The Effect of Peat Moss Amended with Three Engineered Wood Substrate Components on Suppression of Damping-Off Caused by Rhizoctonia solani
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Analysis and Preparation of Substrates
2.3. Pathogen Preparation and Inoculation
2.4. Objective 1: Effects of Differently Processed Wood Products on Damping-Off Disease
2.5. Objective 2: Effects of Peat–Wood Blend Ratio on Damping-Off Disease
2.6. Data Collection
2.7. Data Analysis
3. Results
3.1. Substrate Physiochemical Properties
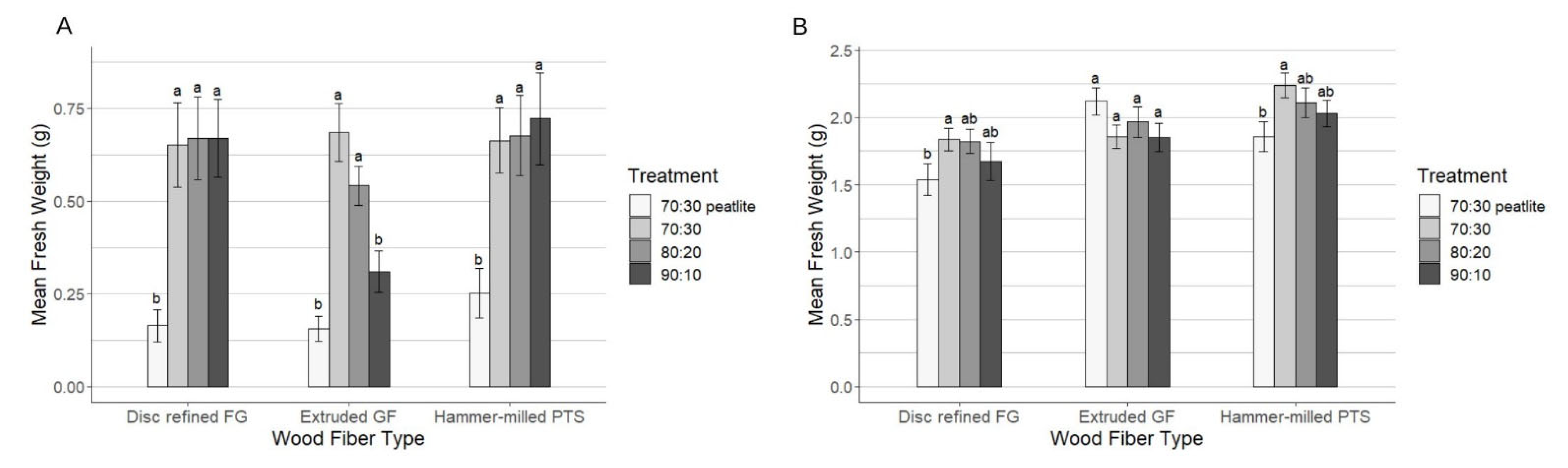
3.2. Objective 1: Effect of Differently Processed Wood Products on Damping-Off Disease Severity
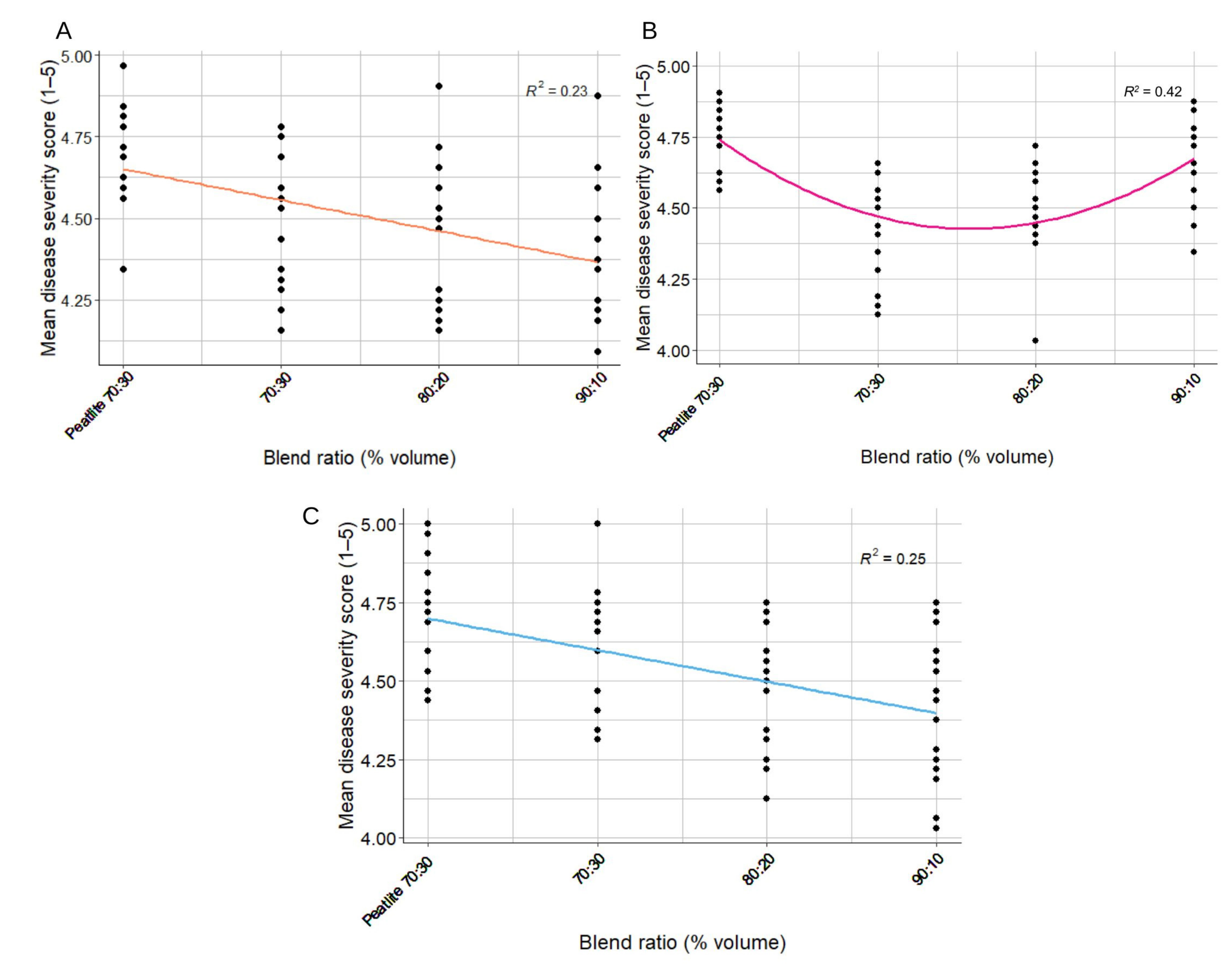
3.3. Objective 2: Effect of Peat:Wood Blend Ratio on Damping-Off Disease Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blok, C.; Eveleens, B.; Winkel, A. van Growing Media for Food and Quality of Life in the Period 2020–2050. Acta Hortic. 2021, 1305, 341–356. [Google Scholar] [CrossRef]
- Jackson, B.E. The Current State of Substrates in 2021. Grow. Talks 2021, 84, 40–44. [Google Scholar]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Jackson, B.E.; Bartley, P. Wood Substrates: The Plant’s Perspective. Grow. Talks 2017, 2, 54–56. [Google Scholar]
- Jackson, B.E. Challenges and Considerations of Using Wood Substrates: Physical Properties. Greenh. Grow. 2018, 36, 28–30. [Google Scholar]
- Jackson, B.E. Challenges and Considerations of Using Wood Substrates: Biological Properties. Greenh. Grow. 2018, 36, 52–54. [Google Scholar]
- Jackson, B.E. Challenges and Considerations of Using Wood Substrates: Chemical Properties. Greenh. Grow. 2018, 36, 46–50. [Google Scholar]
- Jackson, B.E.; Wright, R.D.; Barnes, M.C. Methods of Constructing a Pine Tree Substrate from Various Wood Particle Sizes, Organic Amendments, and Sand for Desired Physical Properties and Plant Growth. HortScience 2010, 45, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Owen, W.G.; Jackson, B.E.; Whipker, B.E.; Fonteno, W.C. Paclobutrazol Drench Activity Not Affected in Sphagnum Peat-Based Substrates Amended with Pine Wood Chip Aggregates. HortTechnology 2016, 26, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Hudgins, J.C.; Jackson, B.E. Chasing PH in Wood Substrates. GrowerTalks, 20 March 2020; 83, 62–64. Available online: https://woodsubstrates.cals.ncsu.edu/wp-content/uploads/sites/147/2020/12/Chasing-pH-in-Wood-Substrates.pdf(accessed on 15 October 2022).
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The Role of Peat-Free Organic Substrates in the Sustainable Management of Soilless Cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Dickson, R.W.; Helms, K.M.; Jackson, B.E.; Machesney, L.M.; Lee, J.A. Evaluation of Peat Blended with Pine Wood Components for Effects on Substrate Physical Properties, Nitrogen Immobilization, and Growth of Petunia (Petunia × Hybrida Vilm.-Andr.). HortScience 2022, 57, 304–311. [Google Scholar] [CrossRef]
- Harris, C.N.; Dickson, R.W.; Fisher, P.R.; Jackson, B.E.; Poleatewich, A.M. Evaluating Peat Substrates Amended with Pine Wood Fiber for Nitrogen Immobilization and Effects on Plant Performance with Container-Grown Petunia. HortTechnology 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. The Use of Wood Fiber for Reducing Risks of Hydrophobicity in Peat-Based Substrates. Agronomy 2021, 11, 907. [Google Scholar] [CrossRef]
- Jackson, B.E. Chemical, Physical, and Biological Factors Influencing Nutrient Availability and Plant Growth in a Pine Tree Substrate. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2008. [Google Scholar]
- Fain, G.B.; Gilliam, C.H.; Sibley, J.L.; Boyer, C.R.; Witcher, A.L. WholeTree Substrate and Fertilizer Rate in Production of Greenhouse-Grown Petunia (Petunia × hybrida Vilm.) and Marigold (Tagetes patula L.). HortScience 2008, 43, 700–705. [Google Scholar] [CrossRef]
- Fields, J.S.; Fonteno, W.C.; Jackson, B.E.; Heitman, J.L.; Owen, J.S. Hydrophysical Properties, Moisture Retention, and Drainage Profiles of Wood and Traditional Components for Greenhouse Substrates. HortScience 2014, 49, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. Soilless Culture Theory and Practice, 2nd ed.; Raviv, M., Lieth, J.H., Eds.; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-444-63696-6. [Google Scholar]
- Owen, W.G.; Jackson, B.E.; Whipker, B.E.; Fonteno, W.C.; Benson, M.D. Assessing the Severity of Damping-off Caused by Pythium ultimum and Rhizoctonia solani in Peat-Based Greenhouse Substrates Amended with Pine Wood Chip Aggregates. Acta Hortic. 2019, 1266, 27–34. [Google Scholar] [CrossRef]
- Fuchs, J.G.; Hedrich, T.; Hofer, V.; Koller, M.; Oberhaensli, T.; Regal, J.R.; Tamm, L.; Thuerig, B.; Schwarze, F.W.M.R.; Herforth-Rahmé, J. Development of Disease-Suppressive Organic Growing Media. Acta Hortic. 2017, 1164, 181–188. [Google Scholar] [CrossRef]
- Baker, K.; Cook, R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: San Francisco, CA, USA, 1974; ISBN 978-0-7167-0589-5. [Google Scholar]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.J. Making Greater Use of Introduced Microorganisms for Biological Control of Plant Pathogens. Annu. Rev. Phytopathol. 1993, 31, 53–80. [Google Scholar] [CrossRef]
- Boehm, M.J.; Hoitink, H.A.J. Sustenance of Microbial Activity in Potting Mixes and Its Impact on Severity of Pythium Root Rot of Poinsettia. Phytopathology 1992, 82, 259–264. [Google Scholar] [CrossRef]
- Hoitinik, H.A.; Boehm, M.J. Biocontrol within the Context of Soil Microbial Communities: A Substrate-Dependent Phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Hunter, P.J.; Petch, G.M.; Calvo-Bado, L.A.; Pettitt, T.R.; Parsons, N.R.; Morgan, J.A.W.; Whipps, J.M. Differences in Microbial Activity and Microbial Populations of Peat Associated with Suppression of Damping-Off Disease Caused by Pythium sylvaticum. Appl. Environ. Microbiol. 2006, 72, 6452–6460. [Google Scholar] [CrossRef] [Green Version]
- Alsanius, B.W.; Wohanka, W. Root Zone Microbiology of Soilless Cropping Systems. In Soilless Culture Theory and Practice; Raviv, M., Lieth, H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 149–194. ISBN 978-0-444-63696-6. [Google Scholar]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Chung, Y.R.; Hoitink, H.A.J.; Dick, W.A.; Herr, L.J. Effects of Organic Matter Decomposition Level and Cellulose Amendment on the Inoculum Potential of Rhizoctonia solani in Hardwood Bark Media. Phytopathology 1988, 78, 836–840. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper, 3rd ed.; Raw Material and Pulp Making; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, ISBN 978-0-12-814240-0. [Google Scholar]
- Jackson, B.E.; Wright, R.D.; Alley, M.M. Comparison of Fertilizer Nitrogen Availability, Nitrogen Immobilization, Substrate Carbon Dioxide Efflux, and Nutrient Leaching in Peat-Lite, Pine Bark, and Pine Tree Substrates. HortScience 2009, 44, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Montagne, V.; Herve, C.; Barret, M.; Cannavo, P.; Charpentier, S.; Grosbellet, C.; Lebeau, T. Bacterial and Fungal Communities Vary with the Type of Organic Substrate: Implications for Biocontrol of Soilless Crops. Environ. Chem. Lett. 2017, 15, 537–545. [Google Scholar] [CrossRef]
- Daughtrey, M.; Buitenhuis, R. Integrated Pest and Disease Management in Greenhouse Ornamentals. In Integrated Pest and Disease Management in Greenhouse Crops; Plant Pathology in the 21st, Century; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 625–679. ISBN 978-3-030-22304-5. [Google Scholar]
- Chase, A.R.; Daughtrey, M.L. Rhizoctonia Rising. Greenhouse Product News, 8 July 2004; 34–38. Available online: https://gpnmag.com/article/rhizoctonia-rising/(accessed on 15 October 2022).
- Krause, M.S.; Madden, L.V.; Hoitink, H.A.J.J. Effect of Potting Mix Microbial Carrying Capacity on Biological Control of Rhizoctonia Damping-off of Radish and Rhizoctonia Crown and Root Rot of Poinsettia. Phytopathology 2001, 91, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Puustjarvi, V.; Robertson, R. Peat in Horticulture. In Physical and Chemical Properties; Robinson, D.W., Lamb, J.G.D., Eds.; Academic Press: London, UK, 1975; pp. 23–38. [Google Scholar]
- Nelson, P.V. Greenhouse Operation and Management, 7th ed.; Prentice Hall, Upper Saddle River: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jackson, B.E. Wood Works as an Alternative to Peat. Grow. Talks 2016, 80, 50–55. [Google Scholar]
- Eliason, R.; Goos, R.J.; Hoskins, B. Recommended Chemical Soil Test Procedures for the North Central Region; North Central Regional Research Publication, No. 221; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 2015. [Google Scholar]
- Warncke, D.D. Analyzing Greenhouse Growth Media by the Saturation Extraction Method. HortScience 1986, 21, 223–225. [Google Scholar] [CrossRef]
- Fonteno, W.C.; Hardin, C.T.; Brewster, J.P. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Jackson, B.E.; Wright, R.D.; Gruda, N. Container Medium PH in a Pine Tree Substrate Amended with Peatmoss and Dolomitic Limestone Affects Plant Growth. HortScience 2009, 44, 1983–1987. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.T.; Jackson, B.E.; Fonteno, W.C. Wettability and Hydrology of Various Wood Fiber Substrates and Substrate Components. Acta Hortic. 2019, 1266, 437–442. [Google Scholar] [CrossRef]
- Montagne, V.; Capiaux, H.; Cannavo, P.; Charpentier, S.; Renaud, S.; Liatard, E.; Grosbellet, C.; Lebeau, T. Protective Effect of Organic Substrates against Soil-Borne Pathogens in Soilless Cucumber Crops. Sci. Hortic. 2016, 206, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.B.; Kuter, G.A.; Hoitnik, H.A. Effects of Fungal Antagonists and Compost Age on Suppression of Rhizoctonia Damping-off in Container Media Amended with Composted Hardwood Bark. Phytopathology 1983, 73, 1457–1462. [Google Scholar] [CrossRef]
- Bugbee, B.; Heins, R. Wood Products in the Root Zone. Greenhouse Product News, 20 February 2019; 22–27. Available online: https://gpnmag.com/article/wood-products-in-the-root-zone/(accessed on 15 October 2022).
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial Activity in Peat-Reduced Plant Growing Media: Identifying Influential Growing Medium Constituents and Physicochemical Properties Using Fractional Factorial Design of Experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Kwok, O.C.H.; Fahy, P.C.; Hoitink, H.A.J.; Kuter, G.A. Interactions between Bacteria and Trichoderma Hamatum in Suppression of Rhizoctonia Damping-off in Bark Compost Media. Phytopathology 1987, 77, 1206. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Hannula, S.E.; Rizaludin, M.S.; Hundscheid, M.P.J.; Klein Gunnewiek, P.J.A.; Schilder, M.T.; Postma, J.; de Boer, W. Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani. Microorganisms 2021, 9, 1285. [Google Scholar] [CrossRef]
- Anothai, J.; Chairin, T. Soil Physicochemical Properties Closely Associated with Fungal Enzymes and Plant Defense Enzymes in Ganoderma-Infected Oil Palm Orchards. Plant Soil 2020, 456, 99–112. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsui, M.; Honjo, H.; Becker, J.O.; Fukui, R. Effects of Soil PH on Rhizoctonia Damping-off of Sugar Beet and Disease Suppression Induced by Soil Amendment with Crop Residues. Plant Soil 2011, 347, 255. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Suitability of Wood Fiber Substrate for Production of Vegetable Transplants: I. Physical Properties of Wood Fiber Substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic Soilless Media Components. In Soilless Culture; Raviv, M., Lieth, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504. ISBN 978-0-444-52975-6. [Google Scholar]
- Witcher, A.L.; Blythe, E.K.; Fain, G.B.; Curry, K.J.; Pounders, C.T. Assessing Phytotoxicity in Fresh and Aged Whole Pine Tree Substrates. Comb. Proc. Int. Plant Propag. Soc. 2011, 61, 6. [Google Scholar]
- Gumy, N. Toresa and Other Wood Fiber Products: Advantages and Drawbacks When Used in Growing Media. In Proceedings of the International Peat Symposium Peat in Horticulture: Peat and Its Alternatives in Growing Media, Amsterdam, The Netherlands, 30 October 2001; pp. 39–46. [Google Scholar]
- Nichols, D.G. The Effect of Pinus radiata Bark Toxicity on the Early Growth of Plants in Containers. Sci. Hortic. 1981, 15, 291–298. [Google Scholar] [CrossRef]
- Daughtrey, M.L.; Benson, D.M. Principles of Plant Health Management for Ornamental Plants. Annu. Rev. Phytopathol. 2005, 43, 141–169. [Google Scholar] [CrossRef] [Green Version]
- Chérif, M.; Tirilly, Y.; Bélanger, R.R. Effect of Oxygen Concentration on Plant Growth, Lipidperoxidation, and Receptivity of Tomato Roots to Pythium F under Hydroponic Conditions. Eur. J. Plant Pathol. 1997, 103, 255–264. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]

| Treatment | SMC (% vol) | pH z | EC (mS cm−1) | Total Porosity y (%) | Container Capacity (% vol) x | Air Space (% vol) w | Bulk Density (lbs ft−3) |
| Peat | 44.82 ± 3.15 b v | 5.80 ± 0.24 ab | 0.354 ± 0.03 a | 81.0 ± 0.83 c | 56.5 ± 0.15 b | 24.5 ± 0.84 a | 5.6 ± 0.07 a |
| Disc-refined FG | 59.04 ± 2.06 a | 5.72 ± 0.20 b | 0.332 ± 0.04 a | 86.5 ± 1.07 a | 62.9 ± 0.98 a | 23.6 ± 0.52 a | 4.3 ± 0.09 c |
| Extruded GF | 57.18 ± 2.63 a | 5.68 ± 0.24 b | 0.373 ± 0.04 a | 85.3 ± 0.20 ab | 63.5 ± 0.55 a | 21.8 ± 0.67 ab | 4.9 ± 0.17 b |
| Hammer-milled PTS | 51.81 ± 1.74 ab | 6.18 ± 0.14 a | 0.789 ± 0.23 b | 82.2 ± 1.04 bc | 62.8 ± 0.80 a | 19.5 ± 0.41 b | 5.5 ± 0.00 a |
| Substrate | Blend Ratio z | SMC (% vol) | pH y | EC (ms cm−1) |
|---|---|---|---|---|
| Disc-refined FG | Peatlite 70:30 | 51.27 ± 0.98 cde x | 5.61 ± 0.11 cde | 0.345 ± 0.02 bcde |
| 70:30 | 57.74 ± 1.23 ab | 5.81 ± 0.12 bcd | 0.357 ± 0.02 abcd | |
| 80:20 | 58.01 ± 1.66 ab | 6.01 ± 0.16 abc | 0.363 ± 0.01 abc | |
| 90:10 | 59.43 ± 0.84 a | 5.47 ± 0.17 de | 0.350 ± 0.02 bcde | |
| Extruded GF | Peatlite 70:30 | 50.30 ± 0.61 de | 6.13 ± 0.07 ab | 0.320 ± 0.01 cde |
| 70:30 | 55.15 ± 1.18 abcde | 5.74 ± 0.05 bcd | 0.296 ± 0.002 de | |
| 80:20 | 56.00 ± 1.16 abcd | 5.58 ± 0.04 cde | 0.292 ± 0.002 e | |
| 90:10 | 57.07 ± 1.69 abc | 5.22 ± 0.04 e | 0.295 ± 0.003 de | |
| Hammer-milled PTS | Peatlite 70:30 | 52.02 ± 1.72 bcde | 6.28 ± 0.07 a | 0.394 ± 0.02 ab |
| 70:30 | 49.49 ± 1.77 e | 6.08 ± 0.06 ab | 0.417 ± 0.03 a | |
| 80:20 | 52.45 ± 1.51 bcde | 6.01 ± 0.05 abc | 0.394 ± 0.02 ab | |
| 90:10 | 51.34 ± 1.63 cde | 5.73 ± 0.07 bcd | 0.415 ± 0.01 a |
| Substrate | Blend Ratio z | Total Porosity y (%) | Container Capacity x (%) | Air Space w (%) | Bulk Density (lbs·ft−3) |
| Peatlite | 70:30 | 78.6 ± 0.86 v | 62.1 ± 0.50 | 16.5 ± 1.33 | 6.6 ± 0.07 bc |
| Disc-refined FG | 70:30 | 81.3 ± 2.42 | 63.7 ± 1.52 | 17.6 ± 1.01 | 4.4 ± 0.00 e |
| 80:20 | 84.7 ± 0.90 | 65.1 ± 2.54 | 19.6 ± 1.73 | 5.3 ± 0.06 de | |
| 90:10 | 84.7 ± 0.83 | 67.4 ± 0.72 | 17.4 ± 0.52 | 5.7 ± 0.20 cd | |
| Extruded GF | 70:30 | 84.2 ± 0.44 | 61.3 ± 0.09 | 25.4 ± 0.49 | 4.7 ± 0.12 de |
| 80:20 | 81.8 ± 1.51 | 65.2 ± 0.43 | 16.6 ± 1.10 | 5.0 ± 0.03 de | |
| 90:10 | 81.9 ± 0.54 | 62.3 ± 0.78 | 19.6 ± 0.99 | 8.1 ± 0.30 a | |
| Hammer-milled PTS | 70:30 | 79.3 ± 1.72 | 60.1 ± 2.85 | 19.2 ± 2.76 | 5.9 ± 0.10 bcd |
| 80:20 | 79.7 ± 3.42 | 63.8 ± 1.40 | 15.9 ± 2.05 | 8.6 ± 0.43 a | |
| 90:10 | 80.1 ± 1.04 | 59.6 ± 2.74 | 20.5 ± 2.08 | 6.8 ± 0.31 b |
| Treatment x | Infested | Non-Infested | Germination (%) z | |||
|---|---|---|---|---|---|---|
| Peatlite | 4.43 y ± 0.0 | b | 1.74 ± 0.09 | bc | 81.6 | bc |
| Disc-refined FG | 4.23 ± 0.06 | a | 1.35 ± 0.07 | a | 91.6 | a |
| Extruded GF | 4.40 ± 0.04 | b | 2.02 ± 0.16 | c | 74.8 | c |
| Hammer-milled PTS | 4.21 ± 0.05 | a | 1.54 ± 0.07 | ab | 86.7 | ab |
| Blend Ratio | Disc-Refined FG z | Extruded GF | Hammer-Milled PTS | |||
|---|---|---|---|---|---|---|
| Infested | Non-Infested | Infested | Non-Infested | Infested | Non-Infested | |
| Peatlite 70:30 | 4.69 ± 0.04 b | 1.73 ± 0.06 | 4.75 ± 0.02 b | 1.56 ± 0.07 | 4.73 ± 0.04 b | 1.6 ± 0.08 |
| 70:30 | 4.51 ± 0.05 a | 1.59 ± 0.08 | 4.39 ± 0.06 a | 1.71 ± 0.07 | 4.56 ± 0.05 a | 1.41 ± 0.06 |
| 80:20 | 4.44 ± 0.06 a | 1.65 ± 0.07 | 4.49 ± 0.04 a | 1.63 ± 0.08 | 4.48 ± 0.05 a | 1.45 ± 0.07 |
| 90:10 | 4.39 ± 0.05 a | 1.81 ± 0.08 | 4.66 ± 0.04 b | 1.72 ± 0.08 | 4.42 ± 0.06 a | 1.38 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poleatewich, A.; Michaud, I.; Jackson, B.; Krause, M.; DeGenring, L. The Effect of Peat Moss Amended with Three Engineered Wood Substrate Components on Suppression of Damping-Off Caused by Rhizoctonia solani. Agriculture 2022, 12, 2092. https://doi.org/10.3390/agriculture12122092
Poleatewich A, Michaud I, Jackson B, Krause M, DeGenring L. The Effect of Peat Moss Amended with Three Engineered Wood Substrate Components on Suppression of Damping-Off Caused by Rhizoctonia solani. Agriculture. 2022; 12(12):2092. https://doi.org/10.3390/agriculture12122092
Chicago/Turabian StylePoleatewich, Anissa, Isobel Michaud, Brian Jackson, Matthew Krause, and Liza DeGenring. 2022. "The Effect of Peat Moss Amended with Three Engineered Wood Substrate Components on Suppression of Damping-Off Caused by Rhizoctonia solani" Agriculture 12, no. 12: 2092. https://doi.org/10.3390/agriculture12122092





