Chemical and Biological Properties of Agricultural Soils Located along Communication Routes
Abstract
:1. Introduction
2. Materials and Methods
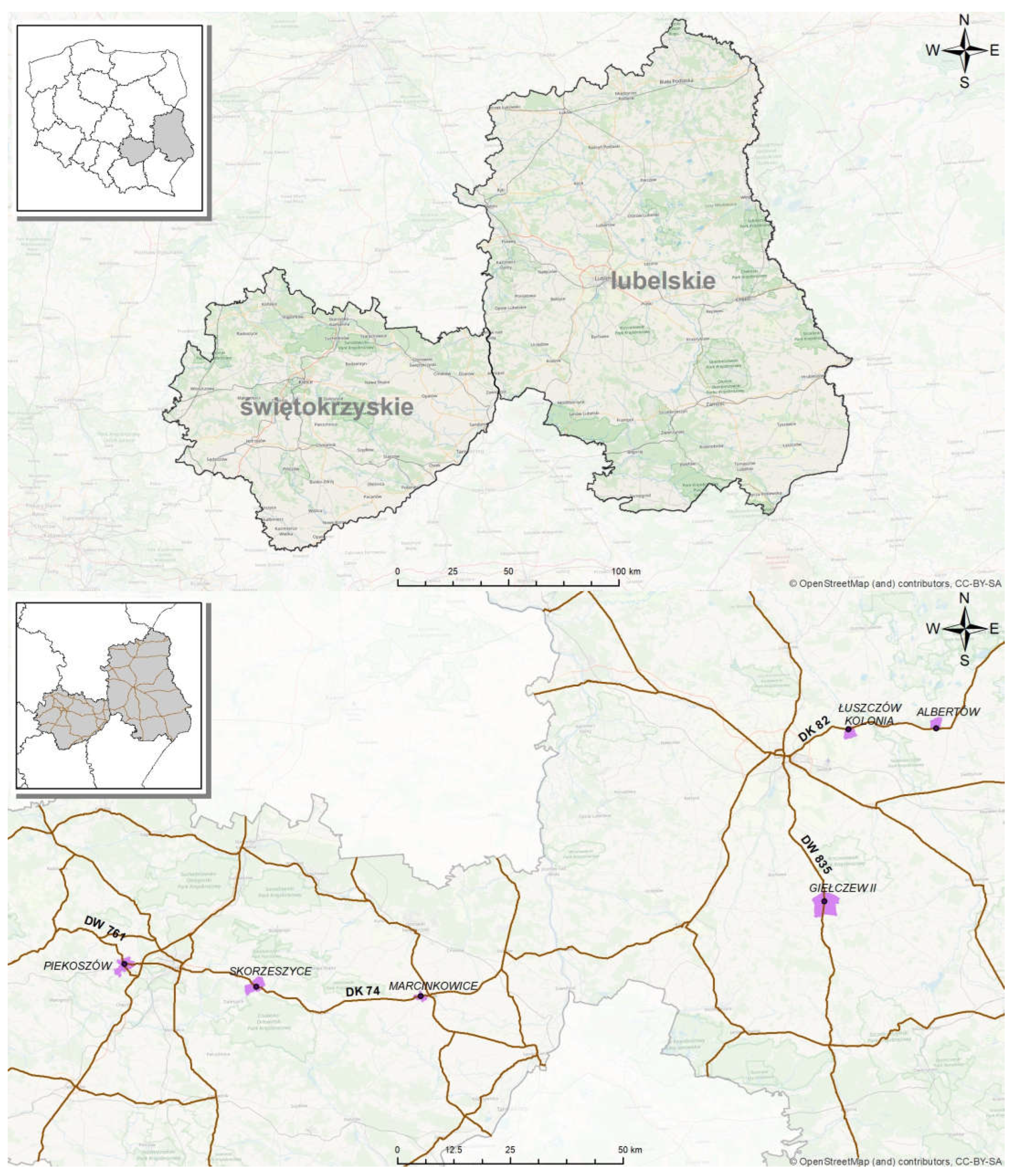
2.1. Study Area and Soil Sampling
2.2. Determination of Soil Properties
2.3. Statistical Analysis
3. Results and Discussion
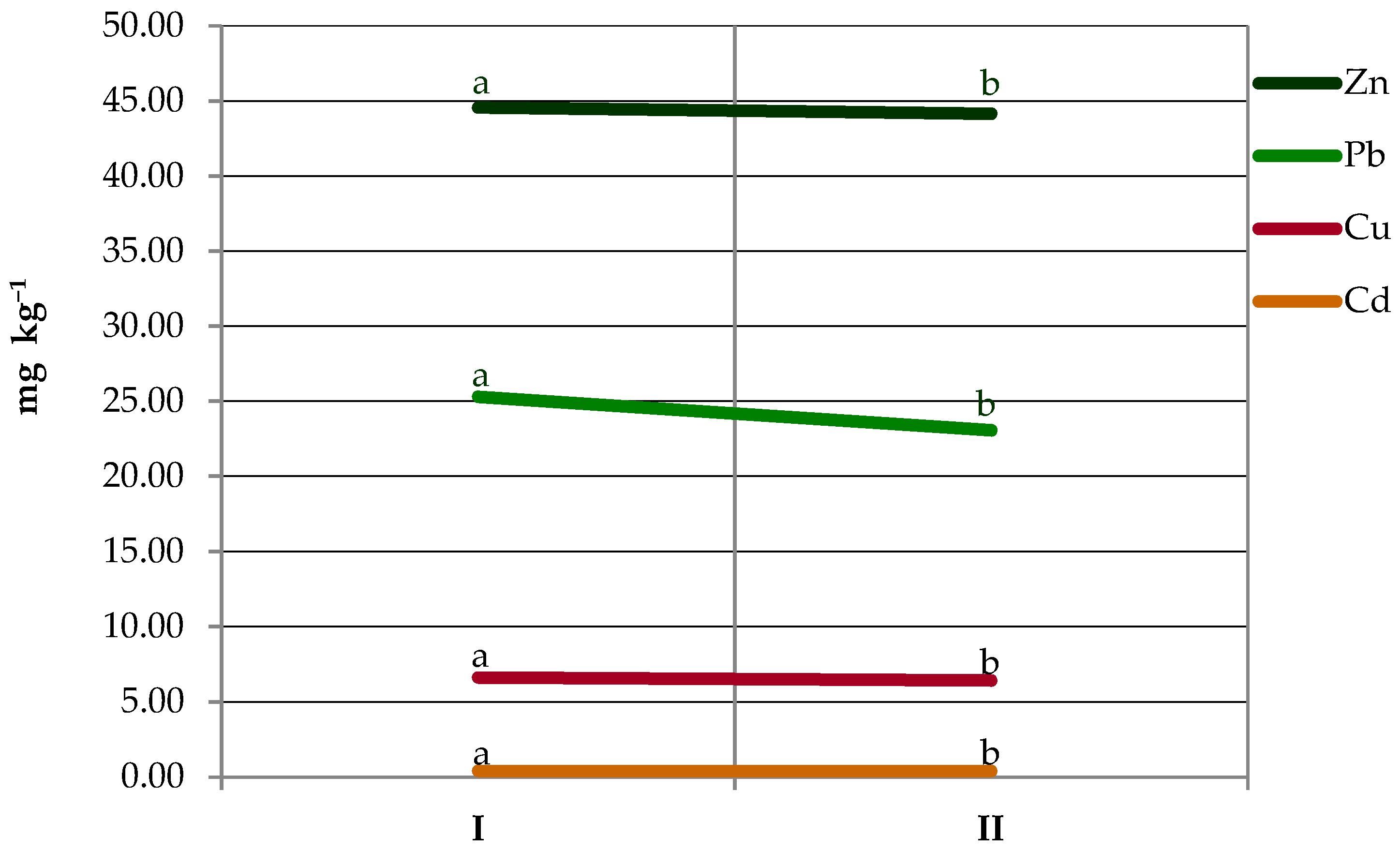
3.1. The Distance from the Edge of the Road
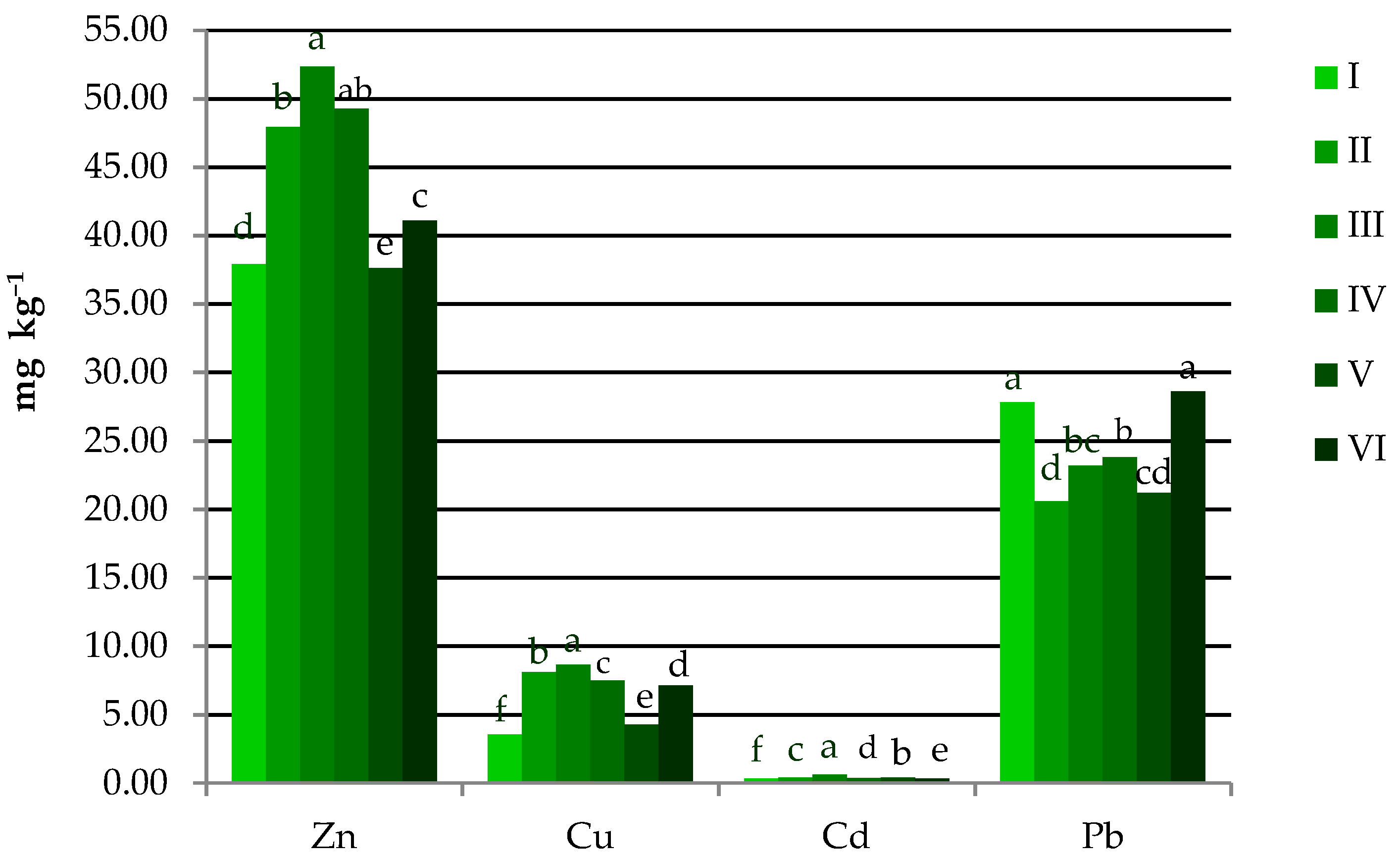
3.2. Location of Sampling Sites
3.3. Seasonal and Yearly Fluctuations in Heavy Metal Contents
3.4. Heavy Metal Contents and Enzymatic Activity
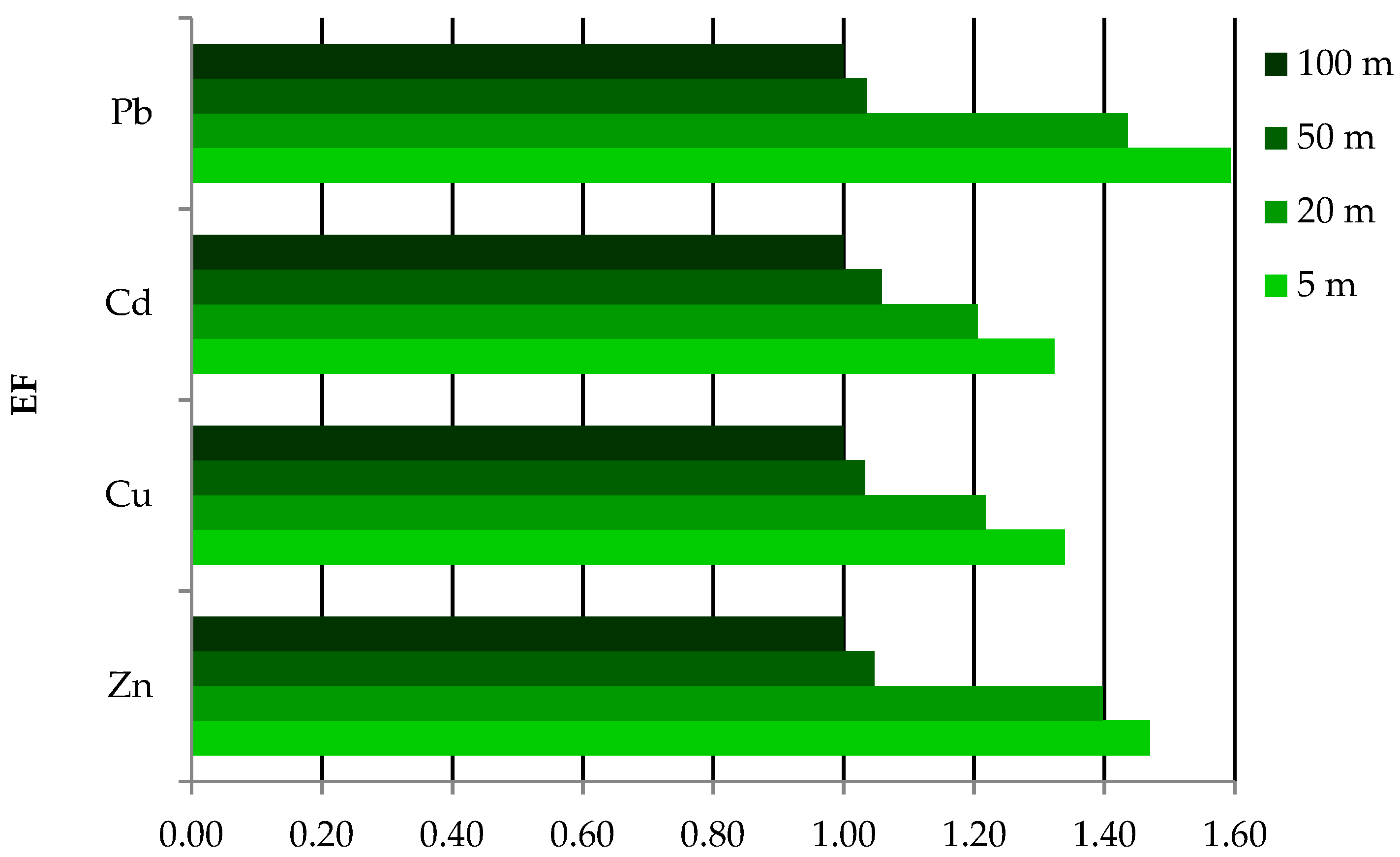
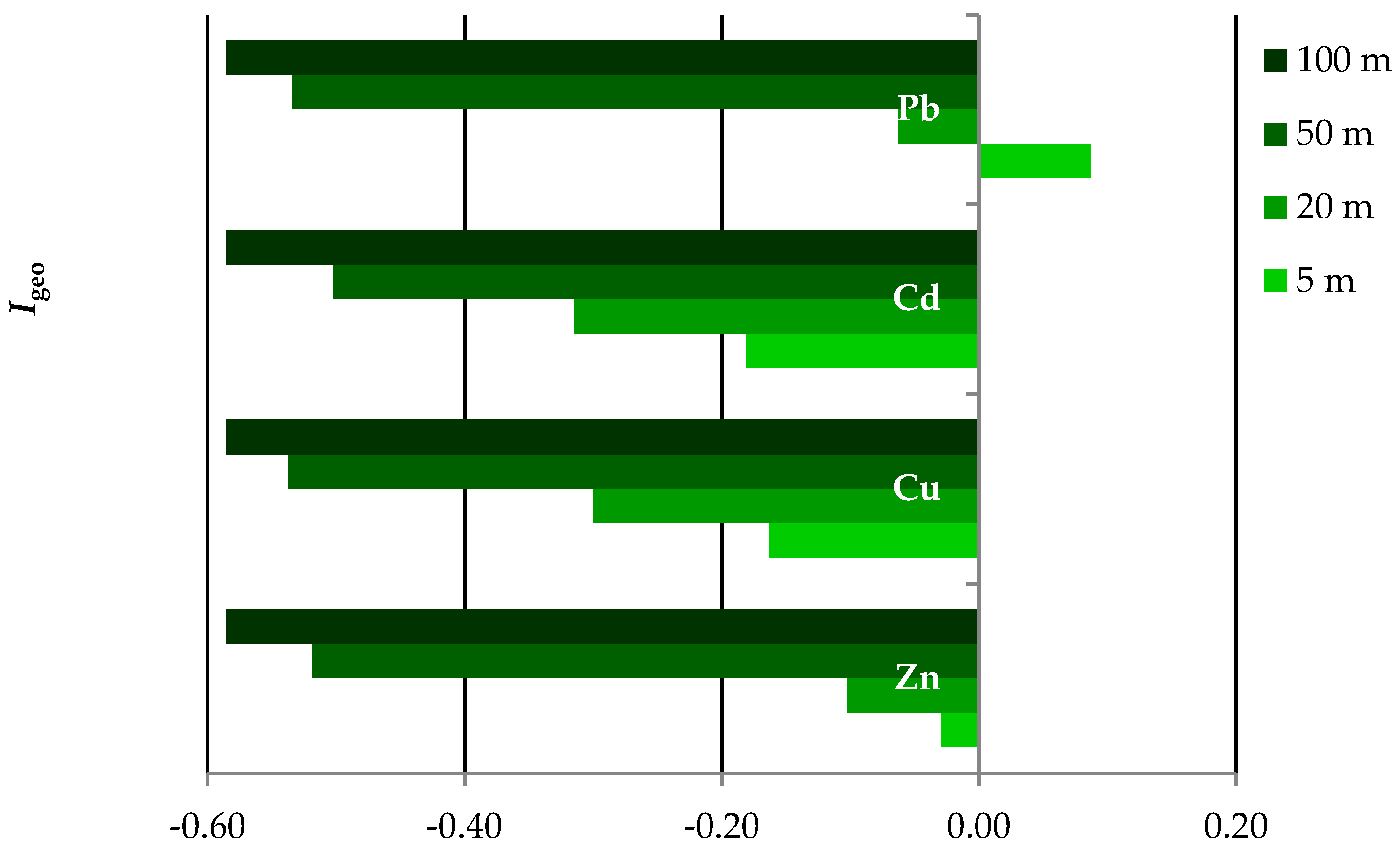
3.5. Enrichment Factor (EF) and Geoaccumulation Index (Igeo)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Silva, S.; Ball, A.S.; Indrapala, D.V.; Reichman, S.M. Review of the interactions between vehicular emitted potentially toxic elements, roadside soils, and associated biota. Chemosphere 2021, 263, 128135. [Google Scholar] [CrossRef]
- Krailertrattanachai, N.; Ketrot, D.; Wisawapipat, W. Distribution of trace metals in roadside agricultural soils, Thailand. Int. J. Environ. Res. Public Health 2019, 16, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Abuduwaili, J.; Li, Y.; Liu, W. Anthropogenically disturbed potentially toxic elements in roadside topsoils of a suburban region of Bishkek, Central Asia. Soil Use Manag. 2019, 35, 283–292. [Google Scholar] [CrossRef]
- Ran, D. Applied research in heavy metals pollution in expressway roadside soil in China. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 032137. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, J.I.A.; Bhat, R.A.; Dervash, M.A.; Ganei, S.A. Vehicular stress a cause for heavy metal accumulation and change in physico-chemical characteristics of road side soils in Pahalgam. Environ. Monit. Assess. 2018, 190, 353. [Google Scholar] [CrossRef]
- Skorbiłowicz, M.; Skorbiłowicz, E.; Rogowska, W. Heavy metal concentrations in roadside soils on the Białystok-Budzisko route in Northeastern Poland. Minerals 2021, 11, 1290. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H. Accumulation of heavy metals in roadside soil in urban area and the related impacting factors. Int. J. Environ. Res. Public Health 2018, 15, 1064. [Google Scholar] [CrossRef] [Green Version]
- Werkenthin, M.; Kluge, B.; Wessolek, G. Metals in European roadside soils and soil solution: A review. Environ. Pollut. 2014, 189, 98–110. [Google Scholar] [CrossRef]
- Ali, M.M.; Hossain, D.; Al-Imran; Khan, M.S.; Begum, M.; Osman, M.H. Environmental Pollution with Heavy Metals: A Public Health Concern. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen Book Series; IntechOpen: Rijeka, Croatia, 2021; pp. 1–20. [Google Scholar]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EU) 2019/1009 of The European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 18 October 2022).
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- Tahir, M.A.; Shaheen, H.; Rathinasabapathi, B. Health risk associated with heavy metal contamination of vegetables grown in agricultural soil of Siran valley, Mansehra, Pakistan—A case study. Environ. Monit. Assess. 2022, 194, 551. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Peng, Y.; Li, R.; Liu, C.; Luo, X.; Zhao, Y. Spatial distribution and source apportionment of agricultural soil heavy metals in a rapidly developing area in East China. Bull. Environ. Contam. Toxicol. 2021, 106, 33–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, D.; Zeng, X.; Li, L.; Wang, A.; Liu, F.; Wang, H.; Zeng, Q.; Xiao, Z. Effect of the direct use of biomass in agricultural soil on heavy metals—Activation or immobilization? Environ. Pollut. 2021, 272, 115989. [Google Scholar] [CrossRef]
- Meng, X.; Ai, Y.; Li, R.; Zhang, W. Effects of heavy metal pollution on enzyme activities in railway cut slope soils. Environ. Monit. Assess. 2018, 190, 197. [Google Scholar] [CrossRef]
- Shen, G.; Lu, Y.; Zhou, Q.; Hong, J. Interaction of polycyclic aromatic hydrocarbons and heavy metals on soil enzyme. Chemosphere 2005, 61, 1175–1182. [Google Scholar] [CrossRef]
- Chu, D. Effects of heavy metals on soil microbial community. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012009. [Google Scholar] [CrossRef]
- De Silva, S.; Ball, A.S.; Huynh, T.; Reichman, S.M. Metal accumulation in roadside soil in Melbourne, Australia: Effect of road age, traffic density and vehicular speed. Environ. Pollut. 2016, 208, 102–109. [Google Scholar] [CrossRef]
- Yan, G.; Mao, L.; Liu, S.; Mao, Y.; Ye, H.; Huang, T.; Li, F.; Chen, L. Enrichment and sources of trace metals in roadside soils in Shanghai, China: A case study of two urban/rural roads. Sci. Total Environ. 2018, 631, 942–950. [Google Scholar] [CrossRef] [PubMed]
- ISO 10390; Soil Quality. Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 14235; Soil Quality. Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 13878; Soil Quality. Determination of Total Nitrogen Content by Dry Combustion. International Organization for Standardization: Geneva, Switzerland, 1998.
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenase Aktivit in Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zantua, M.I.; Bremner, J.M. Comparison of methods of assaying urease activity in soils. Soil Biol. Biochem. 1975, 7, 291–295. [Google Scholar] [CrossRef]
- Ladd, N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil. Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Abderrahmane, B.; Naima, B.; Tarek, M.; Abdelghani, M. Influence of highway traffic on contamination of roadside soil with heavy metals. Civ. Eng. J. 2021, 7, 1459–1471. [Google Scholar] [CrossRef]
- Barbieri, M.; Nigro, A.; Sappa, G. Soil contamination evaluation by Enrichment Factor (EF) and Geoaccumulation Index (Igeo). Senses Sci. 2015, 2, 94–97. [Google Scholar]
- Yang, Z.; Sui, H.; Zhang, Y.; Li, Y.; Sun, L.; Wang, J. Reconstruction, assessment, and calibration of potential toxic elements (PTEs) in a 3500-year-long sedimentary record off the northern coast of Shandong Peninsula, China. Environ. Pollut. 2022, 312, 120075. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-M.; Fiala, M.J.; Park, D.; Wade, T.L. Review of pollutants in urban road dust and stormwater runoff: Part 1. Heavy metals released from vehicles. Int. J. Urban Sci. 2016, 20, 334–360. [Google Scholar] [CrossRef]
- Schuler, M.S.; Relyea, R.A. A review of the combined threats of road salts and heavy metals to freshwater systems. BioScience 2018, 68, 327–335. [Google Scholar] [CrossRef]
- Regulation. Regulation of the Minister of the Natural Environment on the Manner of Conducting the Assessment of the Pollution of the Earth’s Surface Dated 1 September 2016. Journal of Laws 2016, Item 1397. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001397 (accessed on 18 October 2022).
- Available online: https://www.gios.gov.pl/chemizm_gleb/index.php?mod=wyniki&cz=G (accessed on 18 October 2022).
- Wyszkowska, J.; Kucharski, J. Biochemical and physicochemical properties of the soil contaminated with heavy metals. Zesz. Prob. Post. Nauk Rol. 2003, 492, 435–442. (In Polish) [Google Scholar]
- Bielińska, E.J.; Futa, B.; Mocek-Płóciniak, A. Soil Enzymes as Bioindicators of Soil Quality and Health. Scientific Monograph; Society of Scientific Publishers ”LIBROPOLIS”: Lublin, Poland, 2014; p. 107. ISBN 978-83-63761-25-7. (In Polish) [Google Scholar]
- Schmidt, A.; Haferburg, G.; Sineriz, D.M.; Buchel, G.; Kothe, E. Heavy metal resistance mechanisms an actinobacteria for survival in AMD contaminated soils. Chem. Erde. 2005, 65, 131–144. [Google Scholar] [CrossRef]
- Siebielec, G.; Stuczyński, T.; Korzeniowska-Pucułek, R. Metal bioavailability in long-term contaminated Tarnowskie Gory Soils, Polish. J. Environ. Stud. 2006, 15, 121–129. [Google Scholar]
- Egli, M.; Sartori, G.; Mirabella, A.; Giaccai, D.; Favilli, F.; Scherrer, D.; Krebs, R.; Delbos, E. The influence of weathering and organic matter on heavy metals liability in silicatic Alpine soils. Sci. Total Environ. 2010, 408, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dadzie, A.A.; Yuan, L.; Xing, S.; Zhou, X.; Xiao, S. Analysis and potential ecological risk assessment of heavy metals in surface sediments of the freshwater ecosystem in Zhenjiang City, China. SN Appl. Sci. 2022, 4, 258. [Google Scholar] [CrossRef]
- Tong, S.; Li, H.; Li, W.; Tudi, M.; Yang, L. Concentration, spatial distribution, contamination degree and human health risk assessment of heavy metals in urban soils across china between 2003 and 2019—A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 3099. [Google Scholar] [CrossRef]

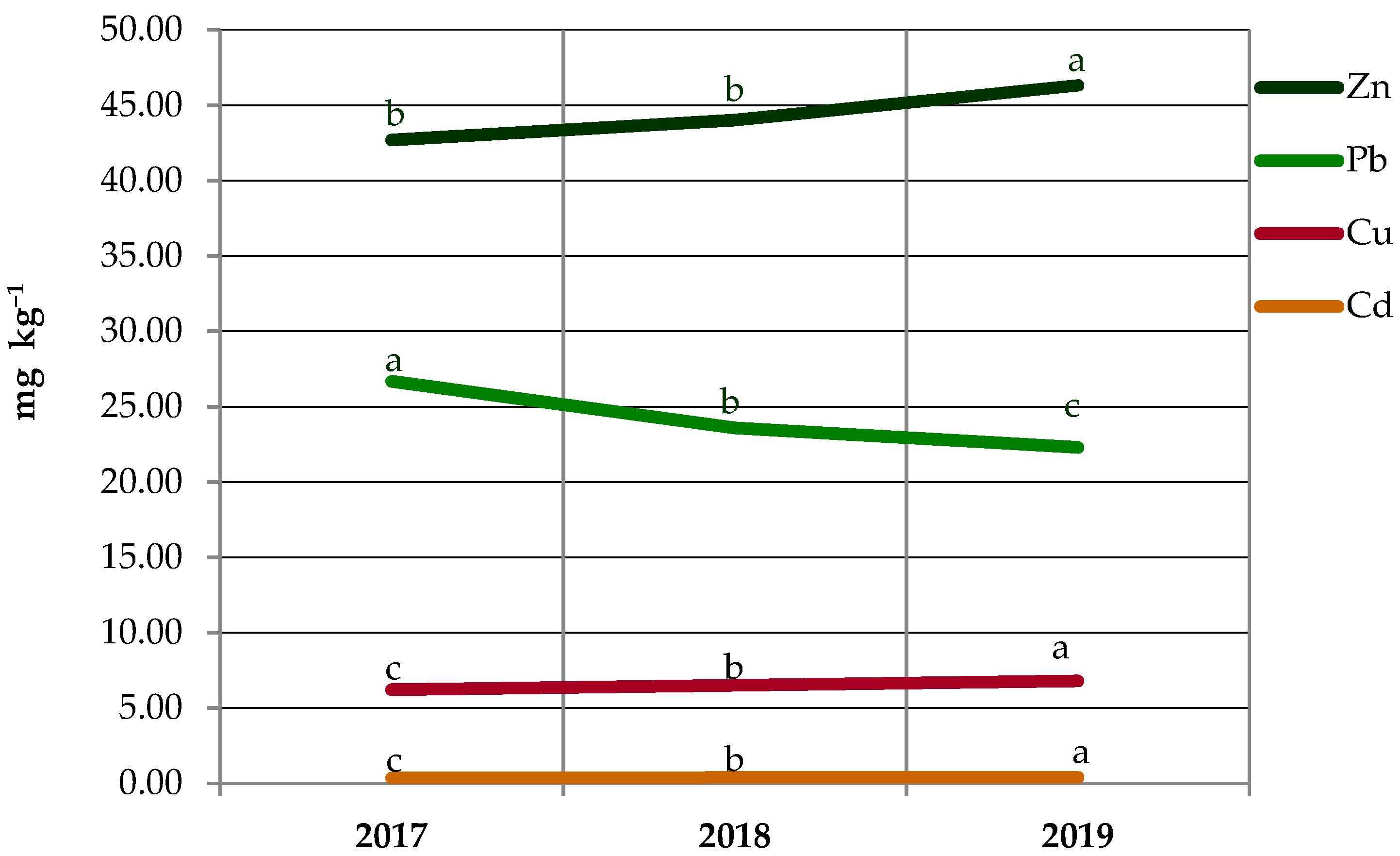
| Parameter | Regression Equation | R2 |
|---|---|---|
| Zn | y = 56.871 − 0.501x + 0.0029x2 | R2 = 0.563 * |
| Pb | y = 32.737 − 0.359x + 0.0022x2 | R2 = 0.557 * |
| Cu | y = 7.913 − 0.059x + 0.0004x2 | R2 = 0.138 * |
| Cd | y = 0.462 − 0.0027x + 1.4127 × 10−5x2 | R2 = 0.179 * |
| Dh | y = 0.763 + 0.081x + 0.0005x2 | R2 = 0.726 * |
| Ph | y = 6.698 + 0.114x + 0.0006x2 | R2 = 0.655 * |
| U | y = 14.28 − 0.143x + 0.001x2 | R2 = 0.456 * |
| P | y = 6.148 + 0.079x + 0.0004x2 | R2 = 0.442 * |
| Parameter | Pb | Cu | Cd | Zn | Dh | Ph | U | P | C | N | pHKCl | C:N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb | 1.000 | 0.144 | 0.108 | 0.432 | −0.608 | −0.720 | 0.374 | −0.486 | −0.299 | −0.521 | 0.314 | 0.604 |
| p = --- | p = 0.086 | p = 0.199 | p = 0.000 | p = 0.000 | p = 0.00 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | |
| Cu | 0.144 | 1.000 | 0.600 | 0.788 | −0.199 | −0.091 | 0.453 | −0.264 | 0.051 | −0.089 | 0.080 | 0.227 |
| p = 0.086 | p = --- | p = 0.000 | p = 0.00 | p = 0.017 | p = 0.277 | p = 0.000 | p = 0.001 | p = 0.547 | p = 0.289 | p = 0.342 | p = 0.006 | |
| Cd | 0.108 | 0.600 | 1.000 | 0.678 | −0.348 | −0.402 | 0.581 | −0.469 | −0.249 | −0.302 | 0.306 | 0.360 |
| p = 0.199 | p = 0.000 | p = --- | p = 0.00 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.003 | p = 0.000 | p = 0.000 | p = 0.000 | |
| Zn | 0.432 | 0.788 | 0.678 | 1.000 | −0.587 | −0.481 | 0.585 | −0.610 | −0.280 | −0.491 | 0.382 | 0.616 |
| p = 0.000 | p = 0.00 | p = 0.00 | p = --- | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.000 | |
| Dh | −0.608 | −0.199 | −0.348 | −0.587 | 1.000 | 0.798 | −0.586 | 0.719 | 0.718 | 0.837 | −0.625 | −0.783 |
| p = 0.000 | p = 0.017 | p = 0.000 | p = 0.000 | p = --- | p = 0.00 | p = 0.000 | p = 0.00 | p = 0.00 | p = 0.00 | p = 0.000 | p = 0.00 | |
| Ph | −0.720 | −0.091 | −0.402 | −0.481 | 0.798 | 1.000 | −0.423 | 0.684 | 0.560 | 0.724 | −0.428 | −0.752 |
| p = 0.00 | p = 0.277 | p = 0.000 | p = 0.000 | p = 0.00 | p = --- | p = 0.000 | p = 0.00 | p = 0.000 | p = 0.00 | p = 0.000 | p = 0.00 | |
| U | 0.374 | 0.453 | 0.581 | 0.585 | −0.586 | −0.423 | 1.000 | −0.398 | −0.380 | −0.488 | 0.274 | 0.515 |
| p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = --- | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.001 | p = 0.000 | |
| P | −0.486 | −0.264 | −0.469 | −0.610 | 0.719 | 0.684 | −0.398 | 1.000 | 0.254 | 0.416 | −0.494 | −0.508 |
| p = 0.000 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.00 | p = 0.00 | p = 0.000 | p = --- | p = 0.002 | p = 0.000 | p = 0.000 | p = 0.000 | |
| C | −0.299 | 0.051 | −0.249 | −0.280 | 0.718 | 0.560 | −0.380 | 0.254 | 1.000 | 0.921 | −0.665 | −0.699 |
| p = 0.000 | p = 0.547 | p = 0.003 | p = 0.001 | p = 0.00 | p = 0.000 | p = 0.000 | p = 0.002 | p = --- | p = 0.00 | p = 0.00 | p = 0.00 | |
| N | −0.521 | −0.089 | −0.302 | −0.491 | 0.837 | 0.724 | −0.488 | 0.416 | 0.921 | 1.000 | −0.615 | −0.902 |
| p = 0.000 | p = 0.289 | p = 0.000 | p = 0.000 | p = 0.00 | p = 0.00 | p = 0.000 | p = 0.000 | p = 0.00 | p = --- | p = 0.000 | p = 0.00 | |
| pHKCl | 0.314 | 0.080 | 0.306 | 0.382 | −0.625 | −0.428 | 0.274 | −0.494 | −0.665 | −0.615 | 1.000 | 0.473 |
| p = 0.000 | p = 0.342 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.001 | p = 0.000 | p = 0.00 | p = 0.000 | p = --- | p = 0.000 | |
| C:N | 0.604 | 0.227 | 0.360 | 0.616 | −0.783 | −0.752 | 0.515 | −0.508 | −0.699 | −0.902 | 0.473 | 1.000 |
| p = 0.000 | p = 0.006 | p = 0.000 | p = 0.000 | p = 0.00 | p = 0.00 | p = 0.000 | p = 0.000 | p = 0.00 | p = 0.00 | p = 0.000 | p = --- |
| Variable | PC1 | PC2 |
|---|---|---|
| Dh | 0.470 | −0.540 |
| Ph | 0.840 | −0.326 |
| U | −0.447 | −0.589 |
| P | 0.839 | −0.130 |
| Zn | −0.808 | −0.502 |
| Pb | −0.809 | 0.253 |
| Cu | −0.420 | −0.874 |
| Cd | −0.500 | −0.509 |
| N | 0.829 | −0.205 |
| pH | −0.590 | 0.216 |
| C:N | −0.422 | 0.837 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawierucha, E.; Skowrońska, M.; Zawierucha, M. Chemical and Biological Properties of Agricultural Soils Located along Communication Routes. Agriculture 2022, 12, 1990. https://doi.org/10.3390/agriculture12121990
Zawierucha E, Skowrońska M, Zawierucha M. Chemical and Biological Properties of Agricultural Soils Located along Communication Routes. Agriculture. 2022; 12(12):1990. https://doi.org/10.3390/agriculture12121990
Chicago/Turabian StyleZawierucha, Elżbieta, Monika Skowrońska, and Marcin Zawierucha. 2022. "Chemical and Biological Properties of Agricultural Soils Located along Communication Routes" Agriculture 12, no. 12: 1990. https://doi.org/10.3390/agriculture12121990





