Early Plant Development in Intermediate Wheatgrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seedling Development
2.2. Statistical Analysis
3. Results
3.1. Half-Sibling Performance
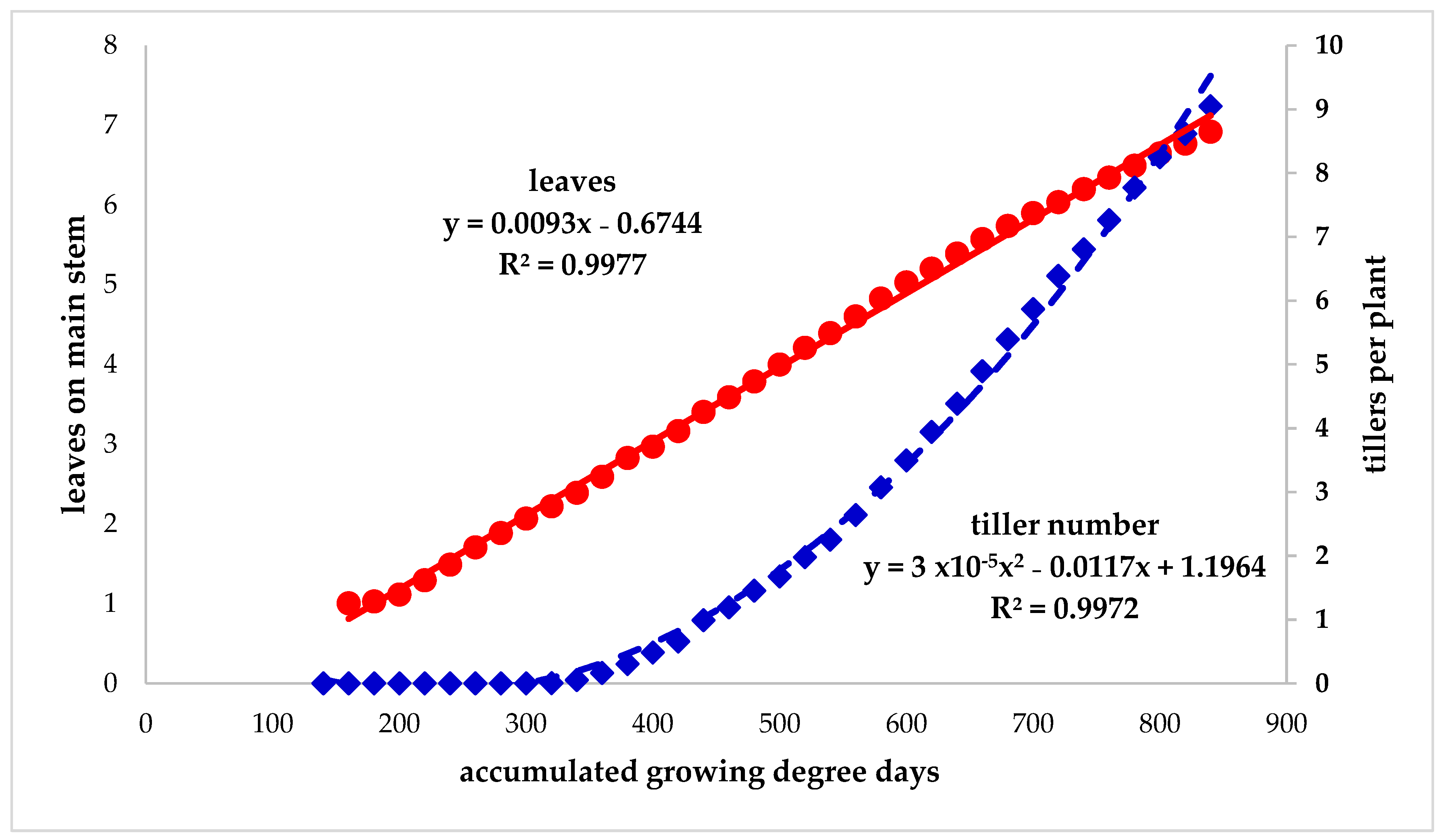
3.2. Seedling Development Study
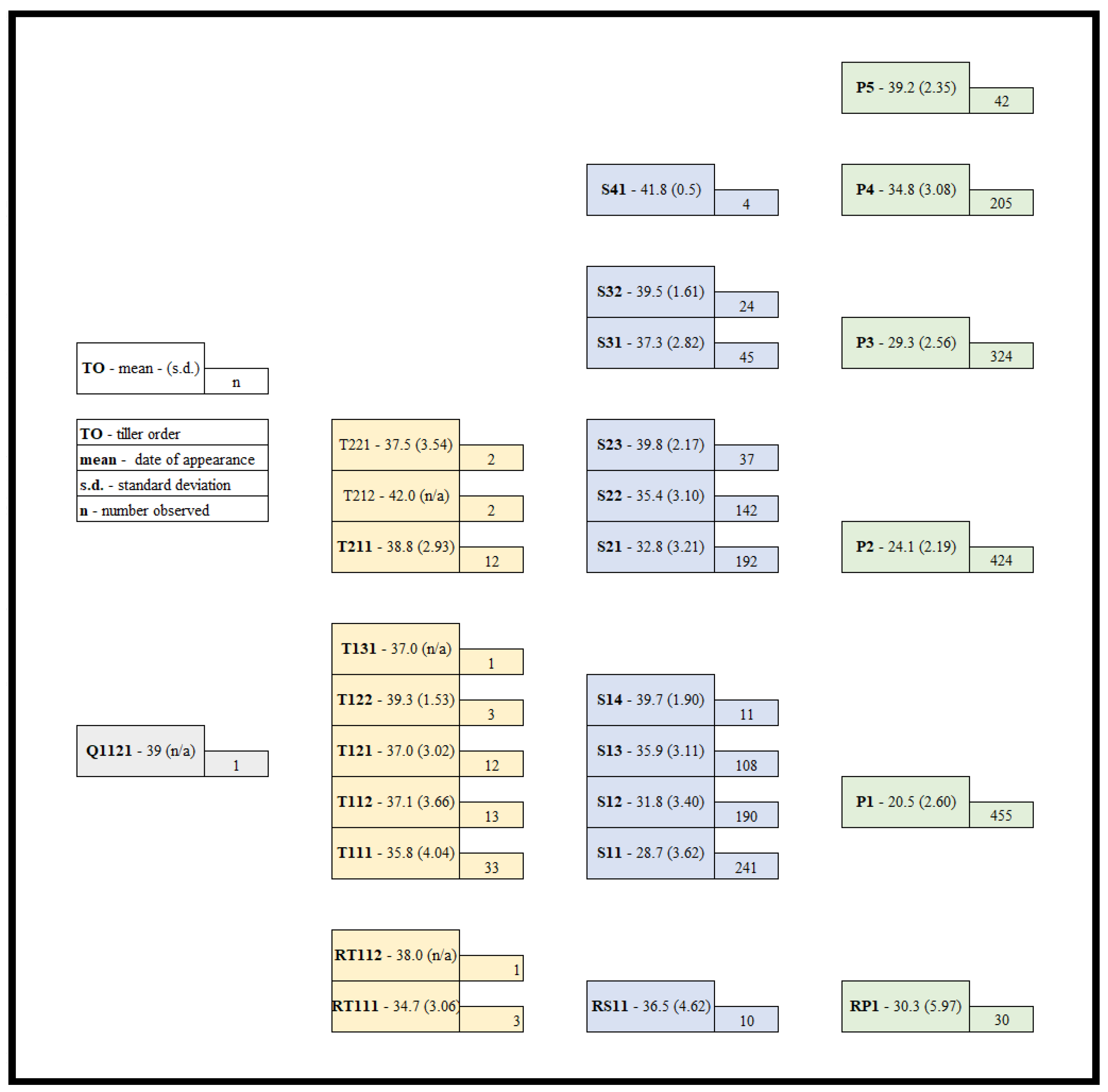
3.3. Plant Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Crews, T.E.; Cattani, D.J. Strategies, advances, and challenges in breeding perennial grain crops. Sustainability 2018, 10, 2192. [Google Scholar] [CrossRef] [Green Version]
- DeHaan, L.R.; Christians, M.; Crain, J.; Poland, J. Development and evolution of an intermediate wheatgrass breeding program. Sustainability 2018, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Bajgain, P.; Zhang, X.; Jungers, J.M.; DeHaan, L.R.; Heim, B.; Sheaffer, C.C.; Wyse, D.L.; Anderson, J.A. ‘MN-Clearwater’, the first food-grade intermediate wheatgrass (Kernza perennial grain) cultivar. J. Plant Regist. 2020, 14, 288–297. [Google Scholar] [CrossRef]
- Huang, G.; Qin, S.; Zhang, S.; Cai, X.; Wu, S.; Dao, J.; Zhang, J.; Huang, L.; Harnpichitvitaya, D.; Wade, L.J.; et al. Performance, Economics and Potential Impact of Perennial Rice PR23 Relative to Annual Rice Cultivars at Multiple Locations in Yunnan Province of China. Sustainability 2018, 10, 1086. [Google Scholar] [CrossRef] [Green Version]
- Wagoner, P. Perennial grain new use for intermediate wheatgrass. J. Soil Water Conserv. 1990, 45, 81–82. [Google Scholar]
- Popkin, G. Satellites document rapid expansion of cropland. Science 2022, 375, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Daly, E.J.; Flesch, T.K.; Coates, T.W.; Hernandez-Ramirez, G. Carbon and water dynamics of a perennial versus an annual grain crop in temperate agroecosystems. Agric. For. Meteor. 2022, 304, 108805. [Google Scholar] [CrossRef]
- Bell, L.W.; Byrne (nee Flugge), F.; Ewing, M.A.; Wade, L.J. A preliminary whole-farm economic analysis of perennial wheat in an Australian dryland farming system. Agric. Systs. 2008, 96, 166–174. [Google Scholar] [CrossRef]
- Bell, L.W.; Harrison, M.T.; Kirkegaard, J.A. Dual-purpose cropping—Capitalising on potential grain crop grazing to enhance mixed-farming profitability. Crop Pasture Sci. 2015, 66, i–iv. [Google Scholar] [CrossRef] [Green Version]
- DeHaan, L.R.; Van Tassel, D.L.; Anderson, J.A.; Asselin, S.R.; Barnes, R.; Baute, G.J.; Cattani, D.J.; Culman, S.W.; Dorn, K.M.; Hulke, B.S.; et al. A pipeline strategy for grain crop domestication. Crop Sci. 2016, 56, 917–930. [Google Scholar] [CrossRef] [Green Version]
- Bajgain, P.; Anderson, J.A.; Crain, J.L.; Cattani, D.J.; Larson, S.R.; Altendorf, K.R.; Poland, J.A.; Westerbergh, A.; Crews, T.E.; Turner, M.K.; et al. Breeding Intermediate Wheatgrass for Grain Production. Plant Breed. Rev. 2022, 46. (In press) [Google Scholar]
- Cattani, D.J. Selection of a perennial grain for seed productivity across years: Intermediate wheatgrass as a test species. Can. J. Plant Sci. 2017, 97, 516–524. [Google Scholar] [CrossRef]
- Ries, R.E.; Svejcar, T.J. The grass seedling: When is it established? J. Range Man. 1991, 44, 574–596. [Google Scholar] [CrossRef]
- Cattani, D.J.; Miller, P.R.; Smith, S.R., Jr. Relationship of shoot morphology between seedlings and established turf in creeping bentgrass. Can. J. Pant Sci. 1996, 76, 283–289. [Google Scholar] [CrossRef]
- Cattani, D.J.; Smith, S.R., Jr.; Miller, P.R.; Feindel, D.E.; Gjuric, R. Seed yield and yield components of creeping bentgrass cultivars. Can. J. Plant Sci. 2004, 84, 117–124. [Google Scholar] [CrossRef]
- Blaser, R.E.; Taylor, T.; Griffith, W.; Skrdla, W. Seedling Competition in Establishing Forage Plants. Agron. J. 1956, 48, 1–6. [Google Scholar] [CrossRef]
- Blaser, R.E.; Griffeths, W.L.; Taylor, T.H. Seedling Competition in Compounding Forage Seed Mixtures. Agron. J. 1956, 48, 118–123. [Google Scholar] [CrossRef]
- Picasso, V.D.; Brummer, E.C.; Liebman, M.; Dixon, P.M.; Wilsey, B.J. Crop species diversity affects productivity and weed suppression in perennial polycultures under two management strategies. Crop Sci. 2008, 48, 331–342. [Google Scholar] [CrossRef]
- Kilcher, M.R. Fall seeding versus spring seeding in the establishment of five grasses and one alfalfa in southern Saskatchewan. J. Range Man. 1961, 14, 320–322. [Google Scholar] [CrossRef]
- Lawrence, T. Emergence of intermediate wheatgrass lines from five depths of seeding. Can. J. Plant Sci. 1957, 37, 216–219. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T.; Kilcher, M.R. Emergence, seedling growth and yield of Altai wild ryegrass and other grasses as influenced by soil temperature and fertility. Can. J. Plant Sci. 1972, 52, 795–800. [Google Scholar] [CrossRef]
- Fraser, M.; Strelkov, S.E.; Turnbull, G.D.; Ahmed, H.U.; Barton, W.; Hwang, S.-F. Evaluation of pyraclostrobin as a component in seed and foliar fungicides for the management of blackleg (Leptosphaeria maculans) of canola (Brassica napus). Can. J. Plant Sci. 2020, 100, 549–559. [Google Scholar] [CrossRef]
- Soltani, N.; Shropshire, C.; Sikkema, P.H. Control of annual ryegrass with spring-applied herbicides prior to seeding corn. Can. J. Plant Sci. 2020, 100, 372–379. [Google Scholar] [CrossRef]
- Tidemann, B.D.; O’Donovan, J.T.; Izydorczyk, M.; Turkington, T.K.; Oatway, L.; Beres, B.; Mohr, R.; May, W.E.; Harker, K.N.; Johnson, E.N.; et al. Effects of plant growth regulator applications on malting barley in western Canada. Can. J. Plant Sci. 2020, 100, 653–665. [Google Scholar] [CrossRef]
- Vollmer, J.; Johnson, B.L.; Deckard, E.L.; Rahman, M. Evaluation of simulated hail damage on seed yield and agronomic traits in canola (Brassica napus L.). Can. J. Plant Sci. 2020, 100, 597–608. [Google Scholar] [CrossRef]
- Whalley, R.D.B.; McKell, C.M.; Green, L.R. Seedling vigor and the early nonphotosynthetic stage of seedling growth in grasses. Crop Sci. 1966, 6, 147–150. [Google Scholar] [CrossRef]
- Barros, J.F.C.; Basch, G.; de Carvalho, M. Effect of reduced doses of a post-emergence herbicide to control grass and broad-leaved weeds in no-till wheat under Mediterranean conditions. Crop Protect. 2007, 26, 1538–1545. [Google Scholar] [CrossRef]
- Casler, M.D.; Undersander, D.J. Selection for establishment capacity in reed canarygrass. Crop Sci. 2006, 46, 1277–1285. [Google Scholar] [CrossRef]
- Neuteboom, J.H.; Lantinga, E.A. Tillering potential and relationship between leaf and tiller production in perennial ryegrass. Ann. Bot. 1989, 63, 265–270. [Google Scholar] [CrossRef]
- Skinner, R.H.; Nelson, C.J. Estimation of potential tiller production and site usage during tall fescue canopy development. Ann. Bot. 1992, 70, 493–499. [Google Scholar] [CrossRef]
- Van Loo, E.N. Tillering, leaf expansion and growth of plants of two cultivars of perennial. Ann. Bot. 1992, 70, 511–518. [Google Scholar]
- Cattani, D.J. Early plant development in ‘Emerald’ and ‘UM67-10′ creeping bentgrass. Crop Sci. 1999, 39, 754–762. [Google Scholar] [CrossRef]
- Cattani, D.J.; Struik, P.C.; Nowak, J.N. Comparative morphological development of divergent flowering types of annual bluegrass and tillering types of creeping bentgrass. Crop Sci. 2002, 42, 1251–1258. [Google Scholar] [CrossRef]
- Robson, M.J. The growth and development of simulated swards of perennial ryegrass. I. Leaf growth and dry weight change as related to ceiling yield of a seedling sward. Ann. Bot. London 1973, 37, 487–500. [Google Scholar] [CrossRef]
- Jonsdottir, G.A. Tiller demography in seashore populations of Agrostis stolonifera, Festuca rubra and Poa ndiangr. J. Veg. Sci. 1991, 2, 89–94. [Google Scholar] [CrossRef]
- Entz, M.H.; Smith, S.R.; Cattani, D.J.; Storgaard, A.K. Influence of post-harvest residue management on tiller dynamics and seed yield in timothy. Can. J. Plant Sci. 1994, 74, 507–513. [Google Scholar] [CrossRef]
- Cattani, D.J.; Entz, M.H.; Bamford, K.C. Tiller production and dry matter accumulation in six creeping bentgrass genotypes grown in Manitoba. Can. J. Plant Sci. 1991, 71, 595–599. [Google Scholar] [CrossRef]
- Heide, O.M. Control of flowering and reproduction in temperate grasses. New Phytol. 1994, 128, 347–362. [Google Scholar] [CrossRef]
- Duchene, O.; Dumont, B.; Cattani, D.J.; Fagnant, L.; Schlautman, B.; DeHaan, L.R.; Barriball, S.; Jungers, J.M.; Picasso, V.D.; David, C.; et al. Process-based analysis of Thinopyrum intermedium phenological development highlights the importance of dual induction for reproductive growth and agronomic performance. Agric. For. Meteorol. 2021, 301–302, 108341. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Ehlke, N.; Sheaffer, C.C.; Jungers, J.M. Effects of nitrogen fertilization and planting density on intermediate wheatgrass yield. Agron. J. 2020, 112, 4159–4170. [Google Scholar] [CrossRef]
- Black, A.L.; Reitz, L.L. Row spacing and fertilization influences on forage and seed yields of intermediate wheatgrass, Canada wildrye, and green needlegrass on dryland. Agron. J. 1969, 61, 801–805. [Google Scholar] [CrossRef]
- Hunter, M.C.; Sheaffer, C.C.; Culman, S.W.; Jungers, J.M. Effects of defoliation and row spacing on intermediate wheatgrass I: Grain production. Agron. J. 2020, 112, 1748–1763. [Google Scholar] [CrossRef]
- Hunter, M.C.; Sheaffer, C.C.; Culman, S.W.; Lazarus, W.F.; Jungers, J.M. Effects of defoliation and row spacing on intermediate wheatgrass II: Forage yield and economics. Agron. J. 2020, 112, 1862–1880. [Google Scholar] [CrossRef]
- Law, R.; Bradshaw, A.D.; Putwain, P.D. Life-history variation in Poa annua. Evolution 1977, 31, 233–246. [Google Scholar] [CrossRef]
- Haun, J.R. Visual quantification of wheat development. Agron. J. 1973, 65, 116–119. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Strauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal scale code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- West, C.P. A proposed growth stage system for bermudagrass. In Proceedings of the Amer. Forage and Grassland Council, Blacksburg, VA, USA, 6–9 June 1990; Am. Forage and Grasslands Council: Georgetown, TX, USA, 1990; pp. 38–42. [Google Scholar]
- Cunniff, J.; Wilkinson, S.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. Functional traits differ between cereal crop progenitors and other wild grasses gathered in the neolithic fertile crescent. PLoS ONE 2014, 9, e87586. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Larson, J.E.; Sheley, R.L.; Hardegree, S.P.; Doescher, P.S.; James, J.J. Do key dimensions of seed and seedling functional trait variation capture variation in recruitment probability? Oecologia 2016, 181, 39–53. [Google Scholar] [CrossRef]
- Happ, K.; McDonald, M.B.; Danneberger, T.K. Vigor testing in perennial ryegrass (Lolium perenne L.) seeds. Seed Sci. Technol. 1993, 21, 375–381. [Google Scholar]
- Atkinson, R.R.L.; Mockford, E.J.; Bennett, C.; Christin, P.-A.; Spriggs, E.L.; Freckleton, R.P.; Thompson, K.; Rees, M.; Osborne, C.P. C4 photosynthesis boosts growth by altering physiology, allocation and size. Nat. Plants 2016, 18, 16038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Larson, S.R.; Gao, L.; Teh, S.L.; DeHaan, L.R.; Fraser, M.; Sallam, A.; Kantarski, T.; Frels, K.; Poland, J.; et al. Uncovering the genetic architecture of seed weight and size in intermediate wheatgrass through linkage and association mapping. Plant Genome 2017, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crain, J.; Bajgain, P.; Anderson, J.; Zhang, X.; DeHaan, L.; Poland, J. Enhancing crop domestication through genomic selection, a case study of intermediate wheatgrass. Front. Plant Sci. 2020, 11, 319. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.; Clemente, A.; Nunes, A.; Correia, O. Suitability and limitations of native species for seed mixtures to re-vegetate degraded areas. Appl. Veg. Sci. 2014, 17, 726–736. [Google Scholar] [CrossRef]
- Gibson, A.; Nelson, C.R.; Atwater, D.Z. Response of bluebunch wheatgrass to invasion: Differences in competitive ability among invader-experienced and invader-naïve populations. Funct. Ecol. 2018, 32, 1857–1866. [Google Scholar] [CrossRef]
- Ross, M.A.; Harper, J.L. Occupation of biological space during seedling establishment. J. Ecol. 1972, 60, 77–88. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. A general leaf area geometric formula exists for plants—Evidence from the simplified Gielis equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef] [Green Version]
- Milla, R.; Reich, P.B. The scaling of leaf area and mass: The cost of light interception increases with leaf size. Proc. R. Soc. B 2007, 274, 2109–2114. [Google Scholar] [CrossRef] [Green Version]
- Sebolai, B.; Vogel, K.B. Evaluation of three breeding cycles for seedling weight of switchgrass, big bluestem, and ndiangrass. Crop Sci. 2014, 54, 1354–1360. [Google Scholar] [CrossRef]
- Ray-Mukherjee, J.; Jones, T.A.; Adler, P.B.; Monaco, T.A. Immature Seedling Growth of Two North American Native Perennial Bunchgrasses and the Invasive Grass Bromus tectorum. Range Ecol. Man. 2011, 64, 358–365. [Google Scholar] [CrossRef]
- Cattani, D.J.; Asselin, S.R. Has selection for grain yield altered intermediate wheatgrass? Sustainability 2018, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Hunt, O.J.; Miller, D.G. Coleoptile Length, Seed Size and Emergence in Intermediate Wheatgrass (Agropyron intermedium Host (Beauv)). Agron. J. 1965, 57, 192–195. [Google Scholar] [CrossRef]
- Altendorf, K.R.; Larson, S.R.; DeHaan, L.R.; Crain, J.; Neyhart, J.; Dorn, K.M.; Anderson, J.A. Nested association mapping reveals the genetic architecture of spike emergence and anthesis timing in intermediate wheatgrass. Genes Genomes Genet. G3 2021, 11, jkab025. [Google Scholar] [CrossRef]
- Jungers, J.M.; DeHaan, L.R.; Betts, K.J.; Sheaffer, C.C.; Wyse, D.L. Intermediate Wheatgrass Grain and Forage Yield Responses to Nitrogen Fertilization. Agron. J. 2017, 109, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Tautges, N.E.; Jungers, J.M.; DeHaan, L.R.; Wyse, D.L.; Sheaffer, C.C. Maintaining grain yields of the perennial cereal intermediate wheatgrass in monoculture vs. bi-culture with alfalfa in the Upper Midwestern USA. 2018. J. Agric. Sci. 2018, 156, 758–773. [Google Scholar] [CrossRef]
- Dick, C.; Cattani, D.; Entz, M. Kernza Intermediate wheatgrass (Thinopyrum intermedium) grain production as influenced by legume intercropping and residue management. Can. J. Plant Sci. 2018, 98, 1376–1379. Available online: http://www.nrcresearchpress.com.uml.idm.oclc.org/doi/pdf/10.1139/CJPS-2018-0146 (accessed on 3 May 2022). [CrossRef]
- Olugbenle, O.; Pinto, P.; Picasso, V.D. Optimal Planting Date of Kernza Intermediate Wheatgrass Intercropped with Red Clover. Agronomy 2021, 11, 2227. [Google Scholar] [CrossRef]
- Ivancic, K.; Locatelli, A.; Tracy, W.F.; Picasso, V. Kernza intermediate wheatgrass (Thinopyrum intermedium) response to a range of vernalization conditions. Can. J. Plant Sci. 2021, 101, 770–773. [Google Scholar] [CrossRef]
- Cattani, D.J. Effect of turf competition on creeping bentgrass seedling establishment. In Proceedings of the 9th International Turfgrass Conference, Toronto, ON, Canada, 15–21 July 2001; pp. 850–854. [Google Scholar]
- Bond, W.J. Ancient grasslands at risk. Science 2016, 351, 120–122. [Google Scholar] [CrossRef]
- Cattani, D.J.; Asselin, S.R. Extending the growing season: Forage seed production and perennial grains. Can. J. Plant Sci. 2018, 98, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.J.; Clark, K.W. Effects of clipping and nitrogen fertilization on tiller development and flowering in Kentucky bluegrass. Can. J. Plant Sci. 1993, 73, 569–575. [Google Scholar] [CrossRef]
- Duchene, O.; Celette, F.; Barreiro, A.; Dimitrova Mårtensson, L.-M.; Freschet, G.T.; David, C. Introducing Perennial Grain in Grain Crops Rotation: The Role of Rooting Pattern in Soil Quality Management. Agronomy 2020, 10, 1254. [Google Scholar] [CrossRef]
- Bullock, J.M.; Clear Hill, B.; Silvertown, J.; Sutton, M. Gap colonization as a source of grassland community change: Effects of gap size and grazing on the rate and mode of colonization by different species. OIKOS 1995, 72, 273–282. [Google Scholar] [CrossRef]
- Silvertown, J.; Bullock, J.M. Do seedlings in gaps interact? A field test of assumptions in ESS seed size models. OIKOS 2003, 101, 499–504. [Google Scholar] [CrossRef]
| Line | All Seed | Large Seed | Seed Yield | Plant Area |
|---|---|---|---|---|
| g thousand seeds−1 | g plant−1 | cm−2 | ||
| Line D | 9.62 ± 1.67 | 11.13 ± 0.68 | 69.52 ± 26.13 | 1039 ± 101 |
| Line E | 8.75 ± 0.81 | 11.12 ± 0.63 | 130.50 ± 38.19 | 1433 ± 493 |
| Line C | 7.96 ± 1.10 | 9.68 ± 0.36 | 47.12 ± 20.36 | 609 ± 51 |
| Line F | 7.92 ± 0.58 | 9.99 ± 0.49 | 76.62 ± 48.70 | 1227 ± 164 |
| Line B | 7.55 ± 0.35 | 8.12 ± 0.33 | 39.93 ± 15.68 | 612 ± 123 |
| Line A | 6.28 ± 0.25 | 8.00 ± 0.28 | 46.33 ± 7.83 | 607 ± 39 |
| Biomass | Harvest Index | Heads | Heads cm−2 | Yield cm−2 | Yield head−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Line | g plant−1 | % | plant−1 | cm−2 | mg cm−2 | mg | ||||||
| Line E | 661.1 ± 154 | 19.1 ± 3.5 | 248.7 ± 63 | 0.177 ± 0.02 | 92.5 ± 10.3 | 525.2 ± 64 | ||||||
| Line D | 654.4 ± 225 | 10.6 ± 1.3 | 245.0 ± 80 | 0.233 ± 0.06 | 66.7 ± 23.5 | 285.5 ± 48 | ||||||
| Line B | 488.4 ± 225 | 8.5 ± 1.4 | 143.3 ± 36 | 0.242 ± 0.08 | 65.5 ± 23.3 | 275.6 ± 62 | ||||||
| Line F | 487.8 ± 173 | 14.5 ± 5.1 | 189.7 ± 72 | 0.156 ± 0.06 | 61.9 ± 40.3 | 382.0 ± 155 | ||||||
| Line C | 466.3 ± 208 | 10.2 ± 0.7 | 165.3 ± 48 | 0.271 ± 0.07 | 77.4 ± 32.0 | 279.0 ± 48 | ||||||
| Line A | 367.8 ± 101 | 12.9 ± 2.0 | 163.0 ± 26 | 0.271 ± 0.06 | 76.2 ± 9.6 | 294.5 ± 100 | ||||||
| Biomass | ||||||||||||
| Line | g plant−1 | |||||||||||
| Line E | 661.1 | a | ||||||||||
| Line D | 654.4 | a | ||||||||||
| Line B | 488.4 | b | ||||||||||
| Line F | 487.8 | b | ||||||||||
| Line C | 466.3 | b | ||||||||||
| Line A | 367.8 | b | ||||||||||
| p value | 0.009 | |||||||||||
| Harvest Dry Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Line | 21 days | 28 days | 35 days | 42 days | ||||
| Line E | 0.080 | a 1 | 0.374 | a | 1.132 | a | 2.455 | a |
| Line C | 0.073 | a | 0.297 | ab | 0.884 | b | 1.992 | a |
| Line F | 0.072 | a | 0.305 | ab | 0.991 | ab | 2.219 | a |
| Line A | 0.064 | a | 0.307 | ab | 0.979 | ab | 2.132 | a |
| Line D | 0.064 | a | 0.249 | b | 1.004 | ab | 2.123 | a |
| Line B | 0.060 | a | 0.267 | b | 0.781 | b | 1.779 | a |
| p value | 0.510 | 0.038 | 0.012 | 0.080 | ||||
| Line | Haun stage on main stem | |||||||
| Line E | 3.27 | a | 4.88 | a | 6.03 | a | 6.95 | ab |
| Line A | 3.18 | ab | 4.60 | ab | 5.93 | a | 7.28 | a |
| Line F | 3.13 | abc | 4.61 | ab | 5.95 | a | 6.92 | ab |
| Line D | 3.05 | abc | 4.30 | c | 5.81 | a | 6.84 | ab |
| Line C | 2.95 | bc | 4.39 | bc | 5.69 | a | 6.74 | b |
| Line B | 2.93 | c | 4.44 | bc | 6.03 | a | 6.78 | b |
| p value | 0.009 | 0.002 | 0.005 | 0.113 | ||||
| Line | Tillers plant−1 | |||||||
| Line E | 2.07 | a | 2.94 | a | 5.11 | ab | 8.66 | a |
| Line F | 1.83 | b | 2.58 | ab | 5.39 | a | 9.71 | a |
| Line A | 1.81 | b | 2.74 | a | 5.43 | a | 9.59 | a |
| Line D | 1.69 | bc | 2.55 | ab | 5.52 | a | 9.80 | a |
| Line C | 1.65 | c | 2.35 | ab | 5.23 | a | 9.44 | a |
| Line B | 1.55 | c | 1.98 | b | 4.01 | b | 8.15 | a |
| p value | 0.001 | 0.013 | 0.002 | 0.442 | ||||
| Line | Length 1 | Length 2 | Length 3 | Length 4 | Length 5 | Length 6 |
|---|---|---|---|---|---|---|
| C | 12.67 a 1 | 19.83 a | 24.75 a | 28.09 a | 30.54 ab | 33.58 ab |
| B | 11.49 ab | 19.54 ab | 24.70 ab | 28.15 a | 32.27 a | 35.84 a |
| E | 11.22 b | 18.40 abc | 23.50 abc | 26.21 bc | 28.90 b | 32.10 b |
| D | 11.00 b | 17.72 c | 22.63 c | 25.72 c | 29.56 ab | 33.27 ab |
| A | 10.72 bc | 18.44 abc | 23.14 c | 26.44 ab | 28.56 b | 31.79 b |
| F | 10.54 c | 18.23 bc | 23.37 bc | 26.72 ab | 29.59 ab | 31.23 b |
| p value | 0.001 | 0.019 | 0.001 | 0.002 | 0.001 | 0.004 |
| Line | Width 1 | Width 2 | Width 3 | Width 4 | Width 5 | Width 6 |
| E | 2.02 a | 3.29 a | 5.9 a | 8.6 a | 10.7 a | 11.8 a |
| D | 2.02 a | 3.28 a | 5.8 a | 8.4 a | 9.9 a | 11.1 a |
| C | 2.00 a | 3.28 a | 5.6 a | 8.4 a | 9.7 a | 10.8 a |
| F | 1.99 a | 3.23 ab | 5.9 a | 8.6 a | 10.3 a | 11.4 a |
| A | 1.97 ab | 3.29 a | 6.2 a | 8.8 a | 10.5 a | 11.5 a |
| B | 1.77 b | 2.95 b | 5.7 a | 8.7 a | 10.0 a | 11.6 a |
| p value | 0.003 | 0.020 | 0.101 | 0.629 | 0.287 | 0.238 |
| Line | Intercept | S.E | p Value | Slope | S.E. | p Value |
|---|---|---|---|---|---|---|
| Line A | 0.1159 | 0.0435 | 0.0098 | 0.00151 | 0.000072 | 0.0001 |
| Line B | 0.1735 | 0.0389 | 0.0001 | 0.00125 | 0.000062 | 0.0001 |
| Line C | 0.1168 | 0.0390 | 0.0001 | 0.00135 | 0.000069 | 0.0001 |
| Line D | 0.1800 | 0.0371 | 0.0001 | 0.00128 | 0.000064 | 0.0001 |
| Line E | 0.1127 | 0.0359 | 0.0026 | 0.00157 | 0.000061 | 0.0001 |
| Line F | 0.1128 | 0.0441 | 0.0131 | 0.00155 | 0.000075 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattani, D.J.; Asselin, S.R. Early Plant Development in Intermediate Wheatgrass. Agriculture 2022, 12, 915. https://doi.org/10.3390/agriculture12070915
Cattani DJ, Asselin SR. Early Plant Development in Intermediate Wheatgrass. Agriculture. 2022; 12(7):915. https://doi.org/10.3390/agriculture12070915
Chicago/Turabian StyleCattani, Douglas John, and Sean Robert Asselin. 2022. "Early Plant Development in Intermediate Wheatgrass" Agriculture 12, no. 7: 915. https://doi.org/10.3390/agriculture12070915






