Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System
Abstract
1. Introduction
2. Materials and Methods
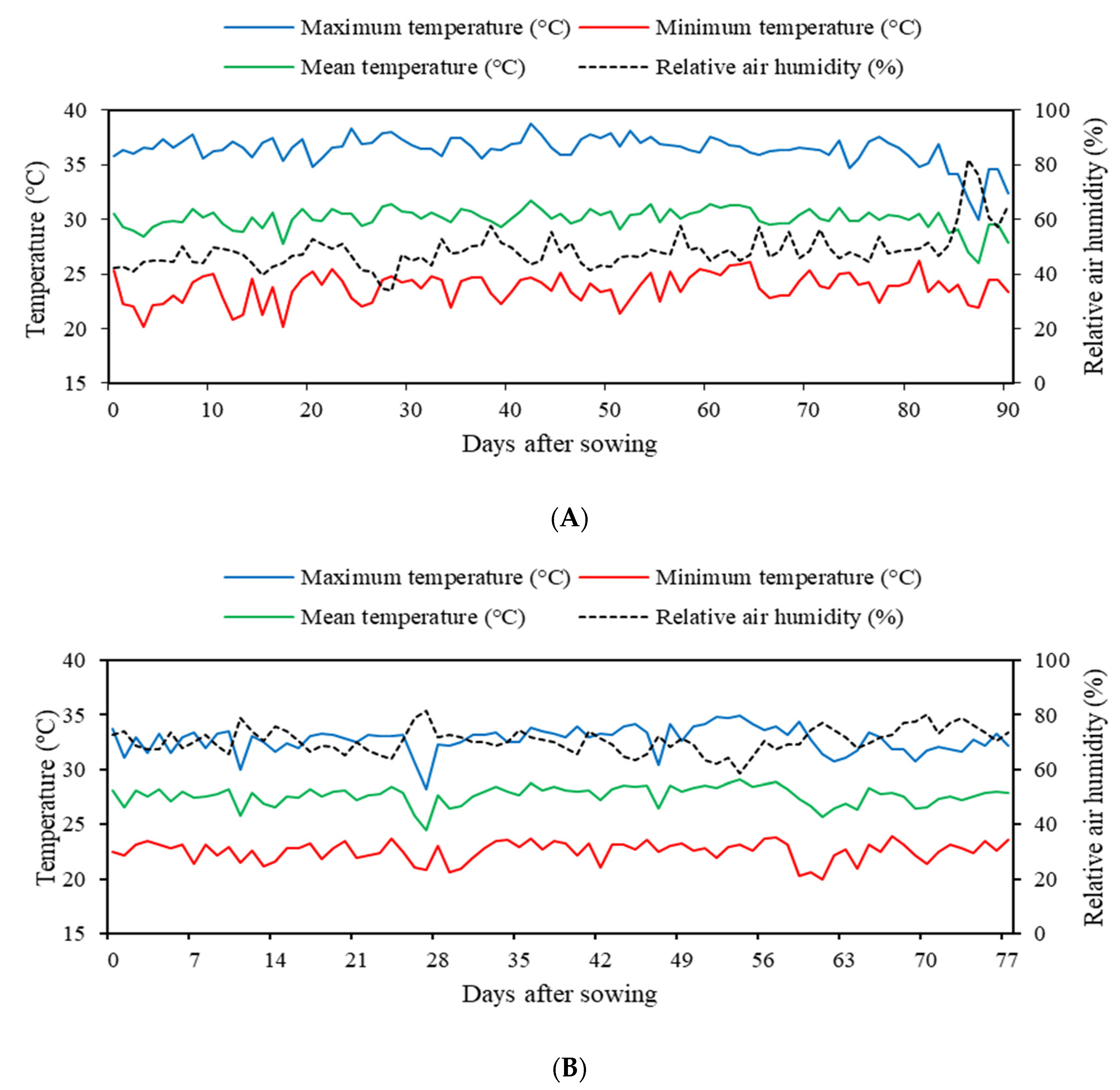
2.1. Location of the Experiment
2.2. Treatments and Experimental Design
2.2.1. Experiment I
2.2.2. Experiment II
2.3. Description of the Experiments
2.4. Variables Analyzed
2.5. Salinity Tolerance
2.6. Statistical Analysis
3. Results
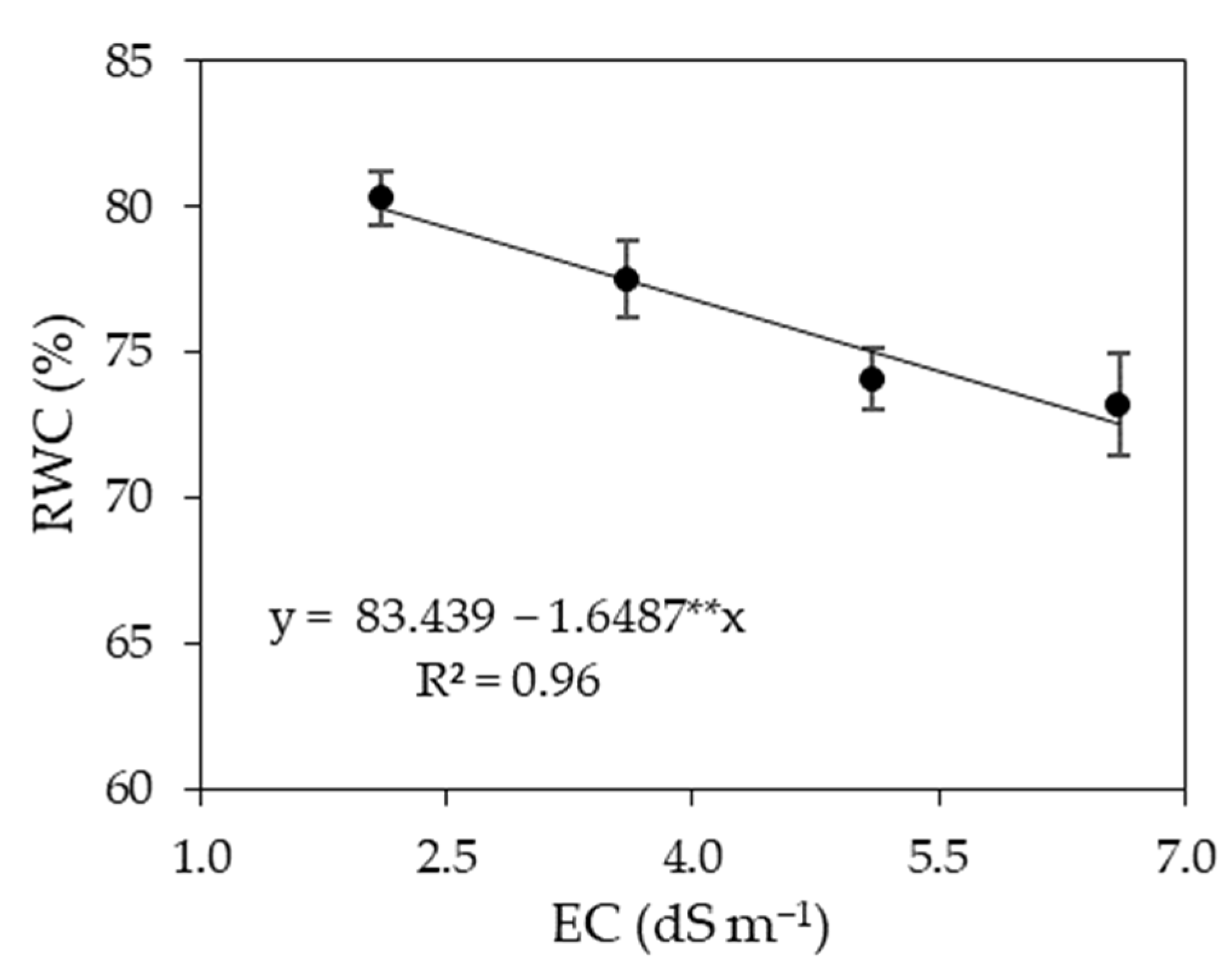
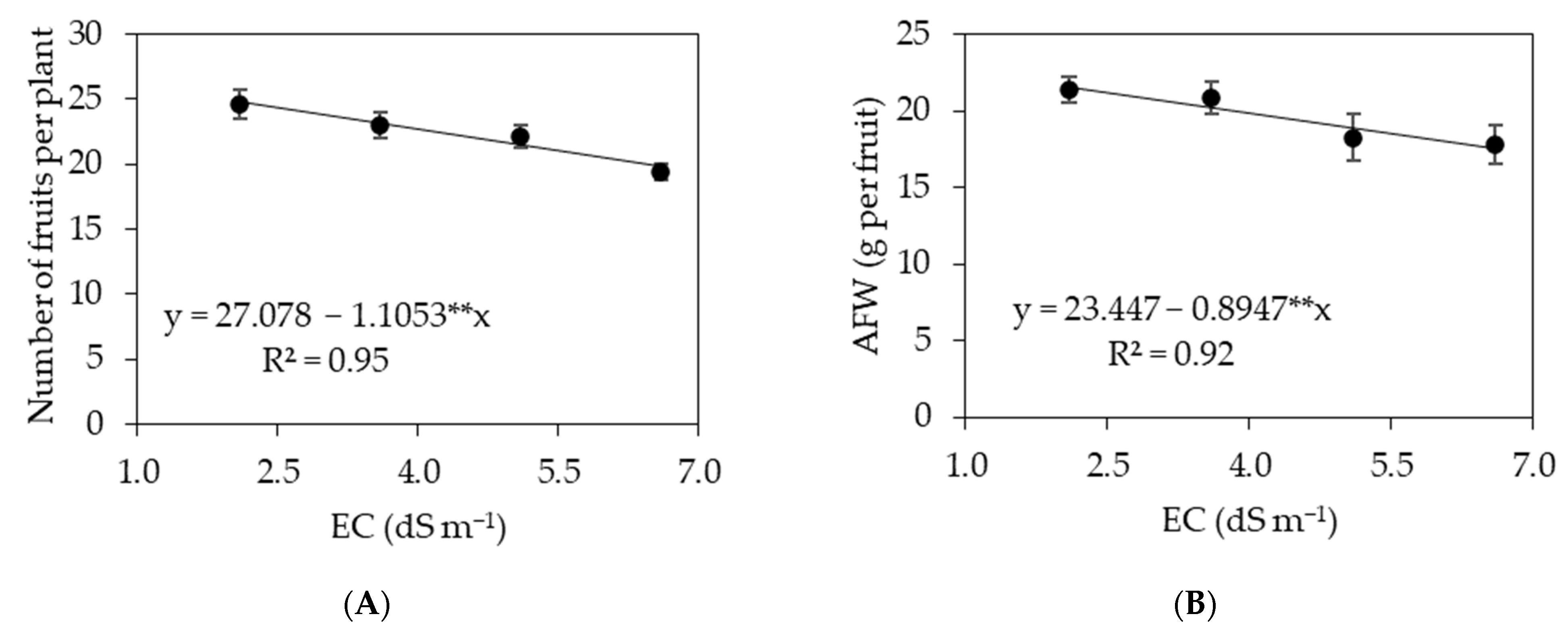
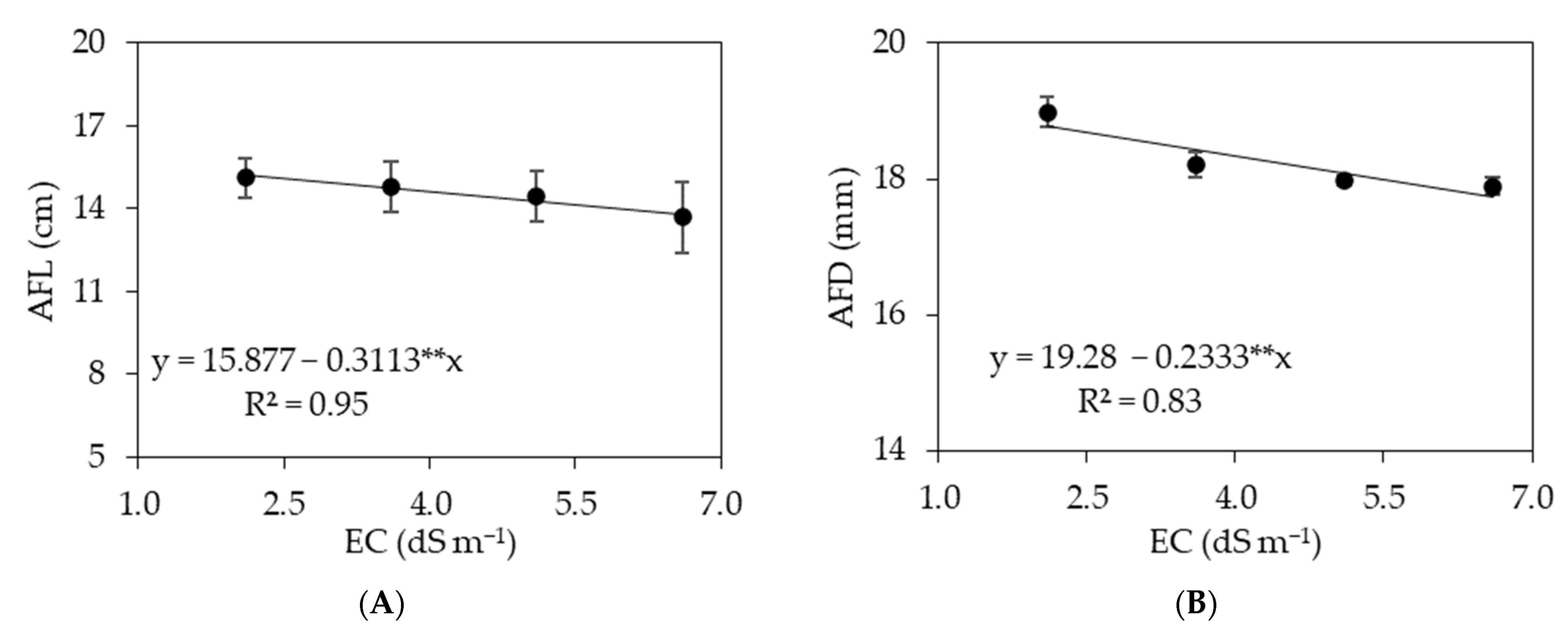
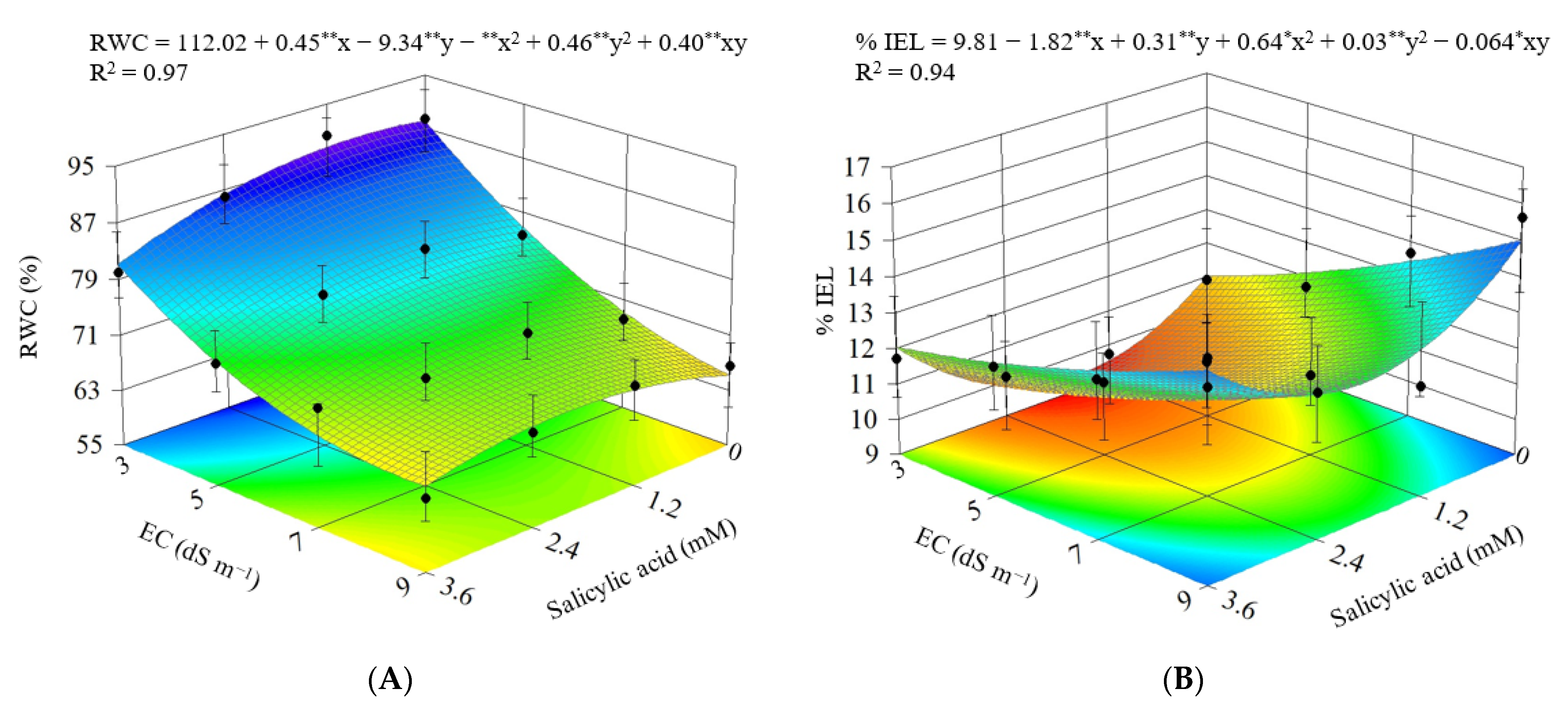
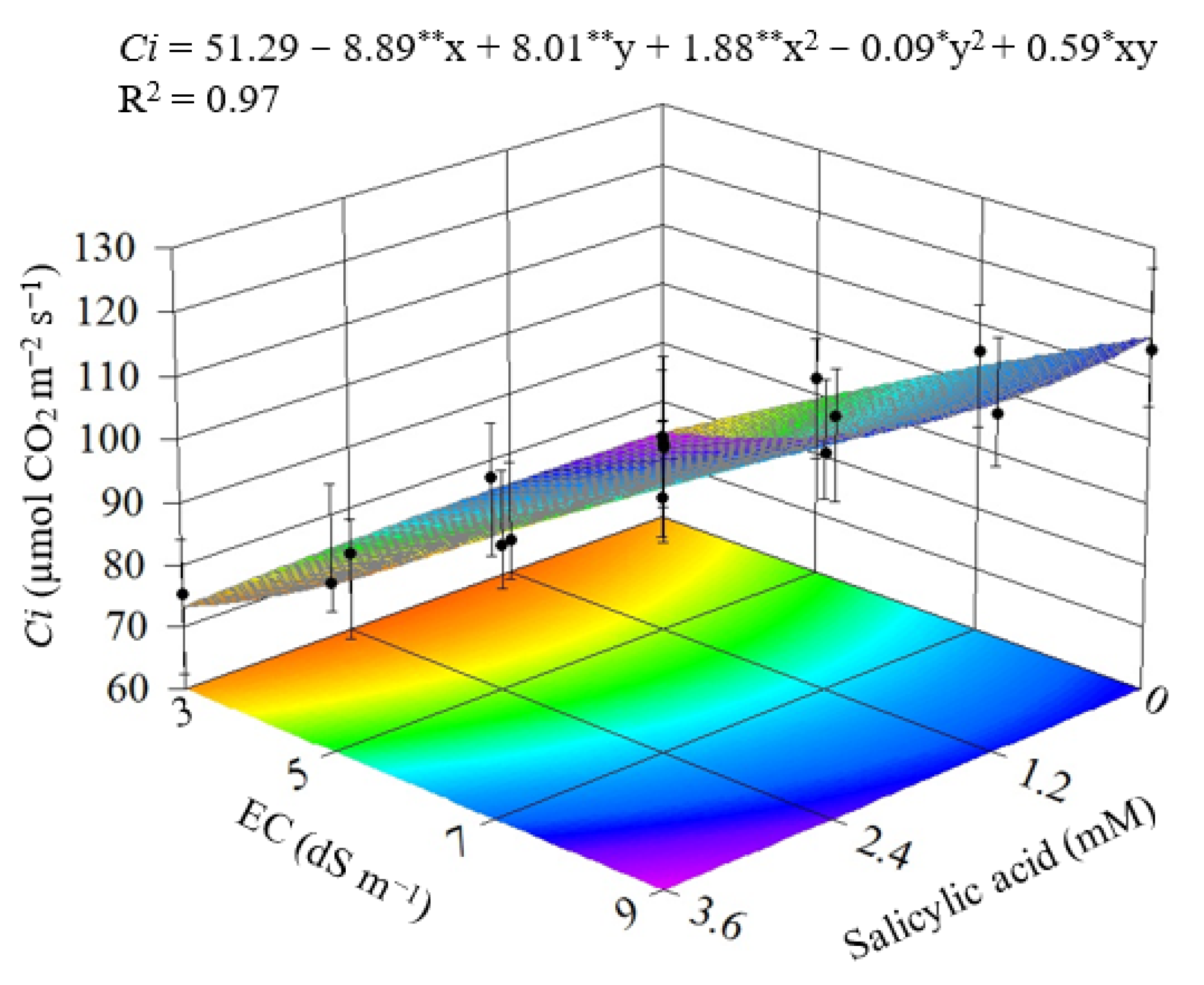
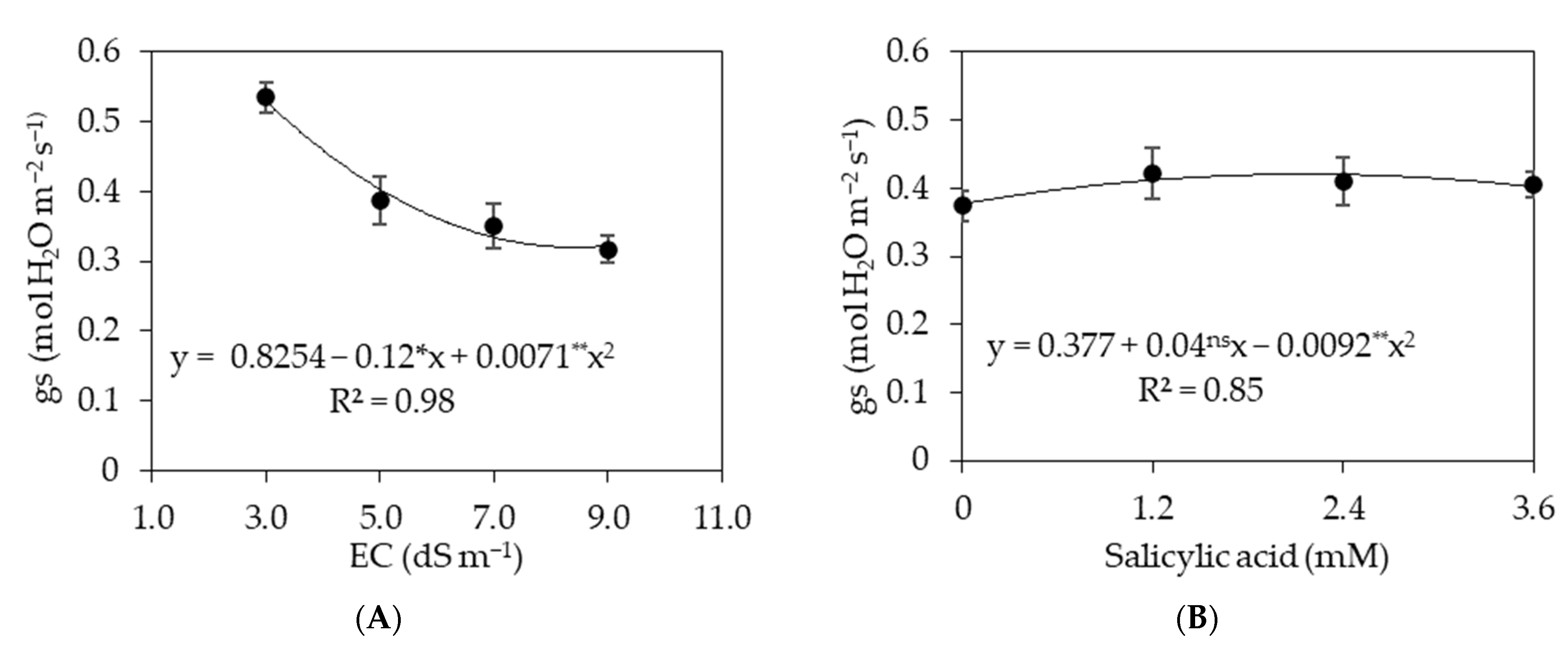
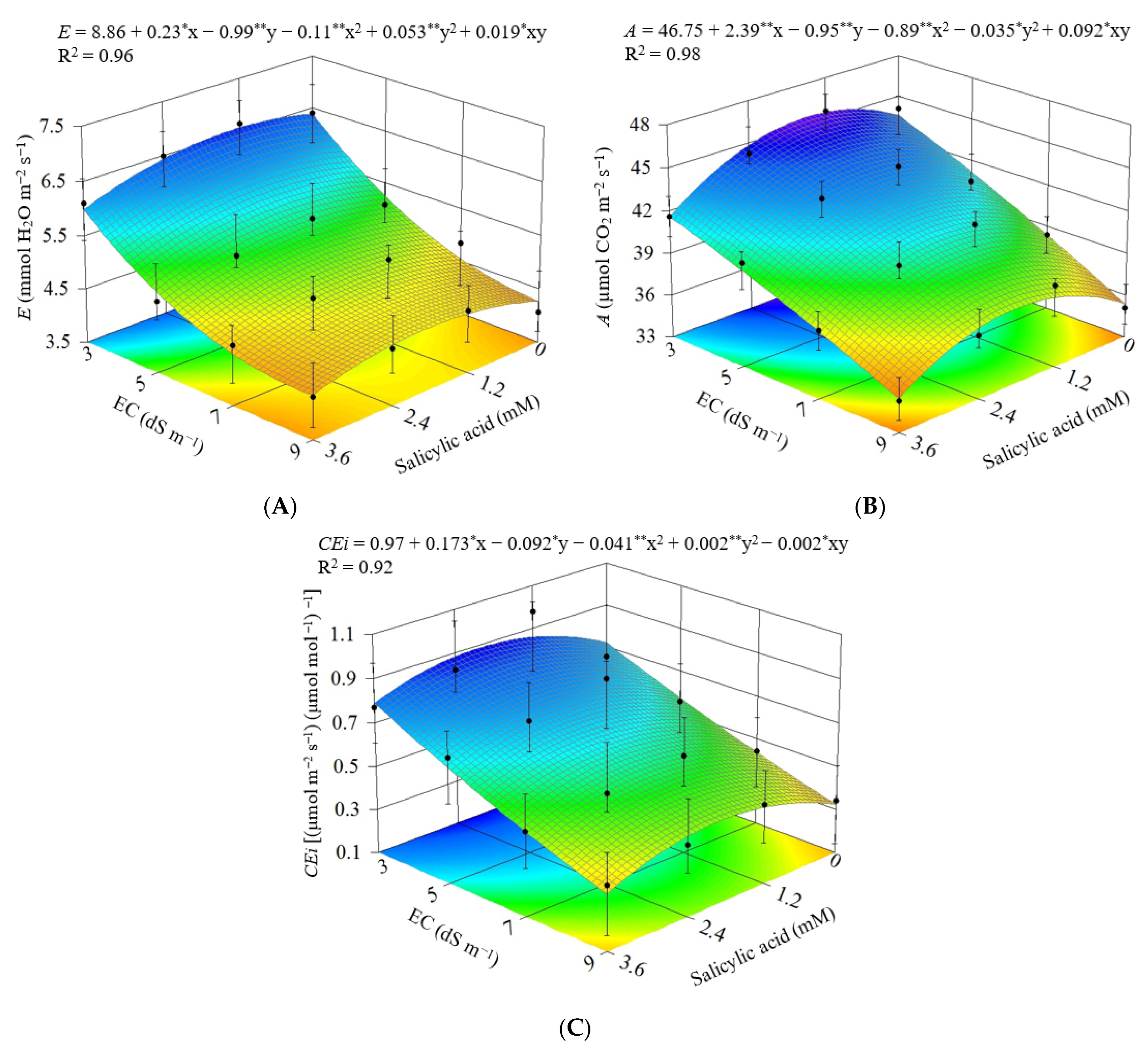
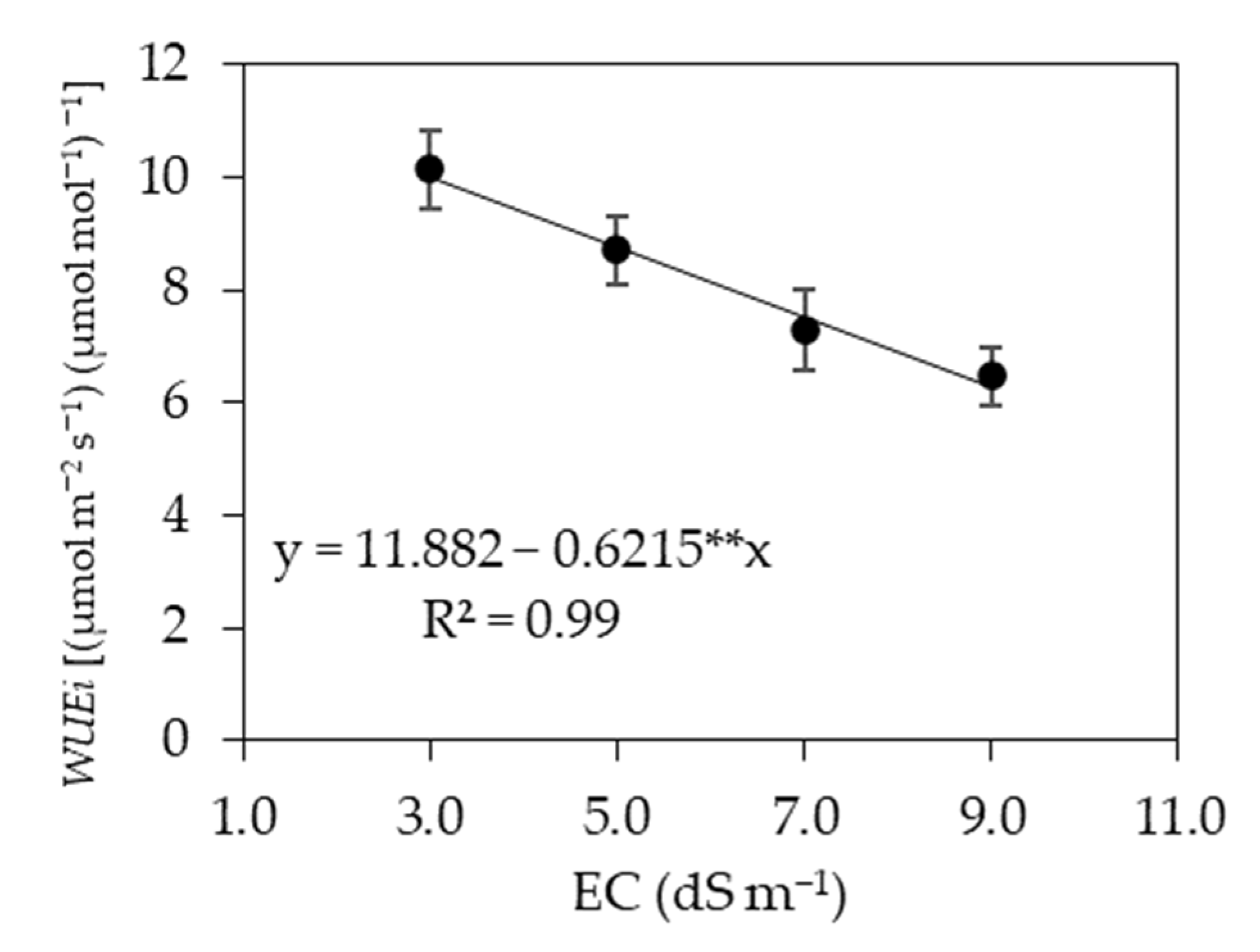
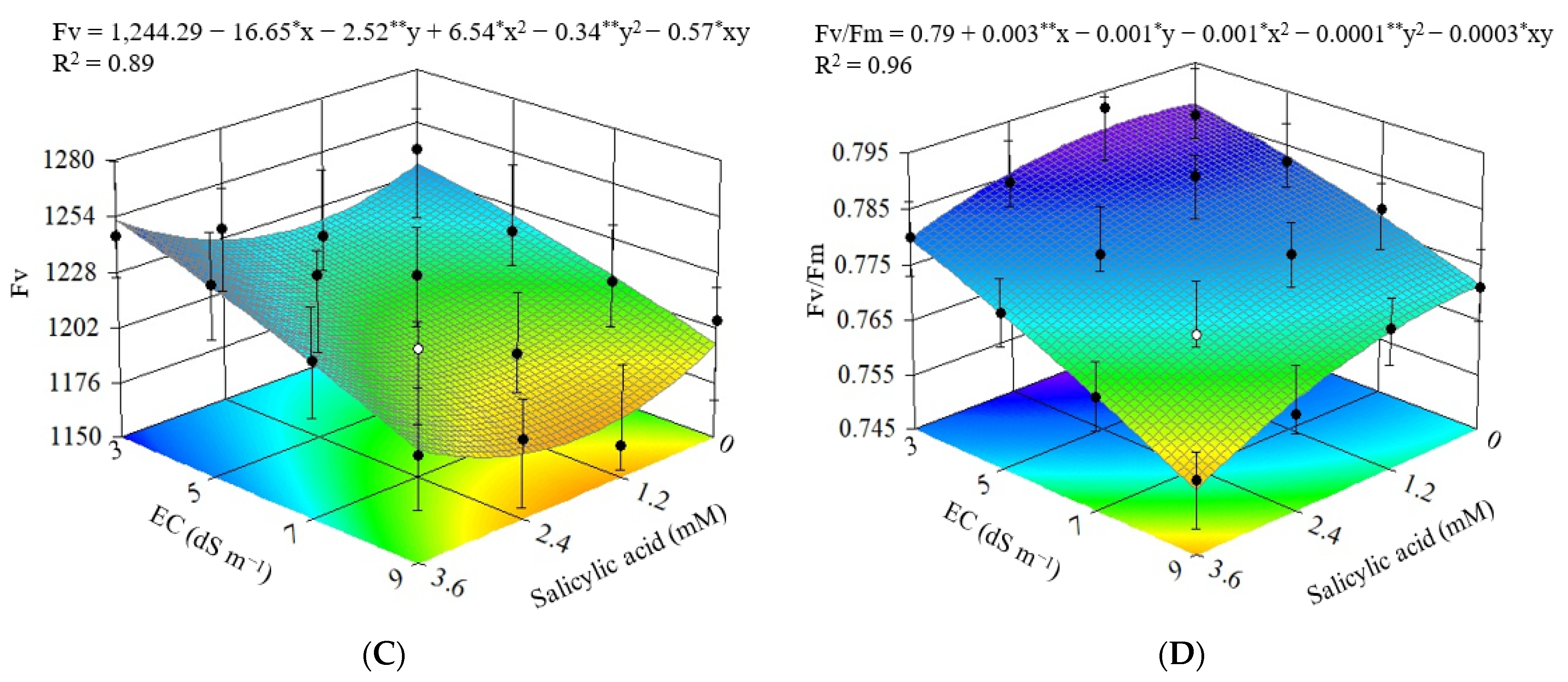
3.1. Experiment I
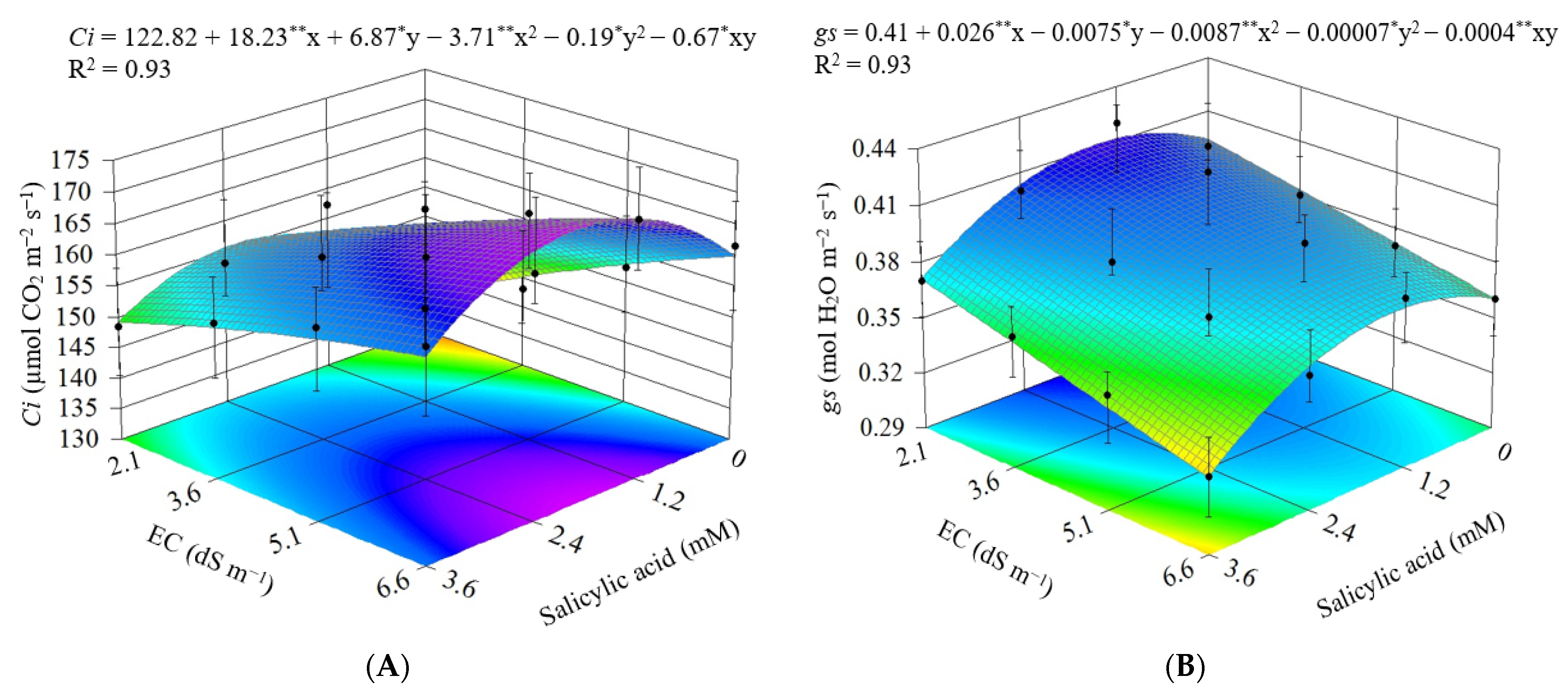
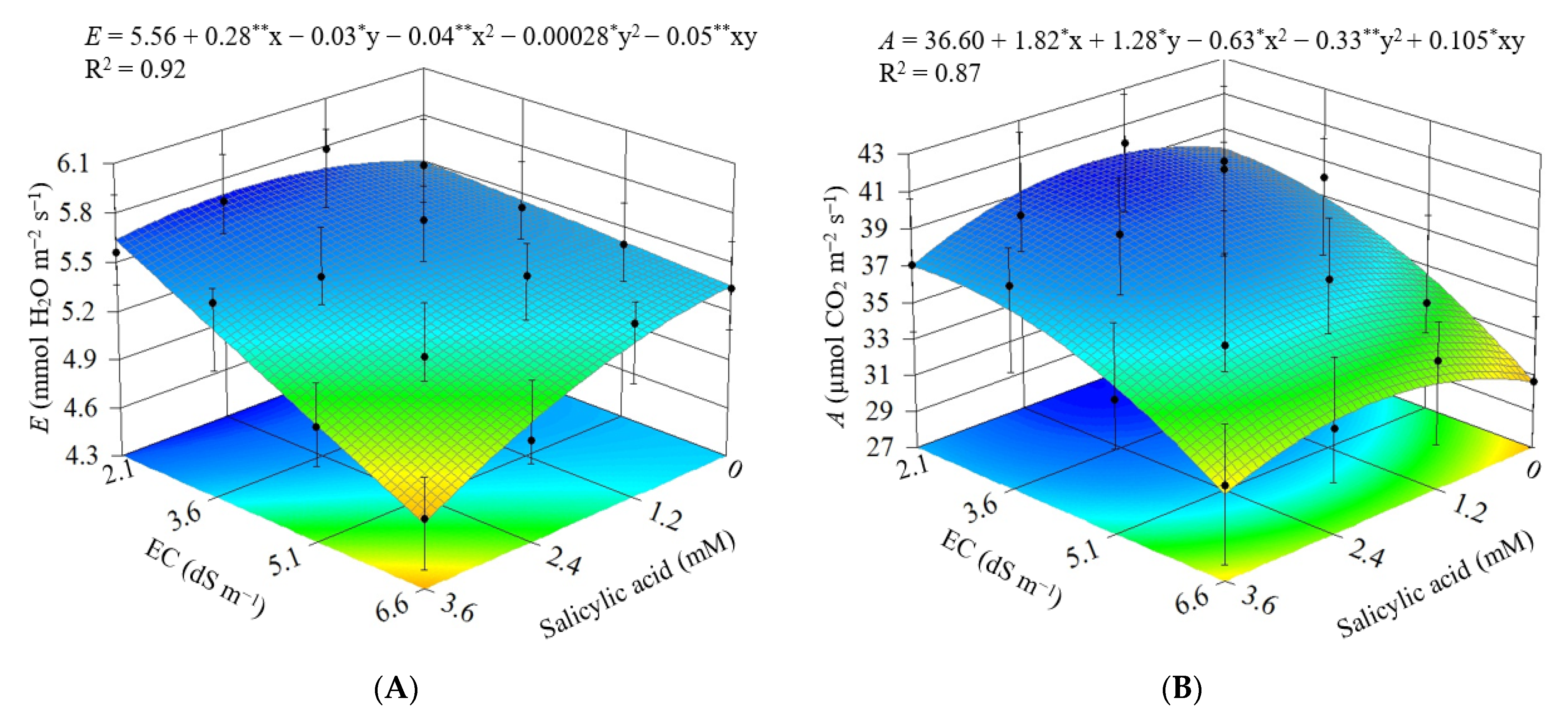
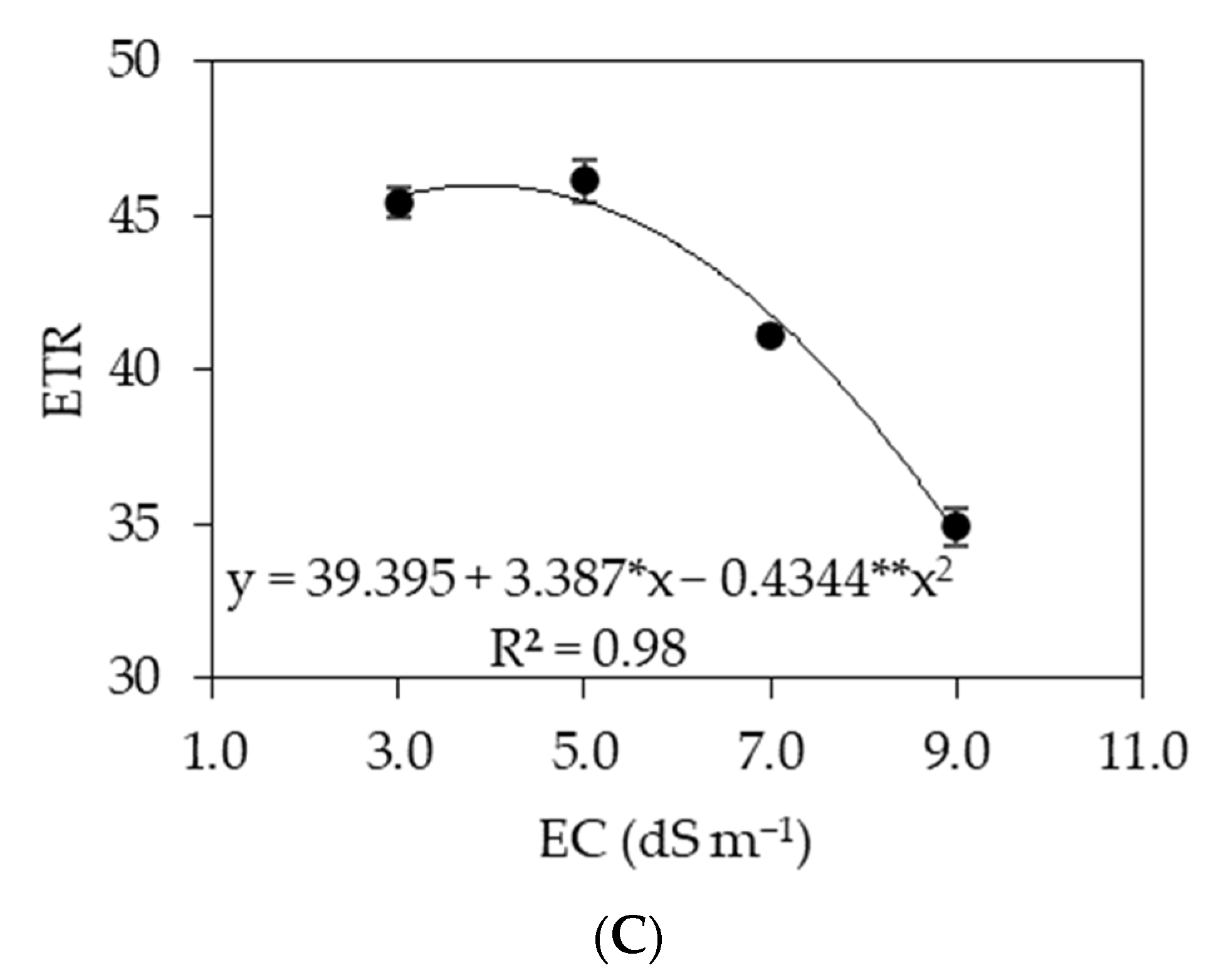
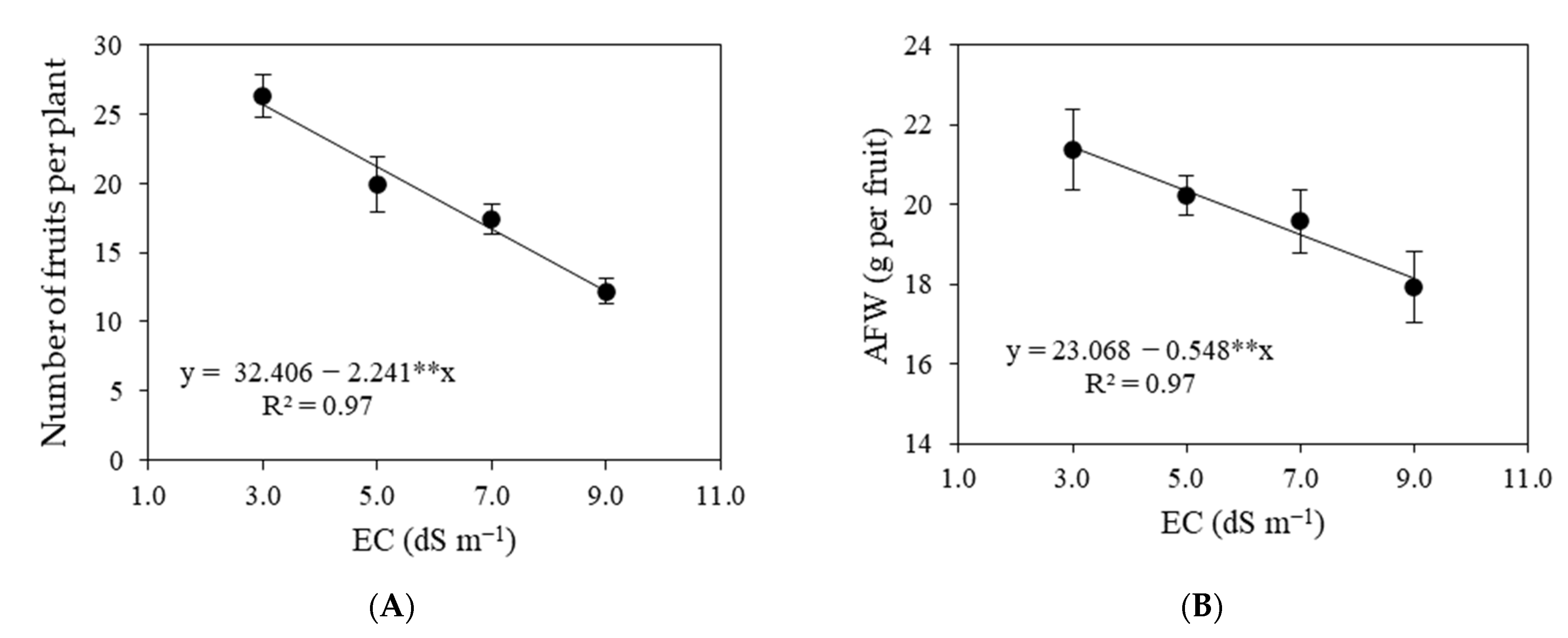
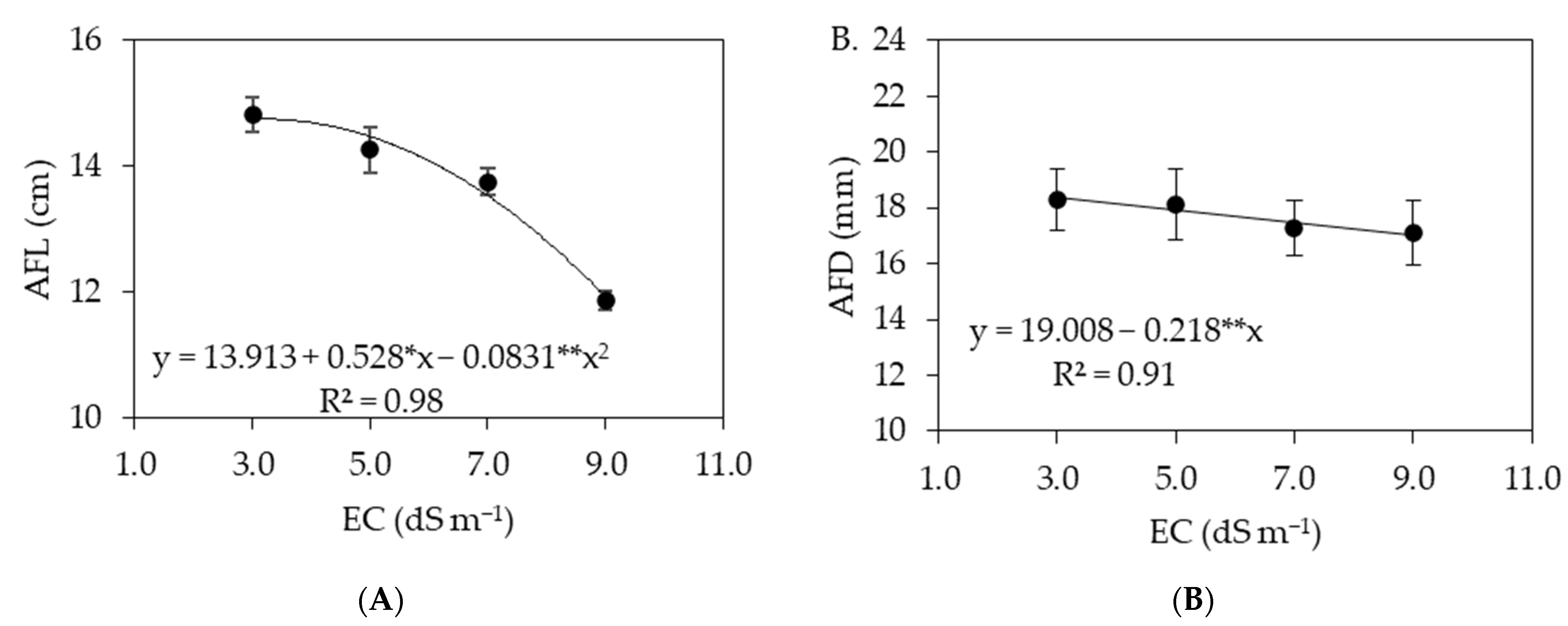
3.2. Experiment II
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nobre, R.G.; de Lima, G.S.; Gheyi, H.R.; Medeiros, E.P.D.; Soares, L.A.A.; Alves, A.N. Oil content and yield of castor bean as affected by nitrogen fertilization and saline water irrigation. Pesqui. Agropecu. Bras. 2012, 47, 991–999. [Google Scholar] [CrossRef]
- Veloso, L.L.S.A.; Nobre, R.G.; de Lima, G.S.; Barbosa, J.L.; Melo, E.M.; Gheyi, H.R.; Gonçalves, E.B.; Souza, C.M.A. Quality of soursop (Annona muricata L.) seedlings under different water salinity levels and nitrogen fertilization. Aust. J. Crop Sci. 2018, 12, 306–310. [Google Scholar] [CrossRef]
- Silva, A.A.R.; Veloso, L.L.S.A.; de Lima, G.S.; Soares, L.A.A.; Chaves, L.H.G.; Silva, F.A.; Fernandes, P.D. Induction of salt stress tolerance in cherry tomatoes under different salicylic acid application methods. Semin. Cienc. Agrar. 2022, 43, 1145–1166. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Najar, R.; Aydi, S.; Sassi-Aydi, S.; Zarai, A.; Abdelly, C. Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula. Plant Biosyst. 2019, 153, 88–97. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, S.W.; Kim, H.; Pan, S.Y.; Fan, C.; Lin, Y.J. Non-conventional water reuse in agriculture: A circular water economy. Water Res. 2021, 199, e117193. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, A.O.; Dahunsi, S.O.; Ayeni, J.F.; Aremu, C.; Aboyeji, C.M.; Okunlola, F.; Oyelami, A.E. Organic and in-organic fertilizers effects on the performance of tomato (Solanum lycopersicum) and cucumber (Cucumis sativus) grown on soilless medium. Sci. Rep. 2022, 12, 12212. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.A.R.; Sousa, P.F.N.; de Lima, G.S.; Soares, L.A.A.; Gheyi, H.R.; Azevedo, C.A.V. Hydrogen peroxide reduces the effect of salt stress on growth and postharvest quality of hydroponic mini watermelon. Water Air Soil Pollut. 2022, 233, 198. [Google Scholar] [CrossRef]
- Soares, T.M.; Duarte, S.N.; Silva, E.F.F.; Paz, V.P.S.; Oliveira, J.L.B. Uso de águas salobras em sistemas hidropônicos de cultivo. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados; Gheyi, H.R., Dias, N.S., Lacerda, C.F., Gomes Filho, É., Eds.; INCT Sal: Fortaleza, Brazil, 2016; pp. 373–393. [Google Scholar]
- Rodrigues, L.R.F. Técnicas de Cultivo Hidropônico e de Controle Ambiental No Manejo de Pragas, Doenças e Nutrição Vegetal em Ambiente Protegido; FUNEP: Jaboticabal, Brazil, 2022; 762p. [Google Scholar]
- De Freitas, F.T.O.; Soares, T.M.; da Silva, M.G.; Rafael, M.R.S. Cultivo de alface sob intervalos de recirculações das soluções nutritivas em sistemas hidropônicos usando água salobra. Irriga 2021, 1, 67–96. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, C.N.; de Lima, G.S.; Soares, L.A.A.; de Fátima, R.T.; Gheyi, H.R.; Azevedo, C.A.V. Morphophysiology and production of guava as a function of water salinity and salicylic acid. Rev. Bras. Eng. Agricola Ambient. 2022, 26, 451–458. [Google Scholar] [CrossRef]
- Roshdy, A.E.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The effect of salicylic acid on the performances of salt stressed strawberry plants, enzymes activity, and salt tolerance index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Veloso, L.L.S.A.; Gheyi, H.R. Salicylic acid as an attenuator of salt stress in soursop. Rev. Caatinga. 2020, 33, 1092–1101. [Google Scholar] [CrossRef]
- Veloso, L.L.S.A.; de Lima, G.S.; da Silva, A.A.R.; Souza, L.P.; de Lacerda, C.N.; Silva, I.J.; Fernandes, P.D. Attenuation of salt stress on the physiology and production of bell peppers by treatment with salicylic acid. Semin. Cienc. Agrar. 2021, 42, 2751–2768. [Google Scholar] [CrossRef]
- Silva, E.B.; Viana, T.V.A.; de Sousa, G.G.; Sousa, J.; dos Santos, M.F.; Azevedo, B.M. Growth and nutrition of peanut crop subjected to saline stress and organomineral fertilization. Rev. Bras. Eng. Agric. Ambient. 2022, 26, 495–501. [Google Scholar] [CrossRef]
- Sousa, V.F.O.; Santos, A.S.; Sales, W.S.; Silva, A.J.; Gomes, F.A.L.; Dias, T.J.; Araújo, J.R.E.S. Exogenous application of salicylic acid induces salinity tolerance in eggplant seedlings. Braz. J. Biol. 2022, 84, e257739. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.D.M.; Dantas, M.V.; de Lima, G.S.; Oliveira, V.K.N.; Soares, L.A.A.; Gheyi, H.R.; Fernandes, P.D. Physiology and yield of ‘Gaúcho’ melon under brackish water and salicylic acid in hydroponic cultivation. Arid. Land Res. Manag. 2022, 1, 1–20. [Google Scholar] [CrossRef]
- Silva, M.G.D.; Soares, T.M.; Gheyi, H.R.; Santos, C.C.D.; Oliveira, M.G.B. Hydroponic cultivation of coriander intercropped with rocket subjected to saline and thermal stresses in the root-zone. Rev. Ceres 2022, 69, 148–157. [Google Scholar] [CrossRef]
- Silva, T.I.; Silva, J.S.; Dias, M.G.; Martins, J.V.S.; Ribeiro, W.S.; Dias, T.J. Salicylic acid attenuates the harmful effects of salt stress on basil. Rev. Bras. Eng. Agric. Ambient. 2022, 26, 399–406. [Google Scholar] [CrossRef]
- Soares, L.A.A.; da Silva, R.G.; de Lima, G.S.; Sales, G.N.B.; da Costa, F.B.; Silva Neta, A.M.S.; Gomes, J.P. Preservation by lactic fermentation and physicochemical characterization of okra produced under water salinity and potassium fertilization. Semin. Cienc. Agrar. 2020, 41, 2495–2508. [Google Scholar] [CrossRef]
- Farias, D.B.S.; da Silva, P.S.O.; Lucas, A.A.T.; de Freitas, M.I.; Santos, T.J.; Fontes, P.T.N.; Oliveira Júnior, L.F.G. Physiological and productive parameters of the okra under irrigation levels. Sci. Hortic. 2019, 252, 1–6. [Google Scholar] [CrossRef]
- Abubaker, B.M.A.; Ahadi, M.; Shuang-En, Y.; Guang-Cheng, S. Different irrigation methods for okra crop production under semi-arid conditions. Int. J. Eng. Res. Technol. 2014, 3, 787–794. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; University of California, Circular, California Agricultural Experiment Station: Oakland, CA, USA, 1950; 32p. [Google Scholar]
- Isla. Available online: https://www.isla.com.br/arquivos-para-download/catalogos (accessed on 5 August 2022).
- Medeiros, J.F.; Lisboa, R.A.; de Oliveira, M.; de Silva Júnior, M.J.; Alves, L.P. Caracterização das águas subterrâneas usadas para irrigação na área produtora de melão da Chapada do Apodi. Rev. Bras. Eng. Agric. Ambient. 2003, 7, 469–472. [Google Scholar] [CrossRef]
- Weatherley, P. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Pham-Thi, A.T.; Semedo, J.N.; Pais, I.P.; Ramalho, J.C.; Matos, M.C. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emir. J. Food Agric. 2013, 25, 1002–1013. [Google Scholar] [CrossRef]
- Sá, F.V.D.S.; Brito, M.; Silva, L.D.A.; Moreira, R.C.L.; Fernandes, P.D.; De Figueiredo, L.C. Fisiologia da percepção do estresse salino em híbridos de tangerineira—Sunki Comum sob solução hidropônica salinizada. Comun. Sci. 2015, 6, 463–470. [Google Scholar] [CrossRef]
- Mota, W.F.; Finger, F.L.; Casali, V.W.D. Olericultura: Melhoramento Genético do Quiabeiro; Universidad Federal de Viçosa, Departamento de Fitotecnia: Viçosa, Brazil, 2000; 144p. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. ASCE. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Bione, M.A.A.; Soares, T.M.; Cova, A.M.W.; Silva Paz, V.P.; Gheyi, H.R.; Rafael, M.R.S.; Neves, B.S.L. Hydroponic production of ‘Biquinho’ pepper with brackish water. Agric. Water Manag. 2021, 245, e106607. [Google Scholar] [CrossRef]
- Fageria, N.K.; Gheyi, H.R. Melhoramento genético das culturas e seleção de cultivares. In Manejo e Controle da Salinidade na Agricultura Irrigada; Gheyi, H.R., Queiroz, J.D., de Medeiros, J.F., Eds.; UFPB: Campina Grande, Brazil, 1997; pp. 365–385. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split-plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Majeedand, A.; Muhammad, Z. Salinity: A major agricultural problem-causes, impacts on crop productivity and management strategies. Plant Abiotic Stress Toler. 2019, 1, 83–99. [Google Scholar] [CrossRef]
- Khatami, S.A.; Kasraie, P.; Oveysi, M.; Moghadam, H.R.T.; Ghooshchi, F. Mitigating the adverse effects of salinity stress on lavender using biodynamic preparations and bio-fertilizers. Ind. Crops Prod. 2022, 183, e114985. [Google Scholar] [CrossRef]
- da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; de Souza, A.R.; Fernandes, P.D. Salicylic acid relieves the effect of saline stress on soursop morphysiology. Ciênc. Agrotecnologia 2021, 45, e007021. [Google Scholar] [CrossRef]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Fahad, S. Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J. Plant Growth Regul. 2021, 40, 2236–2248. [Google Scholar] [CrossRef]
- Batista, M.C.; Nascimento, R.D.; Júnior, S.D.O.M.; Nascimento, E.C.S.; Bezerra, C.V.D.C.; de Lima, R.F. Physiology and production of cherry tomato cultivars in a hydroponic system using brackish water. Rev. Bras. Eng. Agricola Ambient. 2021, 25, 219–227. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Gromova, E.; Nerush, V.; Vodeneev, V.; Sukhov, V. A light-induced decrease in the photochemical reflectance index (PRI) can be used to estimate the energy-dependent component of non-photochemical quenching under heat stress and soil drought in pea, wheat, and pumpkin. Photosynth. Res. 2020, 146, 175–187. [Google Scholar] [CrossRef]
- Sullivan, C.Y. Mechanisms of heat drought resistence in grain sorghum and methods of measurement. In Sorghum in Seventies; Rao, N.G.P., House, L.R., Eds.; Oxford and IBH Publ. Co.: New Delhi, India, 1971; 247p. [Google Scholar]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.C.V.; Pereira, I.M.C.; Paulo-Marinho, S.O.; Canuto, K.M.; Pereira, R.C.A.; Rodrigues, T.H.S.; Daloso, D.M.; Gomes Filho, E.; de Carvalho, H.H. Salicylic acid modulates primary and volatile metabolites to alleviate salt stress-induced photosynthesis impairment on medicinal plant Egletes viscosa. Environ. Exp. Bot. 2019, 167, e103870. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Bezerra, F.M.L.; Lacerda Filho, C.F.; Pereira Filho, J.V.; Freitas, C.A.S. Trocas gasosas em plantas de girassol submetidas à deficiência hídrica em diferentes estádios fenológicos. Rev. Ciênc. Agron. 2013, 44, 86–93. [Google Scholar] [CrossRef]
- Dias, A.S.; de Lima, G.S.; Sá, F.V.S.; Gheyi, H.R.; Soares, L.A.A.; Fernandes, P.D. Gas exchanges and photochemical efficiency of West Indian cherry cultivated with saline water and potassium fertilization. Rev. Bras. Eng. Agricola Ambient. 2018, 22, 628–633. [Google Scholar] [CrossRef]
- Fernandes, C.D.S.; Ferreira-Neto, M.; Dias, N.D.S.; Reges, L.B.L.; Silva, L.D.A.; Moreira, R.C.L.; da Silva, A.; de Paiva, E.P.; Fernandes, P.D.; Sá, F.V.D.S. The appropriate source of nitrogen for Italian Zucchini under salt stress conditions. J. Soil Sci. Plant Nutr. 2022, 22, 560–570. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. Available online: http://www.annualreviews.org/eprint/VN2EH6PPXYXVDSWUTZEM/full/10.1146/annurev-arplant-050718-100005 (accessed on 10 August 2022). [CrossRef] [PubMed]
- Sousa, G.G.; Mendonça, A.M.; Silva, J.R.S.; Silva Junior, F.B.; Moraes, J.G.L.; Sousa, J.T.M. Morphophysiological characteristics of okra plants submitted to saline stress in soil with organic fertilizer. Comun. Sci. 2020, 11, e3241. [Google Scholar] [CrossRef]
- Sales, J.R.S.; Magalhâes, C.L.; Freitas, A.G.; Goes, G.F.; Sousa, H.C.; Sousa, G.G. Physiological indices of okra under organomineral fertilization and irrigated with salt water. Rev. Bras. Eng. Agricola Ambient. 2021, 25, 466–471. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, M.; Li, Y.; Cheng, Z.; Wang, S.; Kong, F. Improvement in salt tolerance of Iris pseudacorus L. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, e113703. [Google Scholar] [CrossRef]
- Lee, S.Y.; Damodaran, P.N.; Roh, K.S. Influence of salicylic acid on rubisco and rubisco activase in tobacco plant grown under sodium chloride in vitro. Saudi J. Biol. Sci. 2014, 21, 417–426. [Google Scholar] [CrossRef]
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Putti, F.F.; Souza, E.P.; Ono, E.O. Foliar application of salicylic acid to mitigate water stress in tomato. Plants 2022, 11, 1775. [Google Scholar] [CrossRef]
- Poór, P.; Borbély, P.G.; Bódi, N.; Bagyánszki, M.; Görgényi, M.T.I. Effects of salicylic acid on photosynthetic activity and chloroplast morphology under light and prolonged darkness. Photosynthetica 2019, 57, 367–376. [Google Scholar] [CrossRef]
- Aires, E.S.; Ferraz, A.K.L.; Carvalho, B.L.; Teixeira, F.P.; Rodrigues, J.D.; Ono, E.O. Foliar application of salicylic acid intensifies antioxidant system and photosynthetic efficiency in tomato plants. Bragantia 2022, 81, e1522. [Google Scholar] [CrossRef]
- Sá, F.V.S.; Gheyi, H.R.; de Lima, G.S.; Pinheiro, F.W.A.; de Paiva, E.P.; Moreira, R.C.L.; Fernandes, P.D. The right combination of NPK fertilization may mitigate salt stress in custard apple (Annona squamosa L.). Acta Physiol. Plant. 2021, 43, 59. [Google Scholar] [CrossRef]
- Silva, M.M.P.; Vasquez, H.M.; Bressan-Smith, R.; da Silva, J.F.C.; Erbesdobler, E.D.A.; Andrade Junior, P.S.C. Eficiência fotoquímica de gramíneas forrageiras tropicais submetidas à deficiência hídrica. Rev. Bras. Zootec. 2006, 35, 67–74. [Google Scholar] [CrossRef][Green Version]
- Tatagiba, S.D.; Moraes, G.A.B.K.; Nascimento, K.J.T.; Peloso, A.F. Limitações fotossintéticas em folhas de plantas de tomateiro submetidas a crescentes concentrações salinas. Eng. Agri. 2014, 22, 138–149. [Google Scholar] [CrossRef]
- Sá, F.V.D.S.; Gheyi, H.R.; De Lima, G.S.; De Paiva, E.P.; Silva, L.D.A.; Moreira, R.C.L.; Fernandes, P.D.; Dias, A.S. Ecophysiology of West Indian cherry irrigated with saline water under phosphorus and nitrogen doses. Biosci. J. 2019, 35, 211–221. [Google Scholar] [CrossRef]
- Larbi, A.; Baccar, R.; Boulal, H. Response of olive tree to ammonium nitrate fertilization under saline conditions. J. Plant Nutr. 2020, 44, 1432–1445. [Google Scholar] [CrossRef]
- da Silva, J.S.; Sá, F.V.D.S.; Dias, N.D.S.; Neto, M.F.; Jales, G.D.; Fernandes, P.D. Morphophysiology of mini watermelon in hydroponic cultivation using reject brine and substrates. Rev. Bras. Eng. Agric. Ambient. 2021, 25, 402–408. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khatri, K.; Patel, D.; Rathore, M.S. Photosynthetic gas exchange and chlorophyll a fluorescence in Salicornia brachiata (Roxb.) under osmotic stress. J. Plant Growth Regul. 2022, 41, 429–444. [Google Scholar] [CrossRef]
- Silva, J.S.; Dias, N.S.; Jales, G.D.; Reges, L.B.L.; de Freitas, J.M.C.; Umbelino, B.F.; Sá, F.V.S. Physiological responses and production of mini-watermelon irrigated with reject brine in hydroponic cultivation with substrates. Environ. Sci. Pollut. Res. 2022, 29, 11116–11129. [Google Scholar] [CrossRef]
- Modesto, F.J.N.; Dos Santos, M.C.M.; Soares, T.M.; Dos Santos, E.P.M. Crescimento, produção e consumo hídrico do quiabeiro submetido à salinidade em condições hidropônicas. Irriga 2019, 24, 2486–2497. [Google Scholar] [CrossRef]
- DA Costa, L.F.; Soares, T.M.; DA Silva, M.G.; Modesto, F.J.N.; Queiroz, L.D.A.; Pereira, J.D.S. Cauliflower growth and yield in a hydroponic system with brackish water. Rev. Caatinga. 2020, 33, 1060–1070. [Google Scholar] [CrossRef]
- Dantas, M.V.; de Lima, G.S.; Gheyi, H.R.; Pinheiro, F.W.A.; Silva, P.C.C.; Soares, L.A.A. Gas exchange and hydroponic production of zucchini under salt stress and H2O2 application. Rev. Caatinga. 2022, 35, 436–449. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef] [PubMed]








| Element | Nutrient Solution mg L−1 | Fertilizer | Nutrient Solution g L−1 |
|---|---|---|---|
| N | 210 | KH2PO4 | 136.09 |
| P | 31 | KNO3 | 101.10 |
| K | 234 | Ca(NO3)2.4H2O | 236.15 |
| Ca | 200 | MgSO4.7H2O | 246.49 |
| Mg | 48 | H3BO3 | 3.10 |
| S | 64 | MnSO4.4H2O | 1.70 |
| B | 0.5 | ZnSO4.7H2O | 0.22 |
| Mn | 0.5 | CuSO4.5H2O | 0.75 |
| Zn | 0.05 | (NH4)6Mo7O24.4H2O | 1.25 |
| Cu | 0.02 | FeSO4 | 13.9 |
| Mo | 0.01 | EDTA—Na | 13.9 |
| Fe | 5 | ||
| Na | 1.2 | ||
| Cl | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Mendonça, A.J.; Silva, A.A.R.d.; Lima, G.S.d.; Soares, L.A.d.A.; Nunes Oliveira, V.K.; Gheyi, H.R.; Lacerda, C.F.d.; Azevedo, C.A.V.d.; Lima, V.L.A.d.; Fernandes, P.D. Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System. Agriculture 2022, 12, 1687. https://doi.org/10.3390/agriculture12101687
Torres Mendonça AJ, Silva AARd, Lima GSd, Soares LAdA, Nunes Oliveira VK, Gheyi HR, Lacerda CFd, Azevedo CAVd, Lima VLAd, Fernandes PD. Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System. Agriculture. 2022; 12(10):1687. https://doi.org/10.3390/agriculture12101687
Chicago/Turabian StyleTorres Mendonça, Allysson Jonhnny, André Alisson Rodrigues da Silva, Geovani Soares de Lima, Lauriane Almeida dos Anjos Soares, Valeska Karolini Nunes Oliveira, Hans Raj Gheyi, Claudivan Feitosa de Lacerda, Carlos Alberto Vieira de Azevedo, Vera Lúcia Antunes de Lima, and Pedro Dantas Fernandes. 2022. "Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System" Agriculture 12, no. 10: 1687. https://doi.org/10.3390/agriculture12101687
APA StyleTorres Mendonça, A. J., Silva, A. A. R. d., Lima, G. S. d., Soares, L. A. d. A., Nunes Oliveira, V. K., Gheyi, H. R., Lacerda, C. F. d., Azevedo, C. A. V. d., Lima, V. L. A. d., & Fernandes, P. D. (2022). Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System. Agriculture, 12(10), 1687. https://doi.org/10.3390/agriculture12101687









