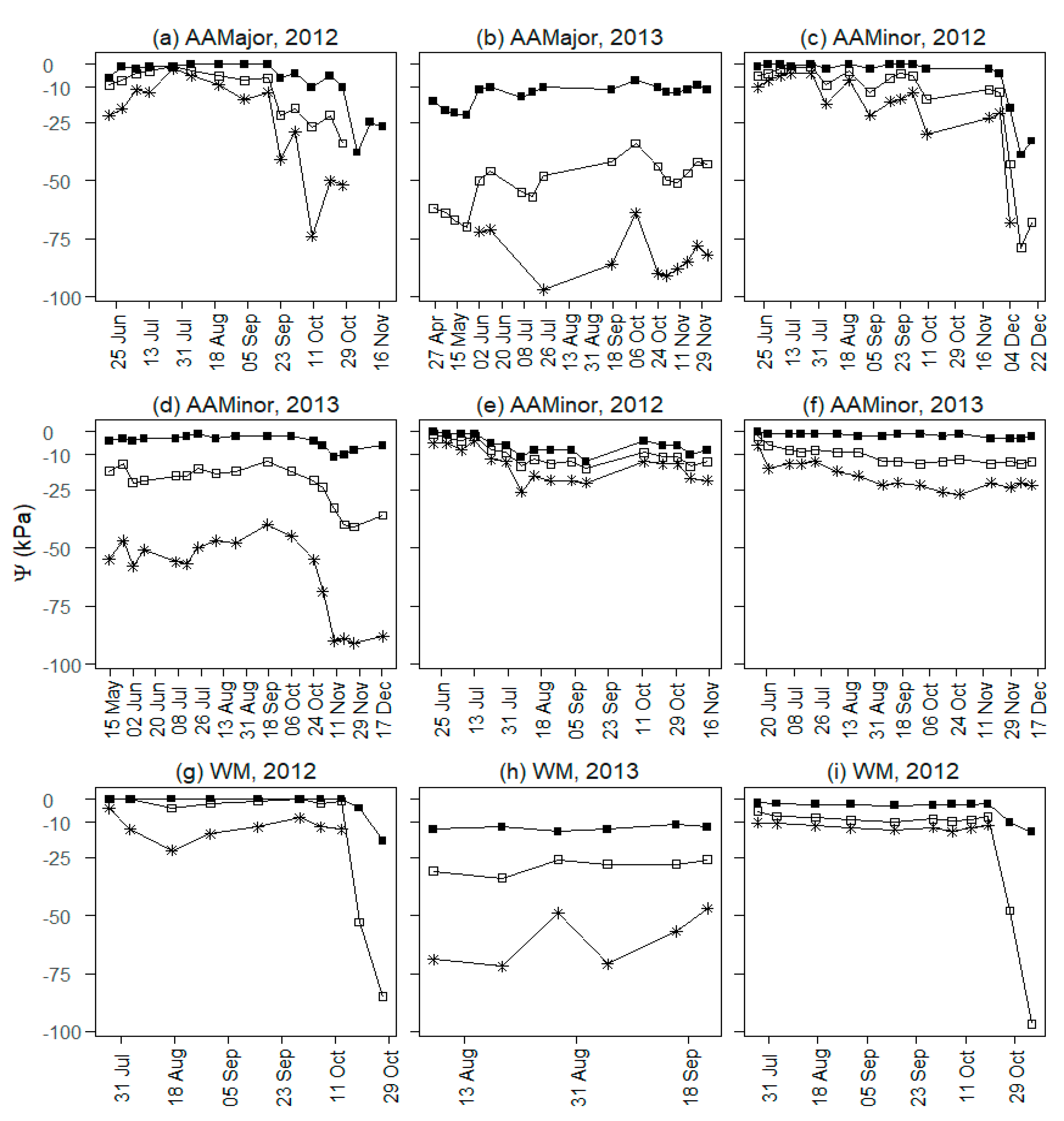
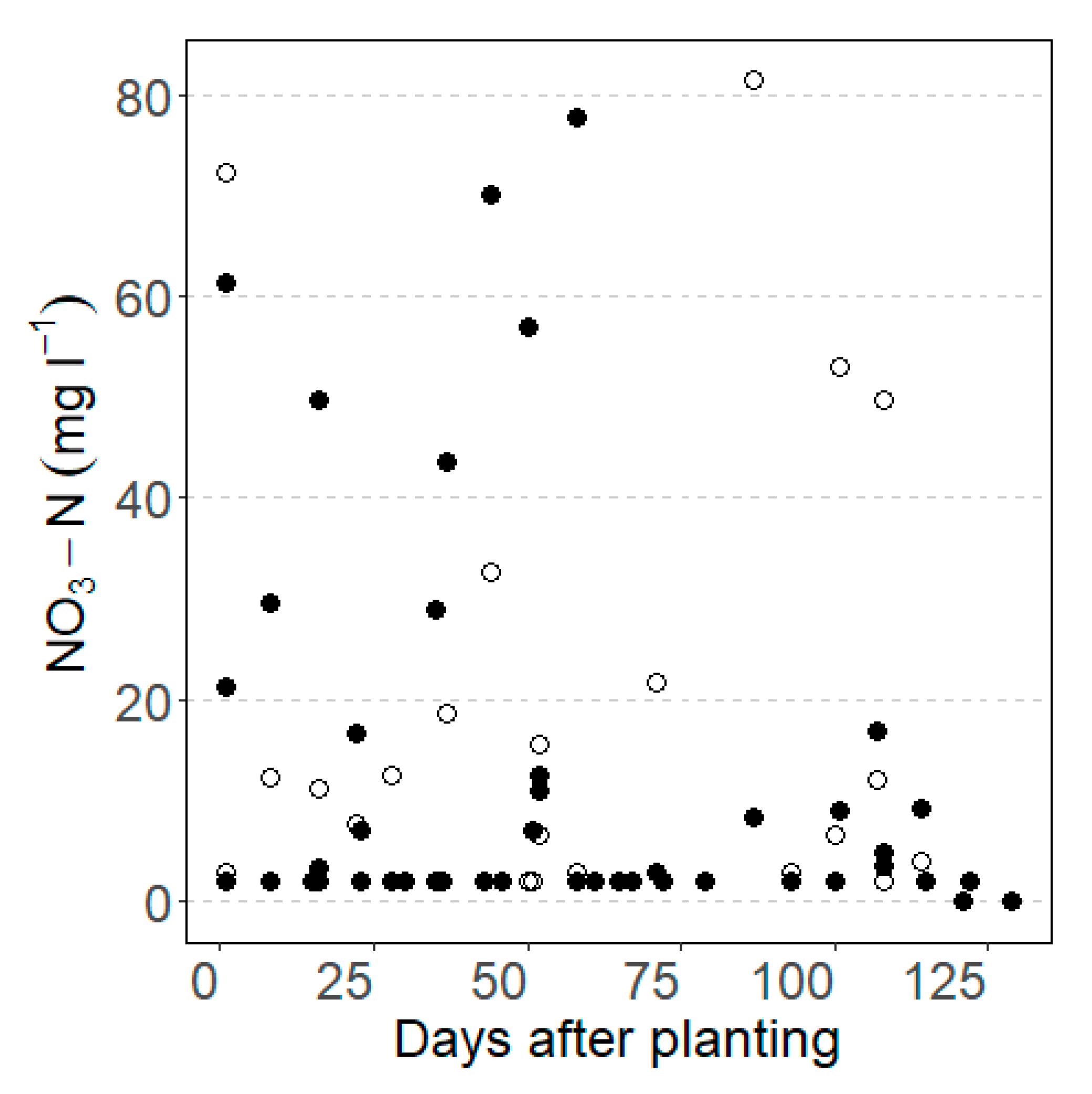
3.1. Observed Yield, Soil Water and Nutrients
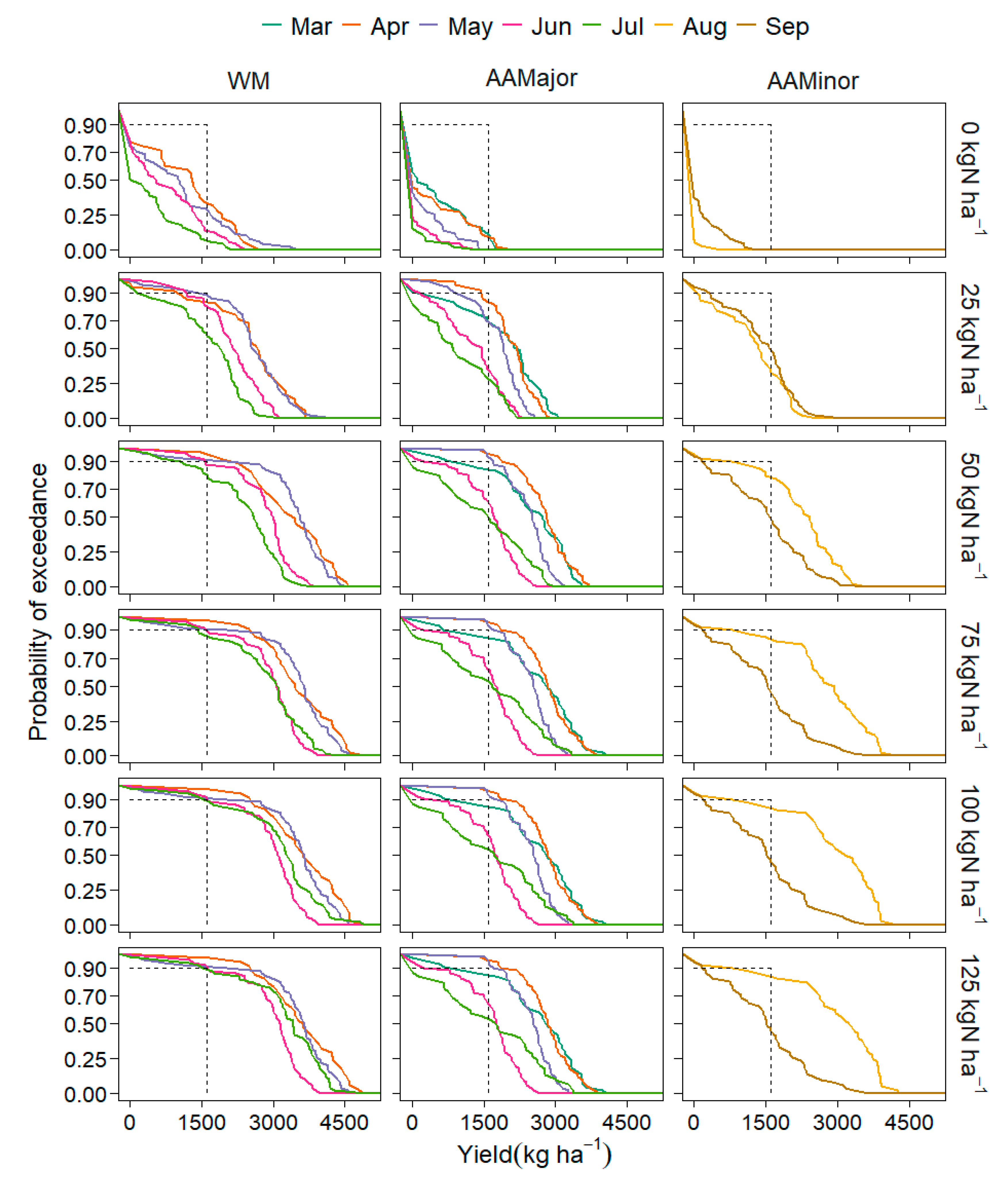
3.2. Modelling Results
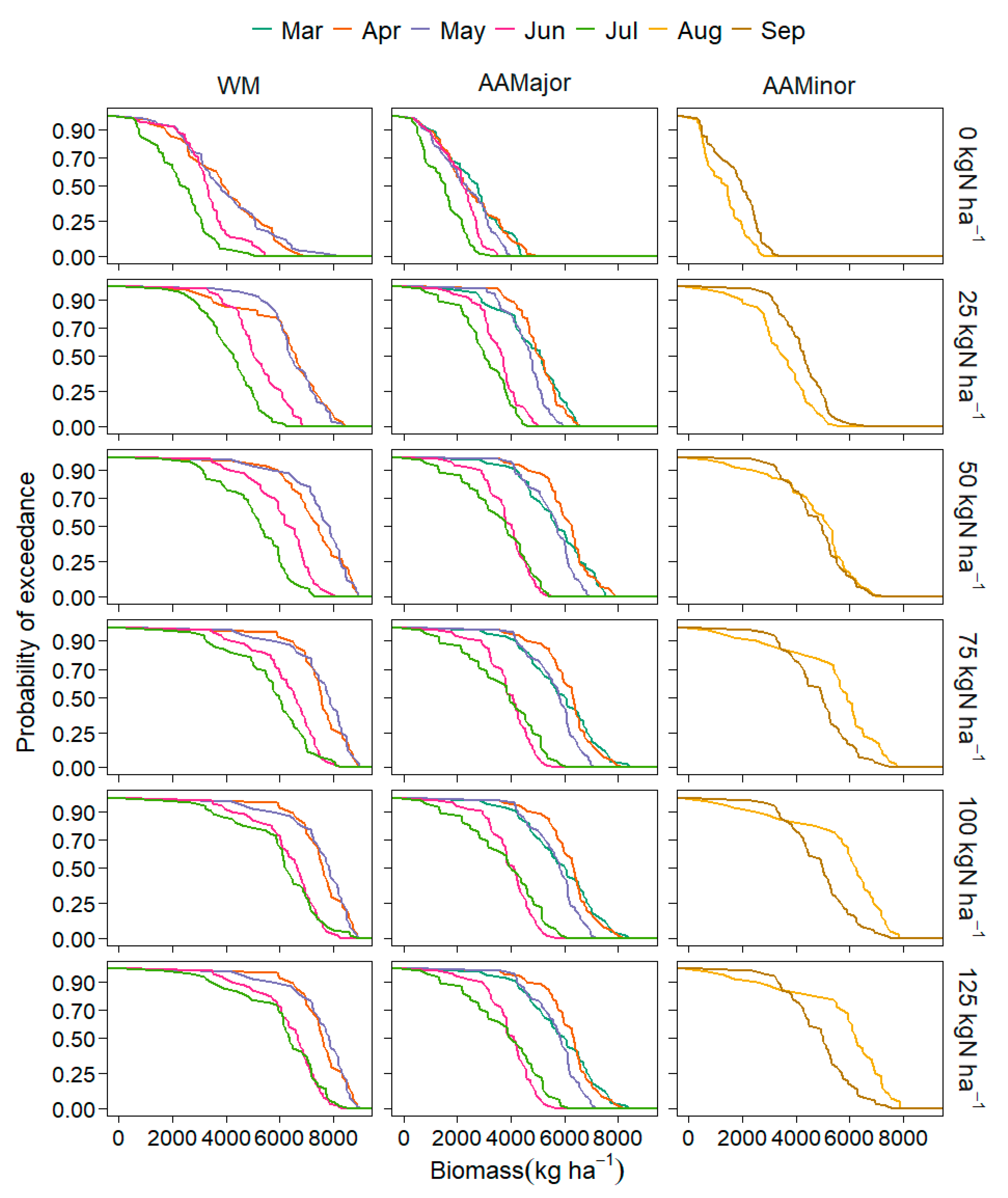
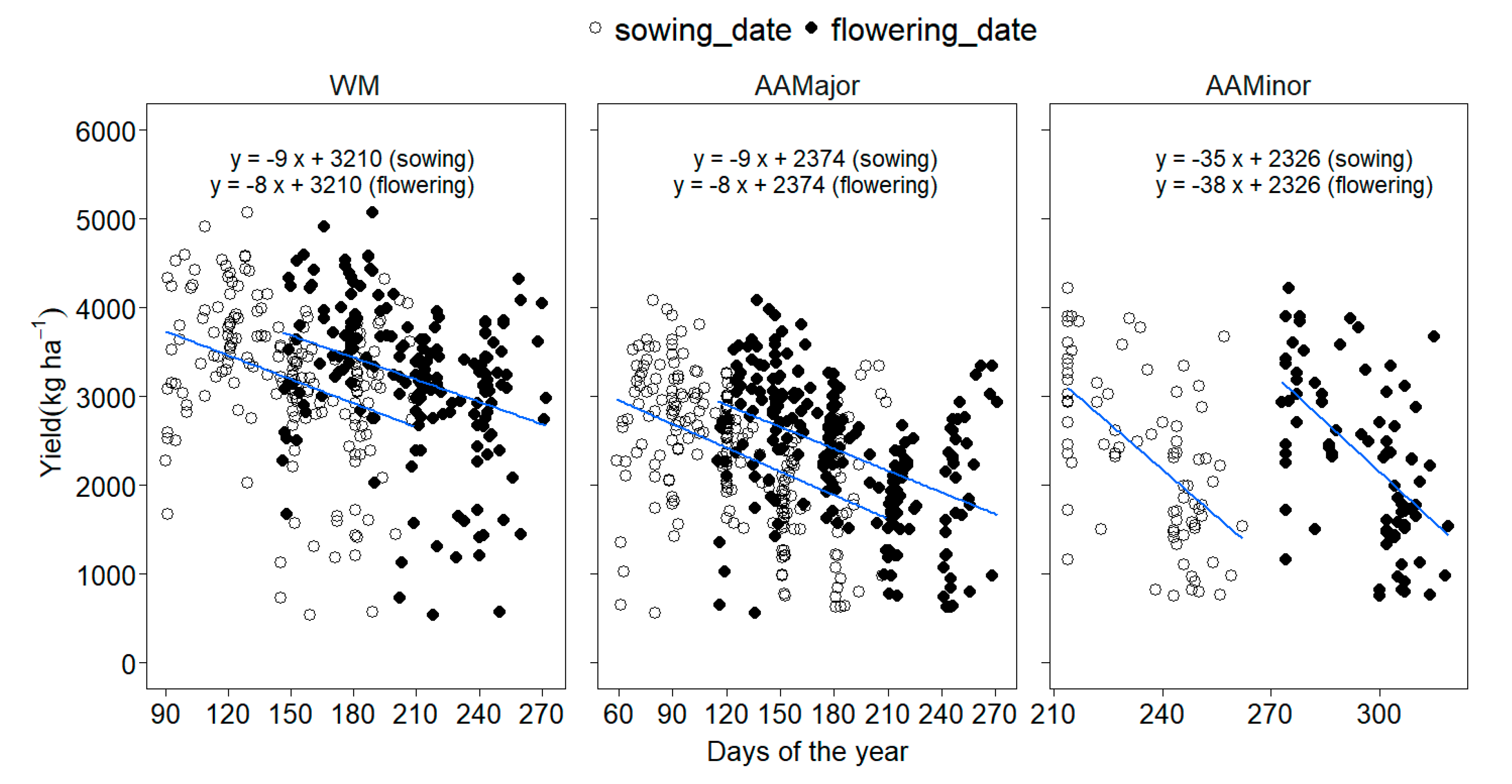
3.2.1. Yield, Sowing Date and Rooting Depth
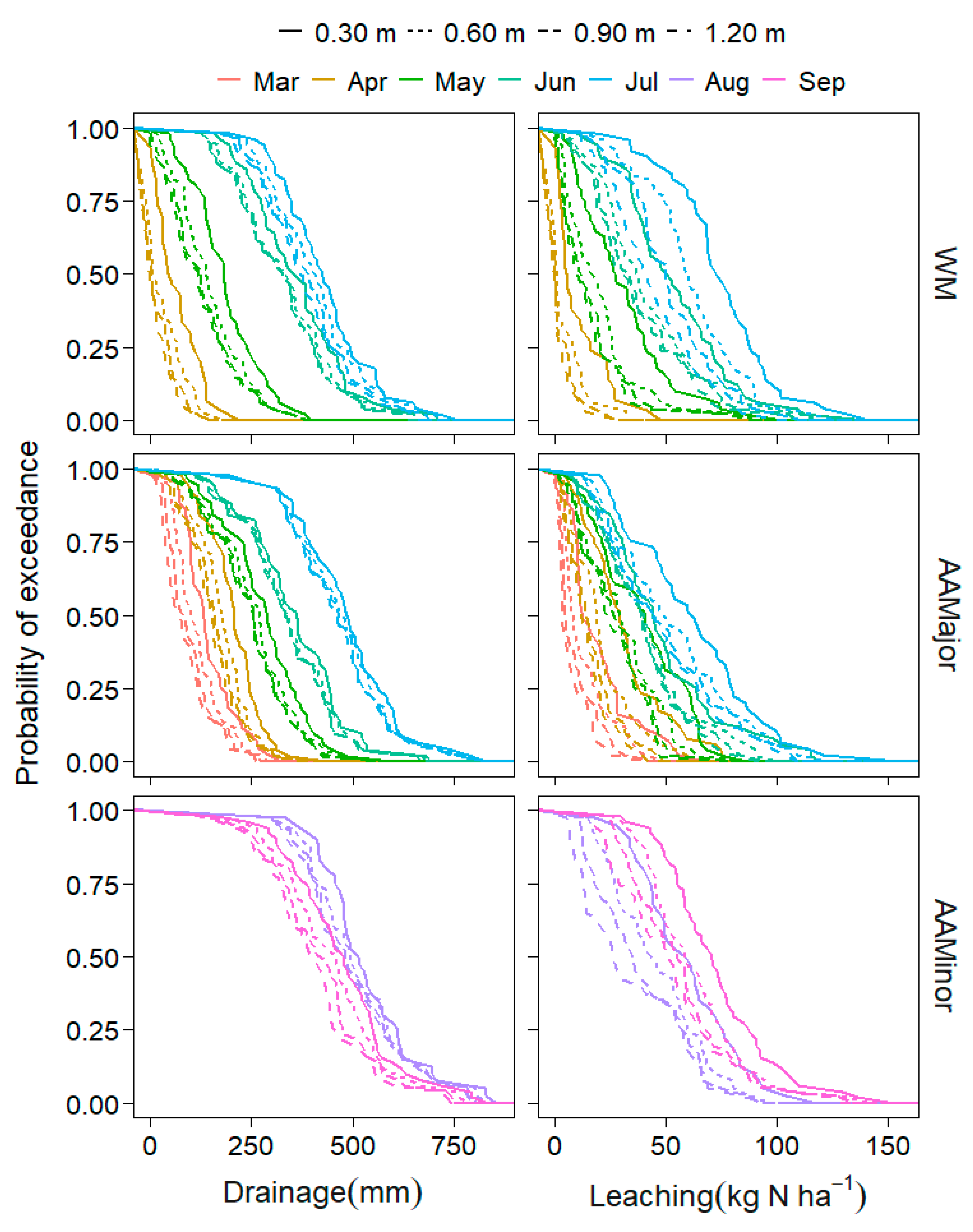
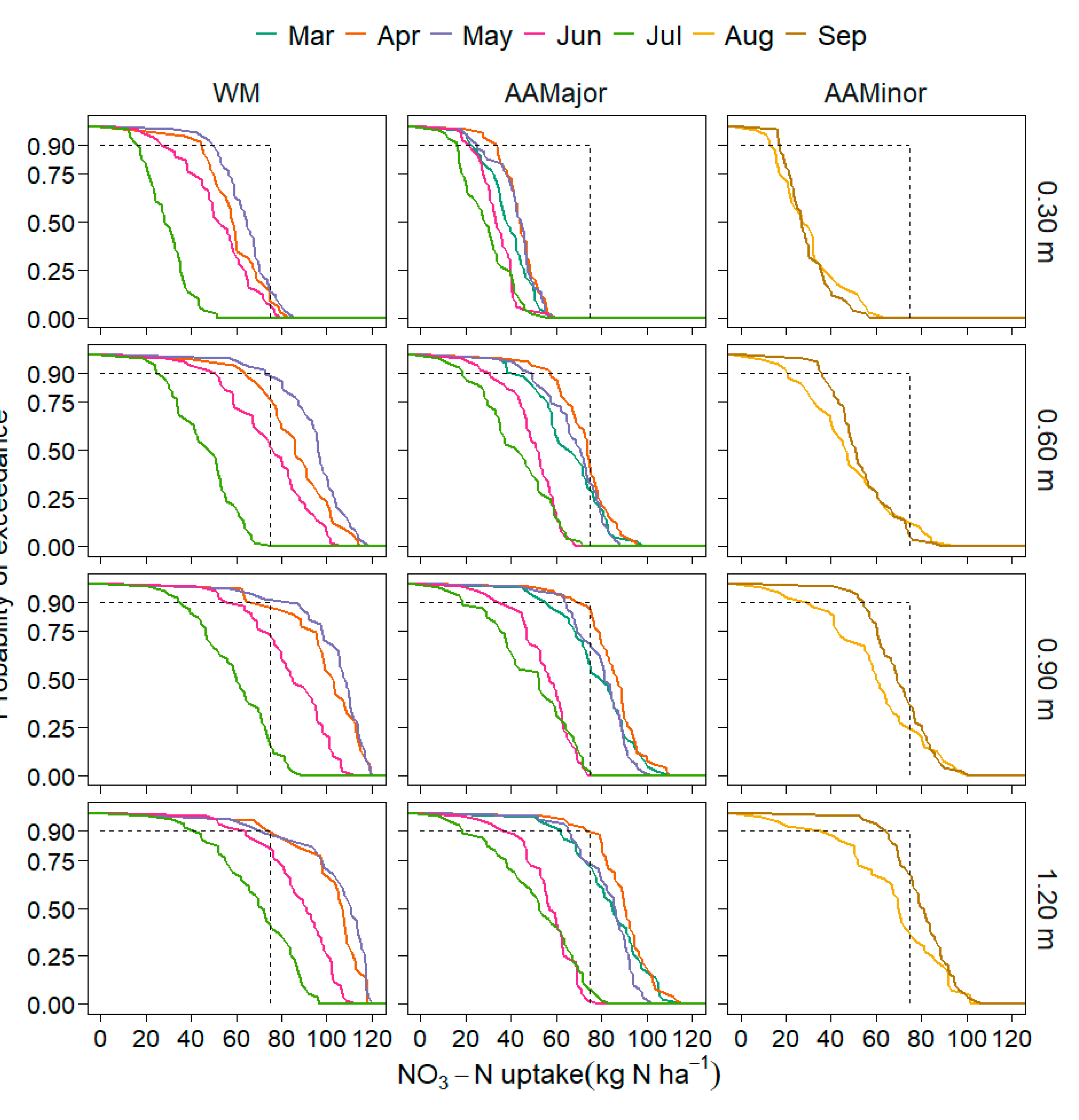
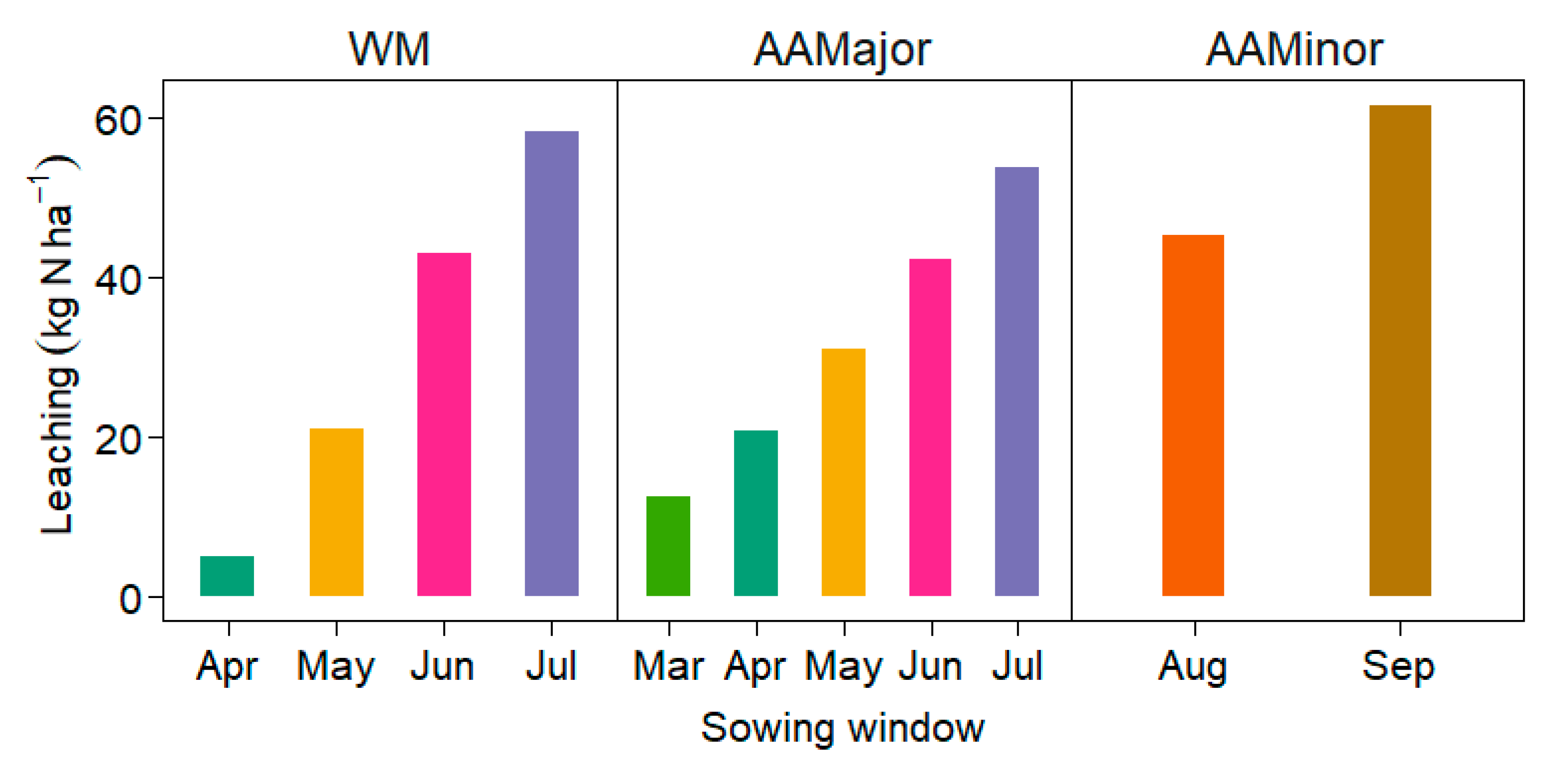
3.2.2. NO3-N Leaching and Uptake, and Water Stress
4. Discussion
4.1. Field Observations
4.2. Modelling Analysis
4.2.1. Yield, Sowing Windows and Climate Variables
4.2.2. Soil Water Stress and NO3-N Leaching
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Sanginga, N.; Dashiell, K.E.; Diels, J.; Vanlauwe, B.; Lyasse, O.; Carsky, R.J.; Tarawali, S.; Asafo-Adjei, B.; Menkir, A.; Schulz, S.; et al. Sustainable resource management coupled to resilient germplasm to provide new intensive cereal–grain–legume–livestock systems in the dry savanna. Agric. Ecosyst. Environ. 2003, 100, 305–314. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological Intensification of Cereal Production Systems: Yield Potential, Soil Quality, and Precision Agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingali, P.L.; Heisey, P.W. Cereal Crop Productivity in Developing Countries: Past Trends and Future Prospects; CIMMYT: Texcoco, Mexico, 1998. [Google Scholar]
- Santpoort, R. The Drivers of Maize Area Expansion in Sub-Saharan Africa. How Policies to Boost Maize Production Overlook the Interests of Smallholder Farmers. Land 2020, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Tittonell, P.; Giller, K.E. When yield gaps are poverty traps: The paradigm of ecological intensification in African smallholder agriculture. Field Crops Res. 2013, 43, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes and causes. Annu. Rev. Environ. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef] [Green Version]
- Van Ittersum, M.K.; Van Bussel, L.G.; Wolf, J.; Grassini, P.; Van Wart, J.; Guilpart, N.; Claessens, L.; de Groot, H.; Wiebe, K.; Mason-D’Croz, D.; et al. Can sub-Saharan Africa feed itself? Proc. Natl. Acad. Sci. USA 2016, 113, 14964–14969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanna, B.M. Maize in the developing world: Trends, challenges, and opportunities. In Addressing Climate Change Effects and Meeting Maize Demand for Asia, Proceedings of the 11th Asian Maze Conference, Nanning, China, 7–11 November 2011; CIMMYT: Texcoco, Mexico, 2011; pp. 26–38. [Google Scholar]
- Pasuquin, J.M.; Pampolino, M.F.; Witt, C.; Dobermann, A.; Oberthür, T.; Fisher, M.J.; Inubushi, K. Closing yield gaps in maize production in Southeast Asia through site-specific nutrient management. Field Crops Res. 2014, 156, 219–230. [Google Scholar] [CrossRef]
- Pasuquin, J.M.C.A.; Witt, C. Yield Potential and Yield Gaps of Maize in Southeast Asia; International Potash Institute: Zug, Switzerland, 2007. [Google Scholar]
- Akramov, K.; Malek, M. Analysing Profitability of Maize, Rice and Soybean Production in Ghana: Results of PAM and DEA Analysis; GSSP Working Paper; International Food Policy Research Institute: Washington, DC, USA, 2012. [Google Scholar]
- MacCarthy, D.S.; Adiku, S.G.K.; Freduah, B.S.; Gbefo, F.; Kamara, A.Y. Using CERES-Maize and ENSO as decision support tools to evaluate climate-sensitive farm management practices for maize production in the northern regions of Ghana. Front Plant Sci. 2017, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water Management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Naab, J.; Mahama, G.; Koo, J.; Jones, J.; Boote, K. Nitrogen and phosphorus fertilization with crop residue retention enhances crop productivity, soil organic carbon, and total soil nitrogen concentrations in sandy-loam soils in Ghana. Nutr. Cycling Agroecosyst. 2015, 102, 33–43. [Google Scholar] [CrossRef]
- Crawford, E.; Kelly, V.; Jayne, T.S.; Howard, J. Input use and market development in Sub-Saharan Africa: An overview. Food Policy. 2003, 28, 277–292. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.Y.; Benedicta, Y.; Mensah, M. The effect of phosphorus and nitrogen fertilizers on grain yield, nutrient uptake and use efficiency of two Maize (Zea mays L.) varieties under rain fed condition on Haplic Lixisol in the forest-savannah transition zone of Ghana. Agric. Ecosyst. Environ. 2016, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Martey, E.; Wiredu, A.N.; Etwire, P.M.; Fosu, M.; Buah, S.S.J.; Bidzakin, J.; Kusi, F. Fertilizer adoption and use intensity among smallholder farmers in Northern Ghana: A case study of the AGRA soil health project. Sustain. Agric. Res. 2014, 3, 24–36. [Google Scholar] [CrossRef]
- Kanton, R.A.L.; Buah, S.S.J.; Larbi, A.; Mohammed, A.A.; Bidzakin, J.K.; Yakubu, E.A. Soil Amendments and Rotation Effects on Soybean and Maize Growths and Soil Chemical Changes in Northern Ghana. Int. J. Agron. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kermah, M.; Franke, A.C.; Adjei-Nsiah, S.; Ahiabor, B.D.K.; Abaidoo, R.C.; Giller, K.E. Maize-grain legume intercropping for enhanced resource use efficiency and crop productivity in the Guinea savanna of northern Ghana. Field Crops Res. 2012, 213, 38–50. [Google Scholar] [CrossRef]
- Adu, G.B.; Akromah, R.; Abdulai, M.S.; Obeng-Antwi, K.; Alidu, H.; Tengan, K.M.L. Trait association for improved grain yield of extra-early maturing maize hybrids evaluated in the forest and transitional zones of Ghana. Aust. J. Crop Sci. 2016, 10, 1127–1135. [Google Scholar] [CrossRef]
- Alhassan, A.; Salifu, H.; Adebanji, A.O. Discriminant analysis of farmers adoption of improved maize varieties in Wa Municipality, Upper West Region of Ghana. SpringerPlus 2016, 5, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DTMA (Drought Tolerant Maize for Africa). A Quarterly Bulletin of the Drought Tolerant Maize for Africa Project; CIMMYT: Texcoco, Mexico, 2013; Volume 2. [Google Scholar]
- MoFA (Ministry of Food and Agriculture). Agriculture in Ghana Facts and Figures; Ministry of Food and Agriculture, Statistics, Research and Information Directorate (SRID): Accra, Ghana, 2017.
- Cakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crops Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Heisey, P.W.; Edmeades, G.O. Maize production in drought-stressed environments: Technical options and research resource allocation. In World Maize Facts and Trends 1997–1998. Part 1; CIMMYT: Texcoco, Mexico, 2019. [Google Scholar]
- Van Ittersum, M.K.; Cassman, K.G.; Grassini, P.; Wolf, J.; Tittonell, P.; Hochman, Z. Yield gap analysis with local to global relevance—A review. Field Crops Res. 2013, 143, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Keating, B.A.; Carberry, P.S.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Smith, C.J. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Brief history of agricultural systems modeling. Agric. Syst. 2017, 155, 240–254. [Google Scholar] [CrossRef]
- Antle, J.M.; Basso, B.; Conant, R.T.; Godfray, H.C.J.; Jones, J.W.; Herrero, M.; Howitt, R.E.; Keating, B.A.; Munoz-Carpena, R.; Rosenzweig, C.; et al. Towards a new generation of agricultural system data, models and knowledge products: Design and improvement. Agric. Syst. 2017, 155, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Dimes, J.P.; van Wijk, M.T.; Twomlow, S.J.; Giller, K.E. Productivity and residual benefits of grain legumes to sorghum under semi-arid conditions in south-western Zimbabwe: Unravelling the effects of water and nitrogen using a simulation model. Field Crops Res. 2009, 110, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Whitbread, A.M.; Robertson, M.J.; Carberry, P.S.; Dimes, J.P. How farming systems simulation can aid the development of more sustainable smallholder farming systems in southern Africa. Eur. J. Agron. 2010, 32, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Fosu-Mensah, B.Y.; MacCarthy, D.S.; Vlek, P.L.G.; Safo, E.Y. Simulating impact of seasonal climatic variation on the response of maize (Zea mays L.) to inorganic fertilizer in sub-humid Ghana. Nutr. Cycl. Agroecosyst. 2012, 94, 255–271. [Google Scholar] [CrossRef]
- Holzworth, D.P.; Huth, N.I.; de Voil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Keating, B.A. APSIM—Evolution towards a new generation of agricultural systems simulation. Environ. Modell. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Beletse, Y.G.; Durand, W.; Nhemachena, C.; Crespo, O.; Tesfuhuney, W.A.; Jones, M.R.; Teweldemedhin, M.Y.; Gamedze, S.M.; Bonolo, P.M.; Jonas, S.; et al. Projected Impacts of Climate Change Scenarios on the Production of Maize in Southern Africa: An Integrated Assessment Case Study of the Bethlehem District, Central Free State, South Africa. In Handbook of Climate Change and Agroecosystems: The Agricultural Model Intercomparison and Improvement Project Integrated Crop and Economic Assessments, Part 2; Imperial College Press: London, UK, 2015; pp. 125–157. [Google Scholar]
- Kihara, J.; MacCarthy, D.S.; Bationo, A.; Koala, S.; Hickman, J.; Koo, J.; Jones, J.W. Perspectives on climate effects on agriculture: The international efforts of AgMIP in sub-Saharan Africa. In Handbook of Climate Change and Agro-ecosystems: The Agricultural Model Inter-comparison and Improvement Project (AgMIP) Integrated Crop and Economic Assessments; Hillel, D., Rosenzweig, C., Eds.; Imperial College Press: London, UK, 2015; pp. 263–280. [Google Scholar] [CrossRef]
- MacCarthy, D.S.; Akponikpe, P.B.I.; Narh, S.; Tegbe, R. Modelling the effect of seasonal climate variability on the efficiency of mineral fertilization on maize in the coastal savannah of Ghana. Nutr. Cycl. Agroecosys. 2015, 102, 45–64. [Google Scholar] [CrossRef]
- MacCarthy, D.S.; Kihara, J.; Masikati, P.; Adiku, S.G.K. Decision support tools for site-specific fertilizer recommendations and agricultural planning in selected countries in sub-Sahara Africa. Nutr. Cycl. Agroecosyst. 2018, 110, 343–359. [Google Scholar] [CrossRef]
- Poss, R.; Noble, A.D.; Dunin, F.X.; Reyenga, W. Evaluation of ceramic cup samplers to measure nitrate leaching in the field. Eur. J. Soil Sci. 1995, 46, 667–674. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1984, 37, 29–38. [Google Scholar] [CrossRef]
- Toth, S.J.; Prince, A.L. Estimation of cation-exchange capacity and exchangeable Ca, K, and Na contents of soils by flame photometer techniques. Soil Sci. 1949, 67, 439–446. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- RQflex Plus 10 Product. Available online: www.sigmaaldrich.com/catalog/product/mm/116957?lang=en®ion=GH&cm_sp=Insite--caContent_prodMerch_gruCrossEntropy_prodMerch10-3 (accessed on 25 March 2020).
- Berchie, J.N.; Tengan, M.L.; Agyeman, K.; Gyasi-Boakye, E.; Adu-Appiah, A. Maize Production Guide; Council for Scientific and Industrial Research—Crops Research Institute (CSIR—CRI): Kumasi, Ghana, 2016. [Google Scholar]
- Owusu Danquah, E.; Ennin, S.A.; Frimpong, F.; Oteng-Darko, P.; Yeboah, S.; Osei-Adu, J. Adoption of good agricultural practices for sustainable maize and cowpea production: The role of enabling policy. World Res. J. Agri. Sci. (WRJAS) 2015, 2, 028–038. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 August 2020).
- SARI (Savanna Agricultural Research Institute). Annual Report for 2005; SARI: Nyankpala, Ghana, 2005. [Google Scholar]
- Buah, S.S.J.; Ibrahim, H.; Derigubah, M.; Kuzie, M.; Segtaa, J.V.; Bayala, J.; Zougmore, R.; Ouedraogo, M. Tillage and fertilizer effect on maize and soybean yields in the Guinea savanna zone of Ghana. Agric. Food Secur. 2017, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Kugbe, J.X.; Kombat, R.; Atakora, W. Secondary and micronutrient inclusion in fertilizer formulation impact on maize growth and yield across northern Ghana. Cogent Food Agric. 2019, 5, 1700030. [Google Scholar] [CrossRef]
- Atakora, K.W.; Fosu, M.; Marthey, F. Modeling Maize Production towards Site Specific Fertilizer Recommendation in Ghana. Glob. J. Sci. Front. Res. Agric. Vet. 2014, 14, 1. [Google Scholar]
- Muchow, R.C.; Davis, R. Effect of nitrogen supply on the comparative productivity of maize and sorghum in a semi-arid tropical environment. II. Radiation interception and bio-mass accumulation. Field Crops Res. 1988, 18, 17–30. [Google Scholar] [CrossRef]
- Danso, E.O.; Yakubu, A.; Darrah, Y.O.K.; Arthur, E.; Manevski, K.; Sabi, E.B.; Abenney-Mickson, S.; Ofori, K.; Plauborg, F.; Andersen, M.N. Impact of rice straw biochar and irrigation on maize yield, intercepted radiation and water productivity in a tropical sandy clay loam. Field Crops Res. 2019, 243, 107628. [Google Scholar] [CrossRef]
- Abubakar, A.W.; Manga, A.A.; Kamara, A.Y.; Tofa, A.I. Physiological Evaluations of Maize Hybrids under Low Nitrogen. J. Adv. Agric. 2019, 2019, 2624707. [Google Scholar] [CrossRef] [Green Version]
- Colombi, T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth—A vicious circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Rostamza, M.; Richards, R.A.; Watt, M. Response of millet and sorghum to a varying water supply around the primary and nodal roots. Ann. Bot. 2013, 112, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hund, A.; Reimer, R.; Messmer, R. A consensus map of QTLs controlling the root length of maize. Plant Soil 2011, 344, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Tai, H.; Lu, X.; Opitz, N.; Marcon, C.; Paschold, A.; Lithio, A.; Nettleton, D.; Hochholdinger, F. Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 1123–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.A.; Zarebanadkouki, M.; Meunier, F.; Javaux, M.; Kaestner, A.; Carminati, A. Root type matters: Measurement of water uptake by seminal, crown, and lateral roots in maize. J. Exp. Bot. 2018, 69, 1199–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perego, A.; Basile, A.; Bonfante, A.; De Mascellis, R.; Terribile, F.; Brenna, S.; Acutis, M. Nitrate leaching under Maize cropping systems in Po Valley (Italy). Agric. Ecosyst. Environ. 2012, 147, 57–65. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on the yield and growth of summer maize under field condition. Can. J. Plant Sci. 2014, 94, 23–31. [Google Scholar] [CrossRef]
- Lopez-Pereira, M.; Morris, M. Impact of International Maize Breeding Research in the Developing World; International Maize and Wheat Improvement Centre (CIMMYT): Texcoco, Mexico, 2019. [Google Scholar]
- Atakora, W.K.; Kwakye, P.K.; Weymann, D.; Brüggemann, N. Stimulus of nitrogen fertilizers and soil characteristics on maize yield and nitrous oxide emission from Ferric Luvisol in the Guinea Savanna agro-ecological zone of Ghana. Sci. Afr. 2019, 6, e00141. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mboh, C.M.; Gaiser, T.; Ewert, F. Impact of climatic variables on the spatial and temporal variability of crop yield and biomass gap in sub-Saharan Africa—A case study in Central Ghana. Field Crop Res. 2017, 203, 33–46. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mboh, G.; Zhao, C.M.; Gaiser, T.; Ewert, F. Climate change impact under alternate realizations of climate scenarios on maize yield and biomass in Ghana. Agric. Sys. 2018, 159, 157–174. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Cameira, M.R.; Fernando, L.; Pereira, S. Monitoring water and NO3-N in irrigated maize fields in the Sorraia Watershed, Portugal. Agric. Water Manag. 2003, 60, 199–216. [Google Scholar] [CrossRef]
- Gheysaria, S.M.; Mirlatifi, M.; Homaeeb, M.E.; Hoogenboomd, A.G. Nitrate leaching in a silage maize field under different irrigation and nitrogen fertilizer rates. Agric. Water Manag. 2009, 96, 946–954. [Google Scholar] [CrossRef]
- Basso, B.; Ritchie, J.T. Impact of compost, manure and inorganic fertilizer on nitrate leaching and yield for a 6-year maize-alfalfa rotation in Michigan. Agric. Ecosyst. Environ. 2005, 108, 329–341. [Google Scholar] [CrossRef]
- Zhou, M.; Klaus, B. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Lin, B.L.; Sakoda, A.; Shibasaki, R.; Suzuki, M. A modelling approach to global nitrate leaching caused by anthropogenic fertilization. Water Res. 2001, 35, 1961–1968. [Google Scholar] [CrossRef]





| Season | Sowing Date | Sowing (DOY) ** | Harvest Date | Harvesting (DOY) | N Applied * (kg ha−1) | Days to Harvesting (Days) |
|---|---|---|---|---|---|---|
| Minor | 31 July 2012 | 213 | 20 December 2012 | 355 | 0 | 143 |
| Major | 5 May 2012 | 126 | 9 September 2012 | 253 | 59 | 128 |
| Minor | 27 August 2013 | 239 | 20 December 2013 | 354 | 38 | 116 |
| Major | 14 April 2012 | 105 | 9 August 2012 | 222 | 65 | 118 |
| Minor | 7 September 2013 | 250 | 20 June 2013 | 354 | 0 | 104 |
| Minor | 5 August 2012 | 218 | 18 December 2012 | 353 | 65 | 136 |
| Minor | 6 August 2012 | 219 | 19 December 2012 | 354 | 101 | 136 |
| Minor | 8 September 2013 | 251 | 18 December 2013 | 352 | 19 | 102 |
| Sowing Date | Sowing (DOY) ** | Harvest Date | Harvesting (DOY) | N Applied * (kg ha−1) | Days to Harvesting (Days) |
|---|---|---|---|---|---|
| 20 June 2012 | 172 | 3 November 2012 | 308 | 76 | 137 |
| 12 July 2013 | 193 | 13 September 2013 | 256 | 19 | 115 |
| 11 July 2012 | 193 | 30 November 2012 | 335 | 0 | 143 |
| 12 July 2013 | 193 | 23 September 2013 | 266 | 0 | 74 |
| 9 July 2012 | 191 | 20 November 2012 | 325 | 62 | 135 |
| 11 July 2013 | 192 | 5 November 2013 | 309 | 25 | 118 |
| 15 June 2012 | 167 | 1 November 2012 | 306 | 40 | 140 |
| 29 June 2013 | 180 | 5 October 2013 | 278 | 41 | 99 |
| Site | Depth (m) | Exchangeable Cations (Centimol+ kg−1) | Org. C (%) | pH | Total N (%) | |||
|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | Na+ | |||||
| A | 0.30 | 1.34 | 1.34 | 0.12 | 0.04 | 0.69 | 5.7 | 0.06 |
| 0.60 | 2.14 | 0.53 | 0.16 | 0.05 | 0.11 | 5.3 | 0.01 | |
| B | 0.30 | 2.67 | 0.53 | 0.03 | 0.02 | 0.75 | 5.6 | 0.06 |
| 0.60 | 2.67 | 0.53 | 0.04 | 0.02 | 0.59 | 5.9 | 0.05 | |
| C | 0.30 | 1.34 | 0.53 | 0.05 | 0.03 | 0.86 | 5.5 | 0.08 |
| 0.60 | 1.34 | 0.53 | 0.03 | 0.02 | 0.48 | 5.4 | 0.04 | |
| D | 0.30 | 3.20 | 1.34 | 0.09 | 0.03 | 0.96 | 6.0 | 0.08 |
| 0.60 | 1.60 | 0.53 | 0.06 | 0.03 | 0.66 | 5.7 | 0.06 | |
| E | 0.30 | 4.27 | 1.87 | 0.11 | 0.04 | 0.86 | 6.3 | 0.08 |
| 0.60 | 2.14 | 1.60 | 0.05 | 0.03 | 0.73 | 5.8 | 0.07 | |
| F | 0.30 | 1.34 | 0.80 | 0.04 | 0.02 | 0.57 | 5.8 | 0.05 |
| 0.60 | 1.34 | 1.07 | 0.05 | 0.03 | 0.50 | 5.5 | 0.04 | |
| G | 0.30 | 1.87 | 1.34 | 0.06 | 0.03 | 0.34 | 6.5 | 0.02 |
| 0.60 | 1.60 | 1.07 | 0.05 | 0.03 | 0.18 | 6.5 | 0.02 | |
| H | 0.30 | 2.40 | 1.07 | 0.19 | 0.05 | 1.19 | 6.17 | 0.06 |
| 0.60 | 2.14 | 1.34 | 0.11 | 0.04 | 1.03 | 6.07 | 0.05 | |
| I | 0.30 | 2.14 | 0.80 | 0.12 | 0.04 | 0.98 | 6.1 | 0.05 |
| 0.60 | 1.60 | 1.07 | 0.11 | 0.04 | 0.71 | 5.9 | 0.04 | |
| J | 0.30 | 4.01 | 0.80 | 0.26 | 0.07 | 0.82 | 6.26 | 0.07 |
| 0.60 | 4.01 | 0.80 | 0.16 | 0.05 | 0.44 | 5.99 | 0.04 | |
| Site | Depth (m) | LL (mm mm−1) | DUL (mm mm−1) | SAT (mm mm−1) | Bulk Density (g cm−3) | PAW (mm) |
|---|---|---|---|---|---|---|
| A | 0.30 | 0.06 | 0.21 | 0.3 | 1.49 | 45 |
| 0.60 | 0.06 | 0.21 | 0.3 | 1.58 | 45 | |
| B | 0.30 | 0.06 | 0.21 | 0.3 | 1.45 | 45 |
| 0.60 | 0.06 | 0.21 | 0.3 | 1.45 | 45 | |
| C | 0.30 | 0.15 | 0.19 | 0.3 | 1.45 | 12 |
| 0.60 | 0.12 | 0.17 | 0.3 | 1.52 | 15 | |
| D | 0.30 | 0.15 | 0.19 | 0.3 | 1.41 | 12 |
| 0.60 | 0.12 | 0.17 | 0.3 | 1.37 | 15 | |
| E | 0.30 | 0.15 | 0.19 | 0.3 | 1.41 | 12 |
| 0.60 | 0.12 | 0.17 | 0.3 | 1.43 | 15 | |
| F | 0.30 | 0.10 | 0.24 | 0.3 | 1.49 | 42 |
| 0.60 | 0.10 | 0.24 | 0.3 | 1.58 | 42 | |
| G | 0.30 | 0.10 | 0.24 | 0.3 | 1.45 | 42 |
| 0.60 | 0.10 | 0.24 | 0.3 | 1.45 | 42 | |
| H | 0.30 | 0.12 | 0.20 | 0.3 | 1.45 | 24 |
| 0.60 | 0.12 | 0.18 | 0.3 | 1.52 | 18 | |
| I | 0.30 | 0.12 | 0.20 | 0.3 | 1.41 | 24 |
| 0.60 | 0.12 | 0.18 | 0.3 | 1.48 | 18 |
| Soil Layers (m) | West Mamprusi (WM) | AA Major and AA Minor | ||||||
|---|---|---|---|---|---|---|---|---|
| LL ** | DUL | SAT | Maize LL * | LL ** | DUL | SAT | Maize LL * | |
| 0–0.10 | 0.06 | 0.24 | 0.3 | 0.06 | 0.1 | 0.21 | 0.3 | 0.1 |
| 0.10–0.20 | 0.06 | 0.24 | 0.3 | 0.06 | 0.1 | 0.21 | 0.3 | 0.1 |
| 0.20–0.30 | 0.06 | 0.24 | 0.3 | 0.06 | 0.1 | 0.21 | 0.3 | 0.1 |
| 0.30–0.45 | 0.07 | 0.24 | 0.3 | 0.07 | 0.11 | 0.21 | 0.3 | 0.11 |
| 0.45–0.60 | 0.07 | 0.24 | 0.3 | 0.08 | 0.11 | 0.2 | 0.3 | 0.12 |
| 0.60–0.90 | 0.07 | 0.22 | 0.3 | 0.10 | 0.11 | 0.19 | 0.3 | 0.13 |
| 0.90–1.20 | 0.07 | 0.20 | 0.3 | 0.12 | 0.11 | 0.18 | 0.3 | 0.14 |
| Soil Parameters | Values |
|---|---|
| kli * | 0–0.06 |
| xfi ** | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owusu Danquah, E.; Beletse, Y.; Stirzaker, R.; Smith, C.; Yeboah, S.; Oteng-Darko, P.; Frimpong, F.; Ennin, S.A. Monitoring and Modelling Analysis of Maize (Zea mays L.) Yield Gap in Smallholder Farming in Ghana. Agriculture 2020, 10, 420. https://doi.org/10.3390/agriculture10090420
Owusu Danquah E, Beletse Y, Stirzaker R, Smith C, Yeboah S, Oteng-Darko P, Frimpong F, Ennin SA. Monitoring and Modelling Analysis of Maize (Zea mays L.) Yield Gap in Smallholder Farming in Ghana. Agriculture. 2020; 10(9):420. https://doi.org/10.3390/agriculture10090420
Chicago/Turabian StyleOwusu Danquah, Eric, Yacob Beletse, Richard Stirzaker, Christopher Smith, Stephen Yeboah, Patricia Oteng-Darko, Felix Frimpong, and Stella Ama Ennin. 2020. "Monitoring and Modelling Analysis of Maize (Zea mays L.) Yield Gap in Smallholder Farming in Ghana" Agriculture 10, no. 9: 420. https://doi.org/10.3390/agriculture10090420






