Decomposition and Nitrogen Release Rates of Foliar Litter from Single and Mixed Agroforestry Species under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
2.2. Field Experimental Procedure
- 100 g fresh leaves of Guazuma, equivalent to 41 g dry weight
- 100 g fresh leaves of Guazuma + Leucaena, equivalent to 40 g dry weight (20 g of Guazuma + 20 g of Leucaena)
- 100 g fresh leaves of Leucaena, equivalent to 38 g dry weight
- 118 g fresh leaves of Moringa + Leucaena, equivalent to 37 g dry weight (19 g Moringa + 18 g of Leucaena)
- 136 g fresh leaves of Moringa, equivalent to 35 g dry weight
2.3. Chemical Analysis of Plant Material Used in the Decomposition Study
2.4. Statistical Analysis
3. Results
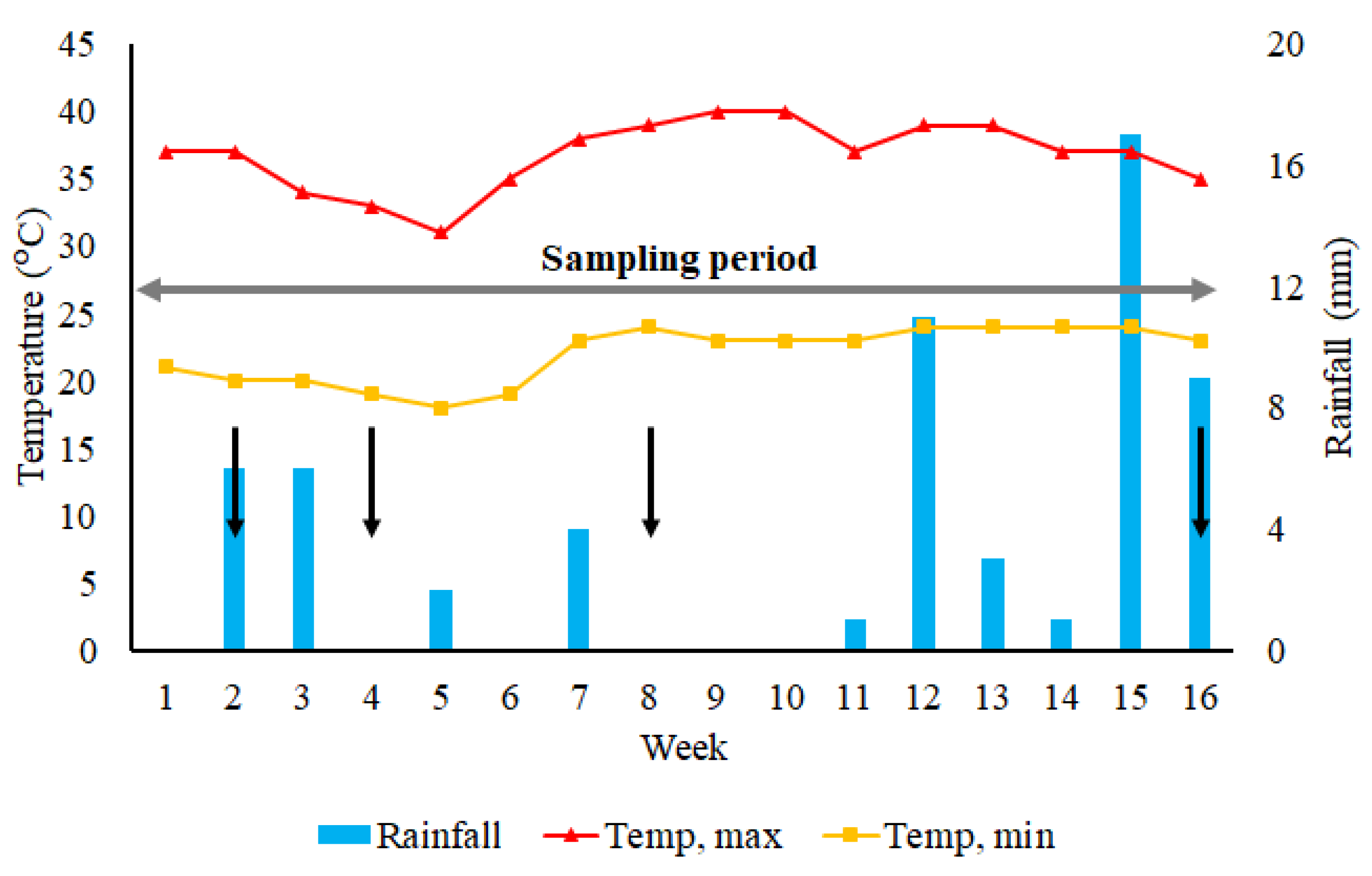
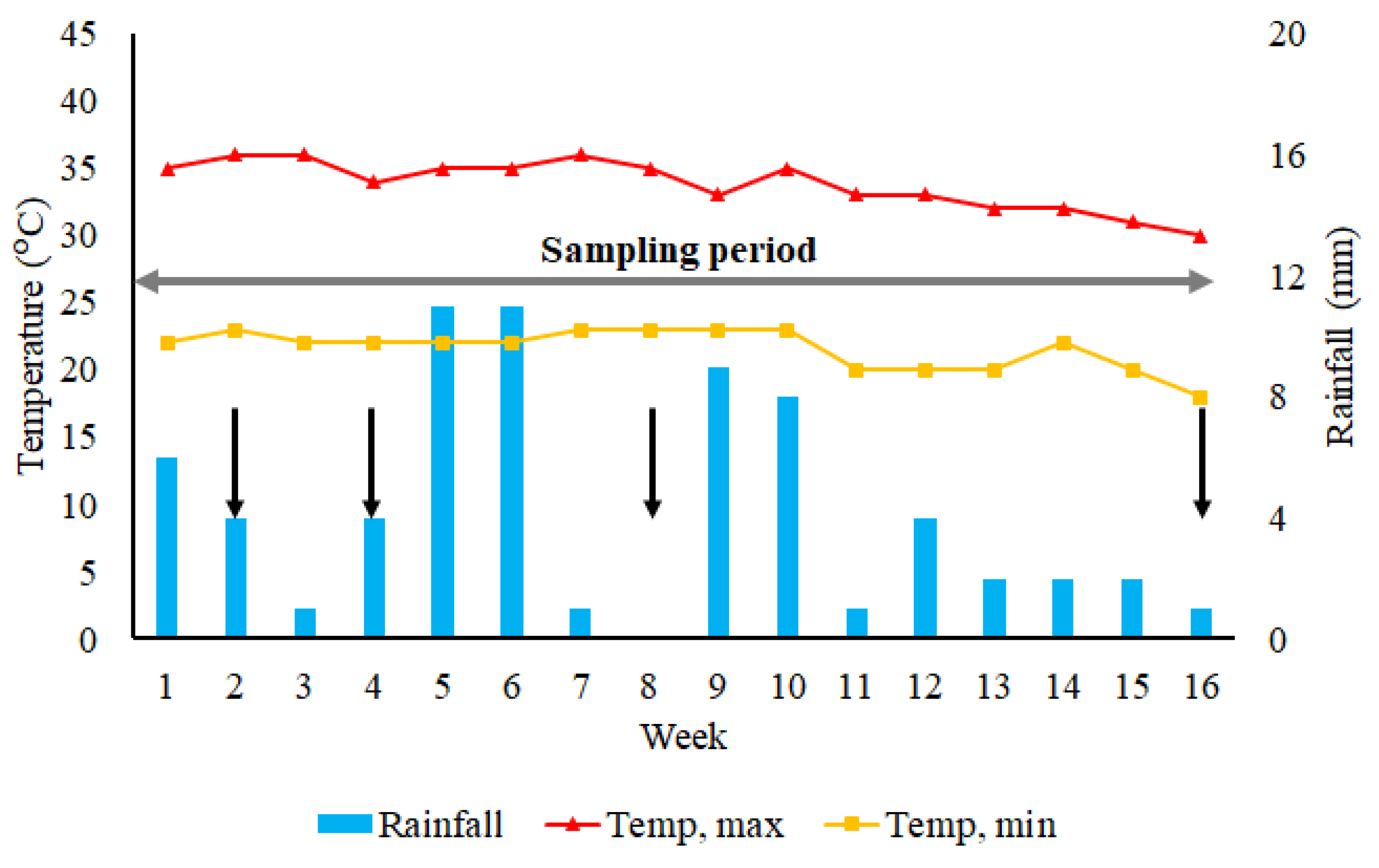
3.1. Climatic Conditions
3.2. Chemical Composition of the Shrub Leaves
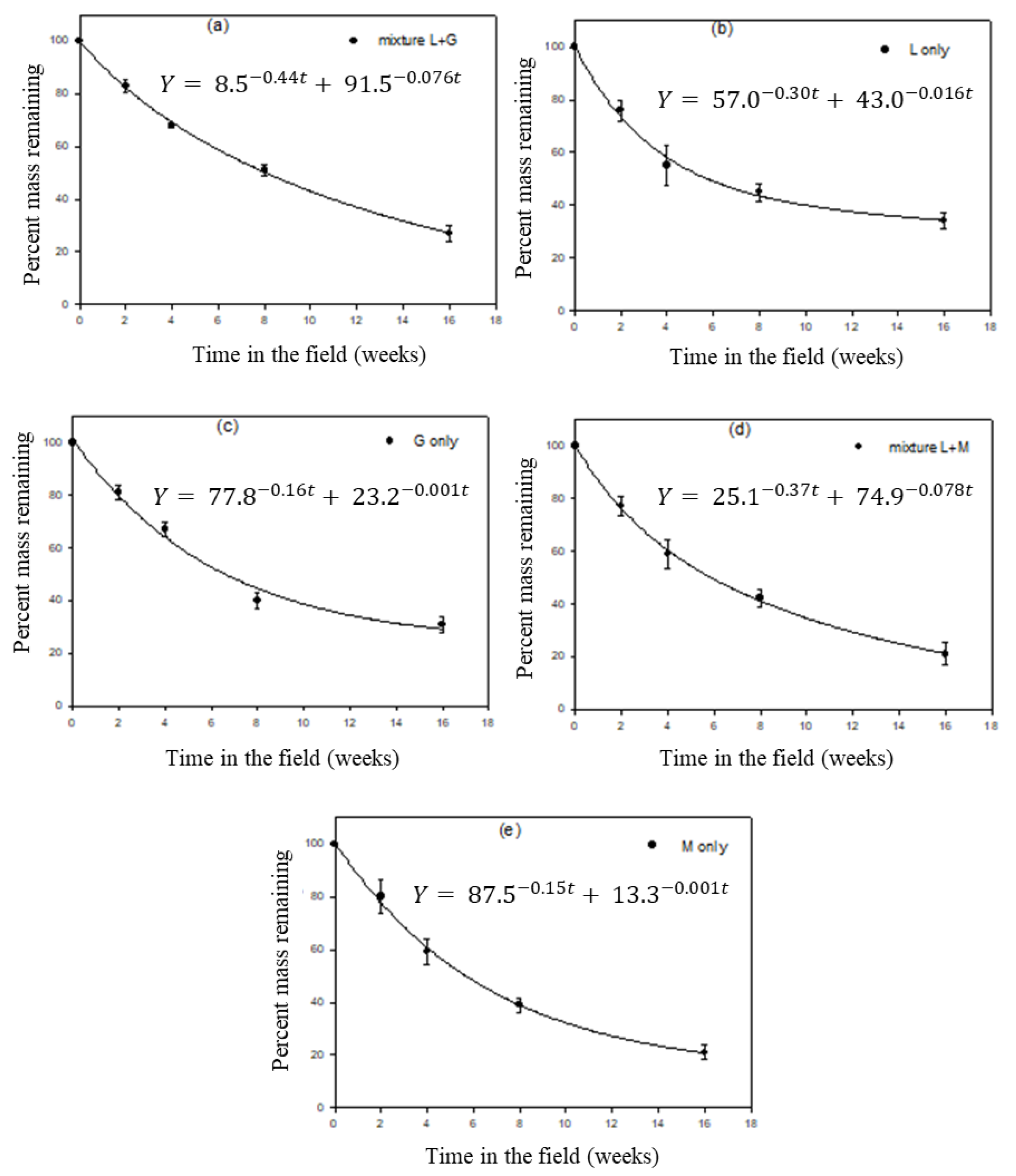
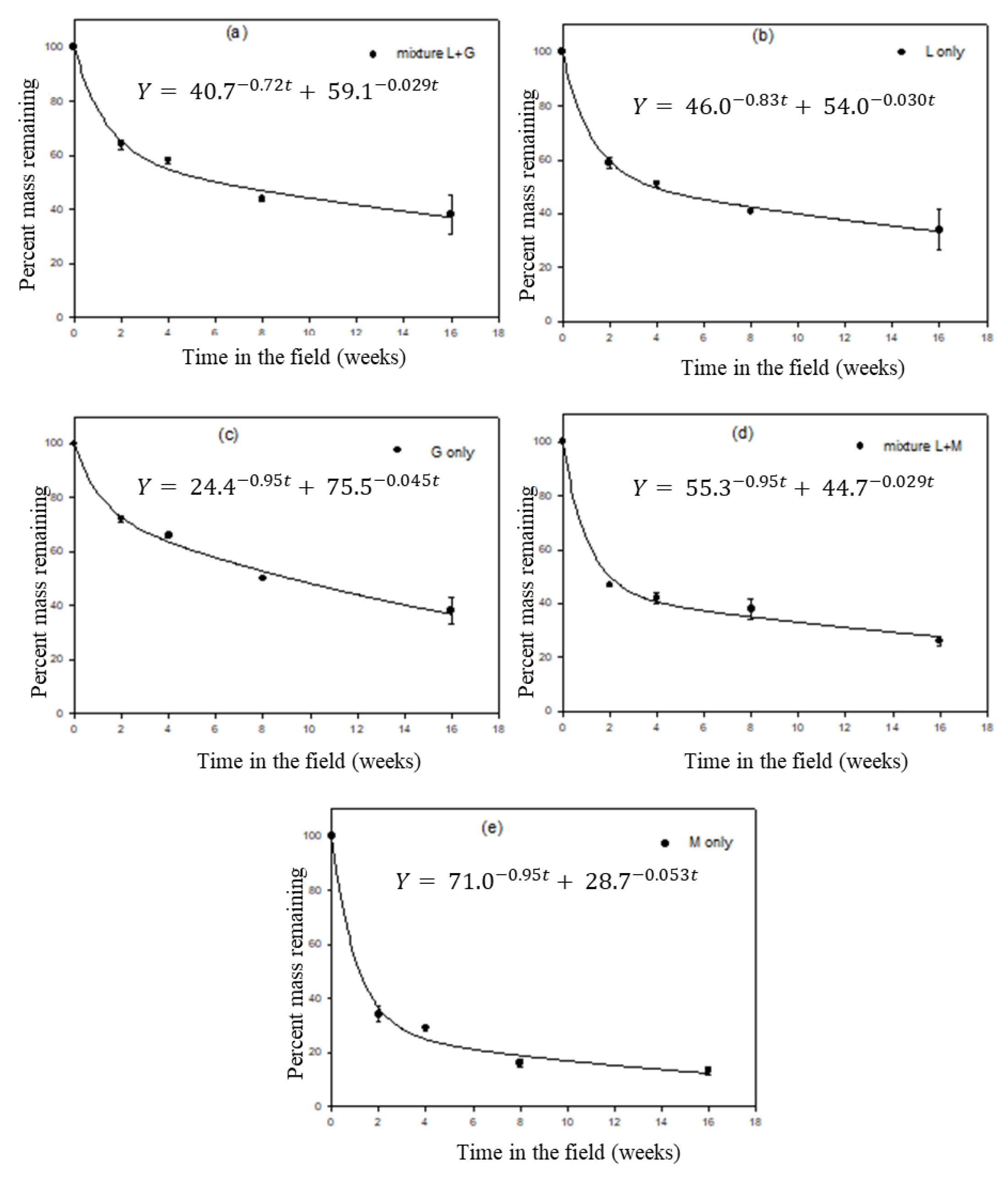
3.3. Leaf Litter Decomposition
3.4. Organic Matter (OM) Decomposition
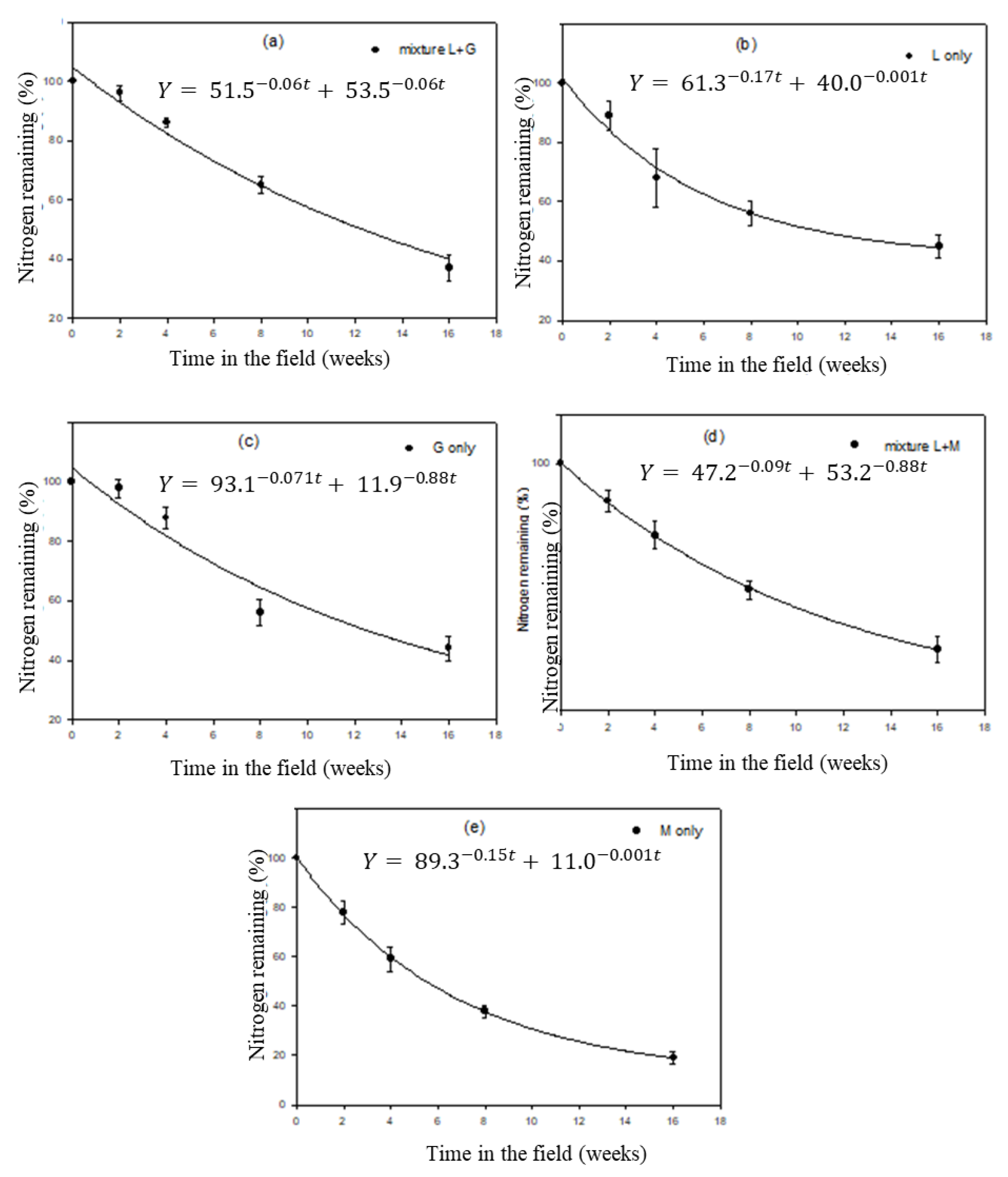
3.5. Nitrogen Release Patterns
3.6. Relationship between Organic Matter Decomposition and Chemical Composition
3.7. Relationship between Nitrogen Release and Leaf Chemical Composition
4. Discussion
4.1. Chemical Composition of Leaves in the Study
4.2. Decomposition Rates and Quality of Shrub Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021; 60p. [Google Scholar] [CrossRef]
- FAO; IFAD; PAHO; UNICEF; WFP. Latin America and the CaribbeanRegional Overview of Food Security and Nutrition 2021: Statistics and Trends. Santiago; FAO: Rome, Italy, 2021; 240p. [Google Scholar] [CrossRef]
- Cairns, M.A.; Dirzo, R.; Zadroza, F. Forests of Mexico: A diminishing resource. J. Forest. 1995, 93, 21–23. [Google Scholar]
- Menegale, M.L.; Rocha, J.H.T.; Harrison, R.; Goncalves, J.L.D.M.; Almeida, R.F.; Piccolo, M.D.C.; Hubner, A.; Arthur, J.C., Jr.; de Vicente, F.A.; James, J.N.; et al. Effect of timber harvest intensities and fertilizer application on stocks of soil C, N, P, and S. Forests 2016, 7, 319. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, P. The climate change-soil fertility-food security nexus. In Proceedings of the Sustainable Food Security for All by 2020: Proceedings of an International Conference, Bonn, Germany, 4–6 September 2001. [Google Scholar]
- Franco, A.A.; de Faria, S.M. The contribution of N2-fixing tree legumes to land reclamation and sustainability in the tropics. Soil Biol. Biochem. 1997, 29, 897–903. [Google Scholar] [CrossRef]
- Buresh, R.J.; Tian, G. Soil improvement by trees in sub-Saharan Africa. Agrofor. Sys. 1998, 38, 51–76. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Akobundu, I.O.; Sanginga, N.; Jutzi, S.C. Effect of mulch from selected multipurpose trees (MPTs) on growth, nitrogen nutrition and yield of maize (Zea mays L.). J. Agron. Crop Sci. 2000, 184, 73–80. [Google Scholar] [CrossRef]
- Schroth, G.; Sinclair, F.L. Impact of trees on the fertility of agricultural soils. In Trees, Crops and Soil Fertility: Concepts and Research Methods, 1st ed.; Schroth, G., Sinclair, F.L., Eds.; CAB International: Wallingford, UK, 2003; pp. 1–11. [Google Scholar]
- González, I.; Sixto, H.; Rodríguez-Soalleiro, R.; Cañellas, I.; Fuertes, A.; Oliveira, N. How can leaf-litter from different species growing in short rotation coppice contribute to the soil nutrient pool? For. Ecol. Manag. 2022, 520, 120405. [Google Scholar] [CrossRef]
- Liu, Y.; Shangguan, Z.; Deng, L. Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession. Forest 2021, 12, 195. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P.; Thevathasan, N.V.; Gordon, A.M.; Kass, D.C.; Schlönvoigt, A.M. Soil carbon dynamics and residue stabilization in a Costa Rican and southern Canadian alley cropping system. Agrofor. Syst. 2006, 68, 27–36. [Google Scholar] [CrossRef]
- Chikowo, R.; Corbeels, M.; Mapfumo, P.; Tittonell, P.; Vanlauwe, B.; Giller, K.E. Nitrogen and Phosphorus Capture and Recovery Efficiencies, and Crop Responses to a Range of Soil Fertility Management Strategies in Sub-Saharan Africa. Innovations as Key to the Green Revolution in Africa; Springer: Dordrecht, The Netherlands, 2011; pp. 571–589. [Google Scholar]
- Cadisch, G.; Giller, K.E. Driven by Nature, Plant Litter Quality and Decomposition; CAB International: Wallingford, CT, USA, 1997. [Google Scholar]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Aihou, K.; Aman, S.; Tossah, B.K.; Diels, J.; Sanginga, N.; Merckx, R. Leaf quality of selected hedgerow species at two canopy ages in the derived savanna zone of West Africa. Agrofor. Sys. 2001, 53, 21–30. [Google Scholar] [CrossRef]
- Bai, S.H.; Gallart, M.; Singh, K.; Hannet, G.; Komolong, B.; Yinil, D.; Field, D.J.; Muqqadas, B.; Wallace, H.M. Leaf litter species affects decomposition rate and nutrient release in a cocoa plantation. Agric. Ecosyst. Environ. 2022, 324, 107705. [Google Scholar] [CrossRef]
- Gartner, T.B.; Zoe, C.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Cattanio, J.H. Soil N Mineralization Dynamics as Affected by Pure and Mixed Application of Leafy Material from Leguminous Trees Used in Planted Fallow in Brazil. Ph.D. Thesis, Faculty of Agricultural Sciences, George-August-University, Gottingen, Germany, 2002. [Google Scholar]
- Prescott, C.E. Do rates of litter decomposition tell us anything we really need to know? For. Ecol. Manag. 2005, 220, 66–74. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L. Carbon allocation in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. For. Ecol. Manag. 2006, 233, 275–284. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna, and decomposition sites effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Hector, A.; Beale, A.J.; Minns, A.; Otway, S.J.; Lawton, J.H. Consequences of the reduction of plant diversity for litter decomposition: Effects through litter quality and microenvironment. Oikos 2000, 90, 357–371. [Google Scholar] [CrossRef]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Litter quality and interactive effects in litter mixtures: More negative interactions under elevated CO2? J. Ecol. 2002, 90, 1009–1016. [Google Scholar] [CrossRef]
- Van Kessel, C. Seasonal accumulation and partitioning of nitrogen by lentil. Plant Soil 1994, 164, 69–76. [Google Scholar] [CrossRef]
- Estrada-Medina, H.; Cobos-Gasca, V.; Acosta-Rodríguez, J.L.; Peña Fierro, S.; Castilla-Martínez, M.; Castillo-Carrillo, C.; Franco-Brito, S.; López-Castillo, D.; López-Díaz, M.; Luna-Flores, W.; et al. La sequía de la península de Yucatán. Tecnol. Cienc. Agua 2016, 7, 151–165. [Google Scholar]
- Patrick, W.H.; Gambrell, R.P.; Faulkner, S.P. Redox measurements of soils. In Methods of Soil Analysis, Part 3: Chemical Analysis; Sparks, D.L., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc: Madison, WI, USA, 1996; pp. 1255–1273. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis, Part 3: Chemical Analysis; Sparks, D.L., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Organic matter. In Methods of Soil Analysis, Part 3: Chemical Analysis; Sparks, D.L., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Kuo, S.P. Phosphorus. In Methods of Soil Analysis, Part 3: Chemical Analysis; Sparks, D.L., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc: Madison, WI, USA, 1996; pp. 869–920. [Google Scholar]
- Suárez, D.L. Beryllium. magnesium, Ca, strontium, and barium. In Methods of Soil Analysis, Part 3: Chemical Analysis; Sparks, D.L., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc: Madison, WI, USA, 1996; pp. 551–574. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Goering, H.K.; van Soest, J.P. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications); USDA Agricultural Handbook Nº. 379; USDA: Washington, DC, USA, 1970. [Google Scholar]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage. a Laboratory Manual for FAO/IAEA Co-ordinated Research Project on “Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on Tanniniferous Tree Foliage”; FAO/IAEA Working Document; IAEA: Vienna, Austria, 2000. [Google Scholar]
- Wieder, R.K.; Lang, G.E. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 1982, 63, 1636–1642. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Peng, X.; Hou, C.; Ma, J.; Guo, J. Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens. Agriculture 2022, 12, 957. [Google Scholar] [CrossRef]
- Armendariz-Yañez, I. Indigenous Fodder Legume Trees: Their Influence on Soil Fertility and Animal Production on Tropical Pastures in Yucatán, México. Ph.D. Thesis, University of London, London, UK, Imperial College at Wye, London, UK, 1998. [Google Scholar]
- Solorio, S.F.J. Soil Fertility and Nutrient Cycling in Pure and Mixed Fodder Bank Systems Using Leguminous and Non-leguminous Shrubs. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2005. [Google Scholar]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadish, G.; Giller, K.E. Organics inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agric. Ecosys. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Handayanto, E.; Cadisch, G.; Giller, K. Regulating N mineralization from plant residues by manipulation of quality. In Driven by Nature: Plant Litter Quality and Decomposition; Cadisch, G., Giller, K.E., Eds.; CAB International: Wallingford, UK, 1997; pp. 175–185. [Google Scholar]
- Baietto, A.; Hernández, J.; del Pino, A. Comparative Dynamics of Above-Ground Litter Production and Decomposition from Eucalyptus grandis Hill ex Maiden and Pinus taeda L., and Their Contribution to Soil Organic Carbon. Forests 2021, 12, 349. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Singh, A.K.; Zeng, H.; Chen, C.; Lu, E.; Liu, W. Reduced litterfall and decomposition alters nutrient cycling following conversion of tropical natural forests to rubber plantations. Ecol. Indic. 2022, 138, 108819. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar] [CrossRef]
- Andrews, E.M.; Kassama, S.; Smith, E.E.; Brown, P.H.; Khalsa, S.D.S. A review of potassium-rich crop residues used as organic matter amendments in tree crop agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Maie, N.; Behrens, A.; Knicker, H.; Kögel-Knabner, I. Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol. Biochem. 2003, 35, 577–589. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Lai, H.; Gao, F.; Su, H.; Zheng, P.; Li, Y.; Yao, H. Nitrogen Distribution and Soil Microbial Community Characteristics in A Legume–cereal Intercropping System: A Review. Agronomy 2022, 12, 1900. [Google Scholar] [CrossRef]
- Palm, C.A.; Sanchez, P.A. Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic content. Soil Biol. Biochem. 1991, 23, 83–88. [Google Scholar] [CrossRef]
- Vahdat, E.; Nourbakhsh, F.; Basiri, M. Lignin content of range plant residues controls N mineralization in soil. Eur. J. Soil Biol. 2011, 47, 243–246. [Google Scholar] [CrossRef]
- Handayanto, E.; Cadish, G.; Giller, K.E. Nitrogen release from prunings of legume hedgerow trees in relation to quality of the prunings and incubation method. Plant Soil 1994, 160, 237–248. [Google Scholar] [CrossRef]
- Hartemink, A.; O’Sullivan, J.N. Leaf litter decomposition of Piper aduncum, Gliricidia sepium and Imperata cylindrica in the humid lowlands of Papua New Guinea. Plant Soil 2001, 230, 115–124. [Google Scholar] [CrossRef]
- Tiang, G.; Olimah, J.A.; Adeoye, G.O.; Kang, B.T. Regeneration of earthworm populations in a degraded soil by natural and planted fallows under humid tropical conditions. Soil Sci. Soc. Am. J. 2000, 64, 222–228. [Google Scholar] [CrossRef]
- Mwangi, M.; Mugendi, D.N.; Kung’u, J.B.; Swift, M.J.; Albrech, A. Soil invertebrate macrofauna composition within agroforestry and forested ecosystems and their role in litter decomposition in Embu, Kenya. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Bationo, A., Ed.; Academic Science Publisher: Brussels, Belgium, 2004; pp. 447–466. [Google Scholar]


| Block | Stone | pH | N | C | C:N | P | Exch K | Exch Ca | Exch Mg |
|---|---|---|---|---|---|---|---|---|---|
| % | % | Ratio | mg kg−1 | ||||||
| I | 78 | 7.8 | 0.89 | 6.4 | 7.2 | 28 | 530 | 872 | 352 |
| II | 60 | 7.8 | 0.98 | 5.0 | 5.1 | 45 | 565 | 824 | 328 |
| III | 79 | 7.9 | 0.99 | 7.2 | 7.3 | 81 | 457 | 1077 | 310 |
| IV | 79 | 7.9 | 0.96 | 6.1 | 6.4 | 111 | 517 | 1573 | 388 |
| Mean | 74 | 7.8 | 0.95 | 6.2 | 6.5 | 66 | 517 | 1086 | 345 |
| Species | Content (%) | Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | N | C | Lignin | Pp | Con. Tannin | Pp:N | (L + Pp):N | C:N | CT:N |
| Dry period | |||||||||
| Guazuma | 2.3 | 44 | 18 | 4.1 | 1.5 | 1.8 | 9.7 | 19 | 0.68 |
| Leucaena + G | 2.8 | 44 | 14 | 4.0 | 2.3 | 1.4 | 6.6 | 16 | 0.82 |
| Leucaena | 3.3 | 45 | 10 | 3.9 | 3.1 | 1.2 | 4.2 | 14 | 0.94 |
| Leucaena + M | 2.8 | 45 | 14 | 4.0 | 2.3 | 1.4 | 6.4 | 16 | 0.83 |
| Moringa | 2.1 | 42 | 10 | 2.2 | 0.2 | 1.0 | 5.8 | 20 | 0.10 |
| Wet period | |||||||||
| Guazuma | 1.8 | 44 | 12 | 2.4 | 2.9 | 1.3 | 8.1 | 25 | 1.61 |
| Leucaena + G | 2.4 | 44 | 13 | 2.9 | 2.7 | 1.2 | 6.5 | 18 | 1.13 |
| Leucaena | 3.2 | 45 | 14 | 3.4 | 2.6 | 1.1 | 5.5 | 14 | 0.83 |
| Leucaena + M | 2.9 | 44 | 13 | 2.7 | 1.5 | 0.9 | 5.2 | 15 | 0.53 |
| Moringa | 2.6 | 42 | 11 | 1.9 | 0.4 | 0.7 | 4.9 | 16 | 0.16 |
| Decomp. Parameter | %N | % Lignin (L) | Pp | CT | Pp/N | (L + Pp)/N | C/N | CT/N | L/N |
|---|---|---|---|---|---|---|---|---|---|
| Dry period | |||||||||
| KL | 0.68 | 0.01 | 0.55 | 0.70 | 0.01 | −0.33 | −0.75 | 0.68 | −0.39 |
| KR | 0.42 | 0.14 | 0.46 | 0.48 | 0.08 | −0.13 | −0.51 | 0.51 | −0.18 |
| CL/CR | −0.78 | −0.25 | −0.85 | −0.89 | −0.34 | 0.12 | 0.83 | −0.93 * | 0.20 |
| K | 0.64 | −0.51 | 0.10 | 0.50 | −0.33 | −0.63 | −0.58 | 0.35 | −0.65 |
| Wet period | |||||||||
| KL | −0.15 | −0.55 | −0.62 | −0.52 | −0.44 | −0.08 | 0.19 | −0.27 | −0.02 |
| KR | −0.48 | −0.92 * | −0.87 * | −0.51 | −0.37 | 0.09 | 0.39 | −0.18 | 0.15 |
| CL/CR | 0.34 | −0.58 | −0.60 | 0.97 ** | −0.97 ** | −0.78 | −0.52 | −0.92 * | −0.73 |
| K | 0.46 | −0.47 | −0.52 | −0.98 ** | −0.99 *** | −0.84 | −0.60 | −0.96 ** | −0.80 |
| Rate Constants | %N | % Lignin (L) | Pp | CT | Pp/N | (L + Pp)/N | C/N | CT/N | L/N |
|---|---|---|---|---|---|---|---|---|---|
| Dry period | |||||||||
| KnL | 0.22 | −0.86 * | −0.54 | −0.04 | −0.76 | −0.75 | −0.15 | −0.27 | −0.72 |
| KnR | 0.18 | 0.15 | 0.27 | 0.23 | 0.08 | −0.04 | −0.25 | 0.28 | −0.06 |
| NL/NR | −0.86 * | 0.16 | −0.58 | −0.85 * | 0.08 | 0.50 | 0.91 * | −0.78 | 0.57 |
| Kn | 0.13 | −0.01 | 0.11 | 0.14 | −0.07 | −0.14 | −0.19 | 0.02 | 0.15 |
| Wet period | |||||||||
| KnL | −0.11 | −0.51 | −0.58 | −0.50 | −0.44 | −0.09 | 0.16 | −0.027 | −0.04 |
| KnR | 0.01 | −0.79 | −0.77 | −0.86 * | −0.79 | −0.48 | −0.19 | −0.69 | −0.43 |
| NL/NR | 0.33 | −0.57 | −0.59 | −0.95 ** | −0.94 ** | −0.77 | −0.53 | −0.91 * | −0.73 |
| Kn | 0.33 | −0.60 | −0.63 | −0.99 *** | −0.98 ** | −0.76 | −0.49 | −0.92 * | −0.71 |
| Specie | Initial | Pruning Leaves | Initial N | %N and kg N Released ha−1 (Time in Weeks) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | ||||||||
| Dry Period | %N | kg ha−1 | kg ha−1 | % | kg | % | kg | % | kg | % | kg |
| Moringa | 2.1 | 919 | 19 | 22 | 4 | 41 | 8 | 62 | 12 | 81 | 15 |
| Leuc (M) | 2.8 | 3914 | 62 | 15 | 9 | 29 | 18 | 51 | 32 | 75 | 47 |
| Guazuma | 2.3 | 2681 | 110 | 0 | 0 | 12 | 13 | 44 | 48 | 56 | 62 |
| Leucaena | 3.3 | 4559 | 150 | 11 | 17 | 32 | 48 | 44 | 66 | 55 | 83 |
| Leuc (G) | 2.8 | 5653 | 158 | 4 | 6 | 14 | 22 | 35 | 55 | 63 | 100 |
| Wet period | |||||||||||
| Moringa | 2.6 | 919 | 24 | 64 | 15 | 67 | 16 | 88 | 21 | 88 | 21 |
| Guazuma | 1.8 | 2681 | 48 | 11 | 5 | 14 | 7 | 25 | 12 | 44 | 21 |
| Leuc (M) | 2.9 | 3914 | 114 | 43 | 49 | 47 | 54 | 55 | 63 | 65 | 74 |
| Leuc (G) | 2.4 | 5653 | 136 | 21 | 29 | 25 | 34 | 40 | 54 | 46 | 63 |
| Leucaena | 3.2 | 4559 | 146 | 26 | 38 | 36 | 53 | 42 | 61 | 52 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzec-Gamboa, M.d.C.; Álvarez-Rivera, O.O.; Ramírez y Avilés, L.; Solorio-Sánchez, F.J. Decomposition and Nitrogen Release Rates of Foliar Litter from Single and Mixed Agroforestry Species under Field Conditions. Agriculture 2023, 13, 222. https://doi.org/10.3390/agriculture13010222
Tzec-Gamboa MdC, Álvarez-Rivera OO, Ramírez y Avilés L, Solorio-Sánchez FJ. Decomposition and Nitrogen Release Rates of Foliar Litter from Single and Mixed Agroforestry Species under Field Conditions. Agriculture. 2023; 13(1):222. https://doi.org/10.3390/agriculture13010222
Chicago/Turabian StyleTzec-Gamboa, Magnolia del Carmen, Oscar Omar Álvarez-Rivera, Luis Ramírez y Avilés, and Francisco Javier Solorio-Sánchez. 2023. "Decomposition and Nitrogen Release Rates of Foliar Litter from Single and Mixed Agroforestry Species under Field Conditions" Agriculture 13, no. 1: 222. https://doi.org/10.3390/agriculture13010222





