Does Sodium Nitroprusside Alleviate Water Deficit Stress in Impatiens walleriana Shoots Grown In Vitro?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Media, Culture Conditions and Treatments Pattern
2.2. Analyses of Biochemical Parameters during Different SNP Treatments
2.2.1. Protective Metabolite: Free Proline Content
2.2.2. Oxidative Damage: Photosynthetic Pigments, MDA and H2O2 Content
2.2.3. Nonenzymatic Antioxidants: Total Polyphenol Content and Antioxidant Activity of Secondary Metabolites
2.2.4. Enzymatic Antioxidants: SOD, CAT and POX Activity
2.3. Statistical Analysis
3. Results
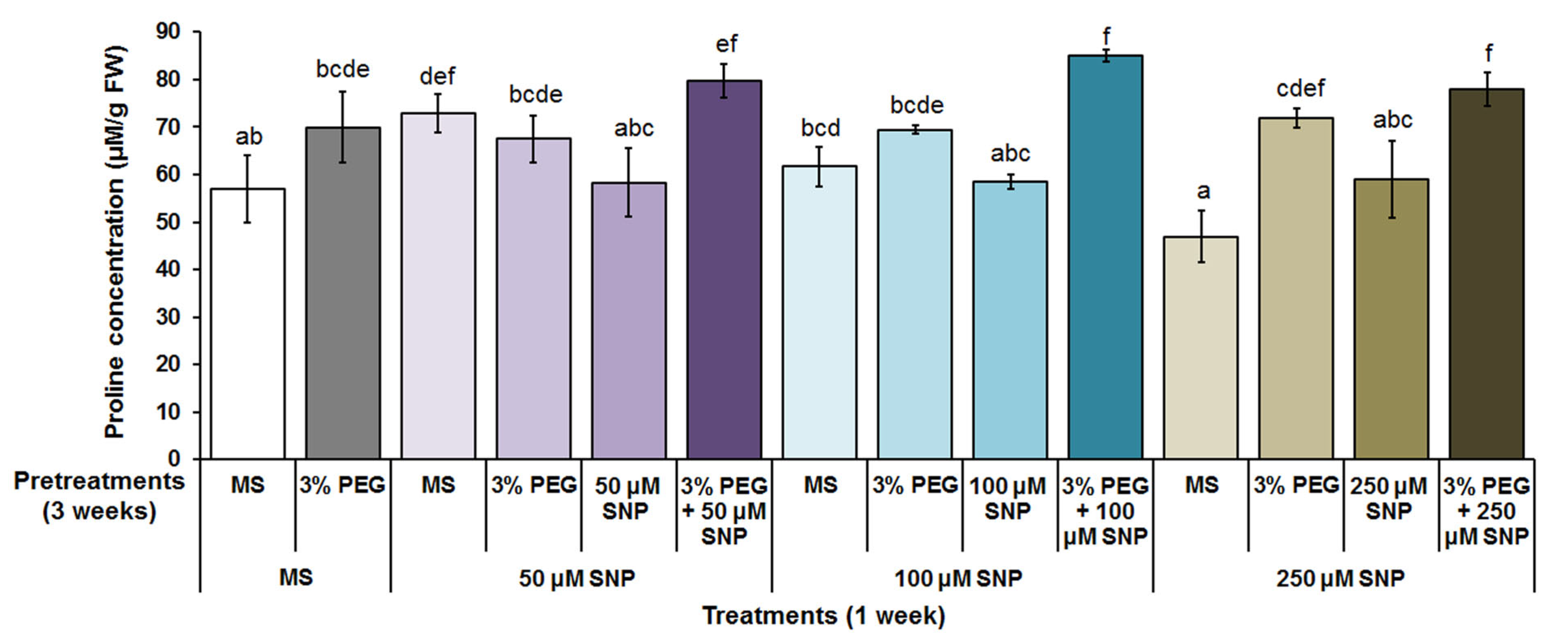
3.1. Free Proline Content
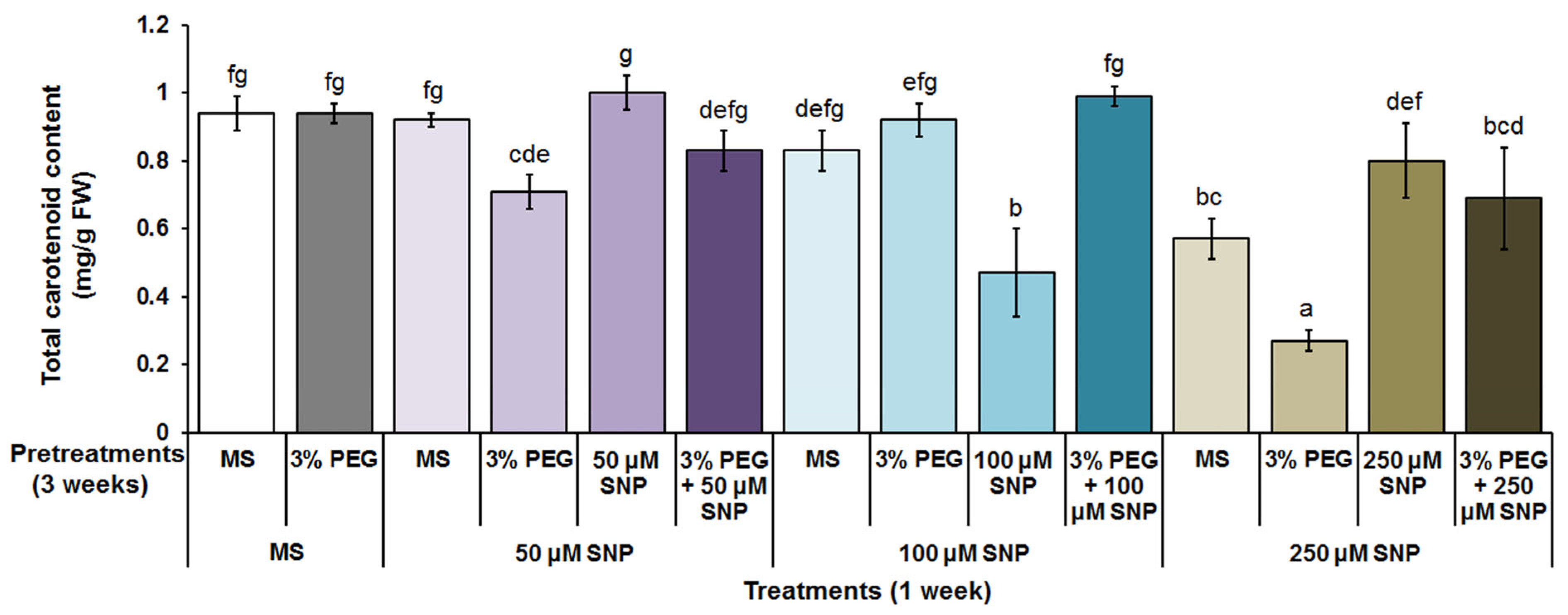
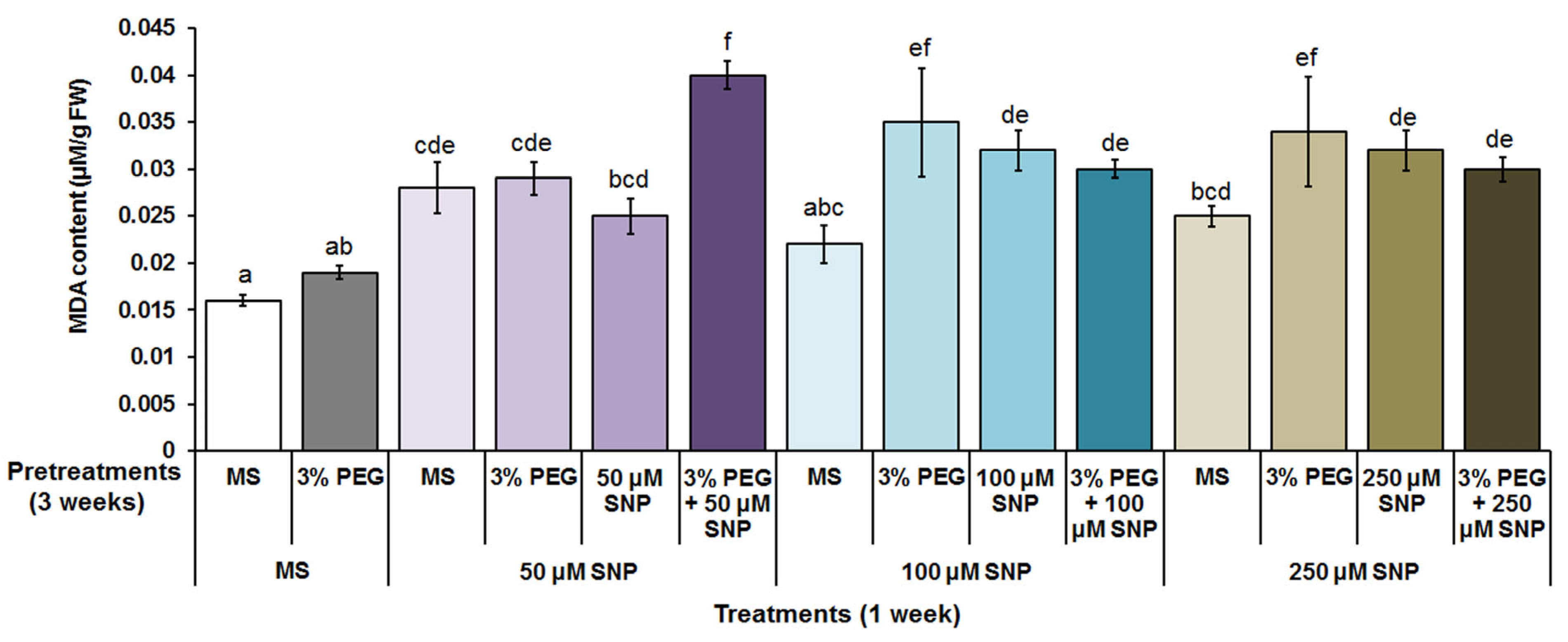
3.2. Photosynthetic Pigments, MDA and H2O2 Content
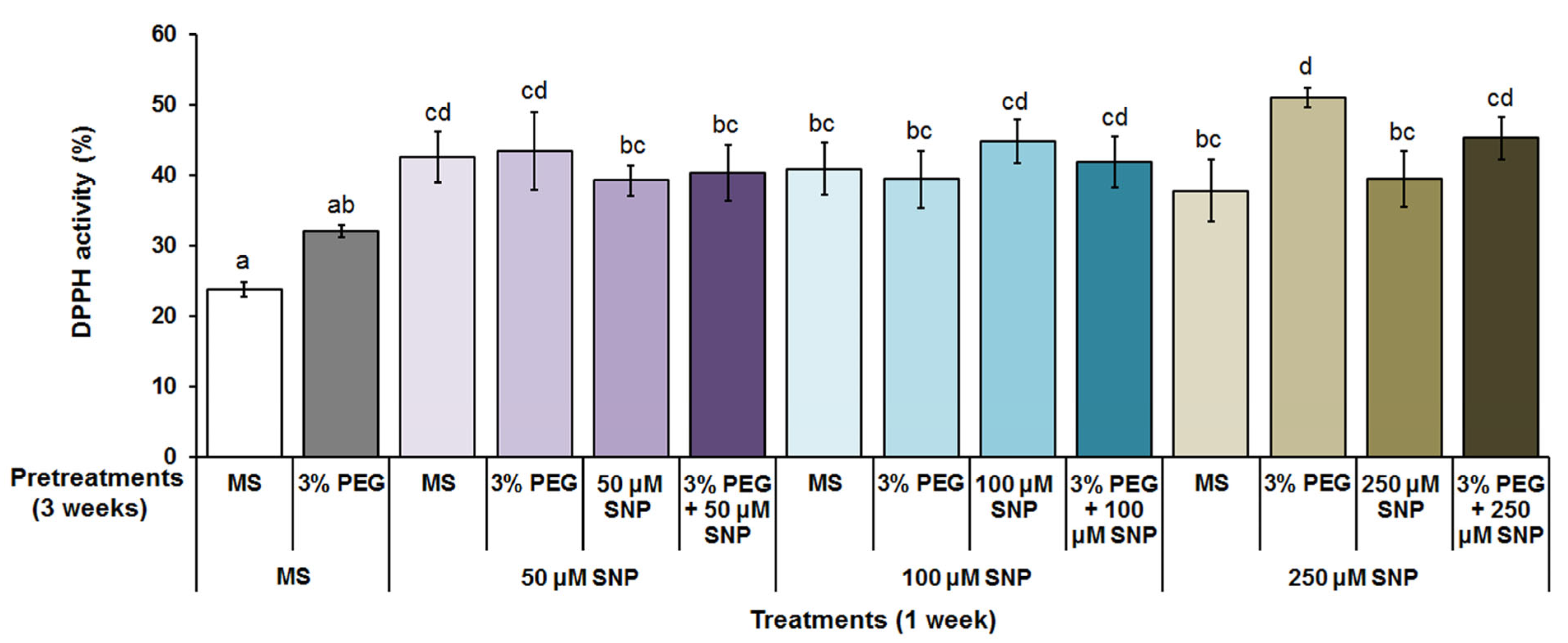
3.3. Total Polyphenol Content and Antioxidant Activity
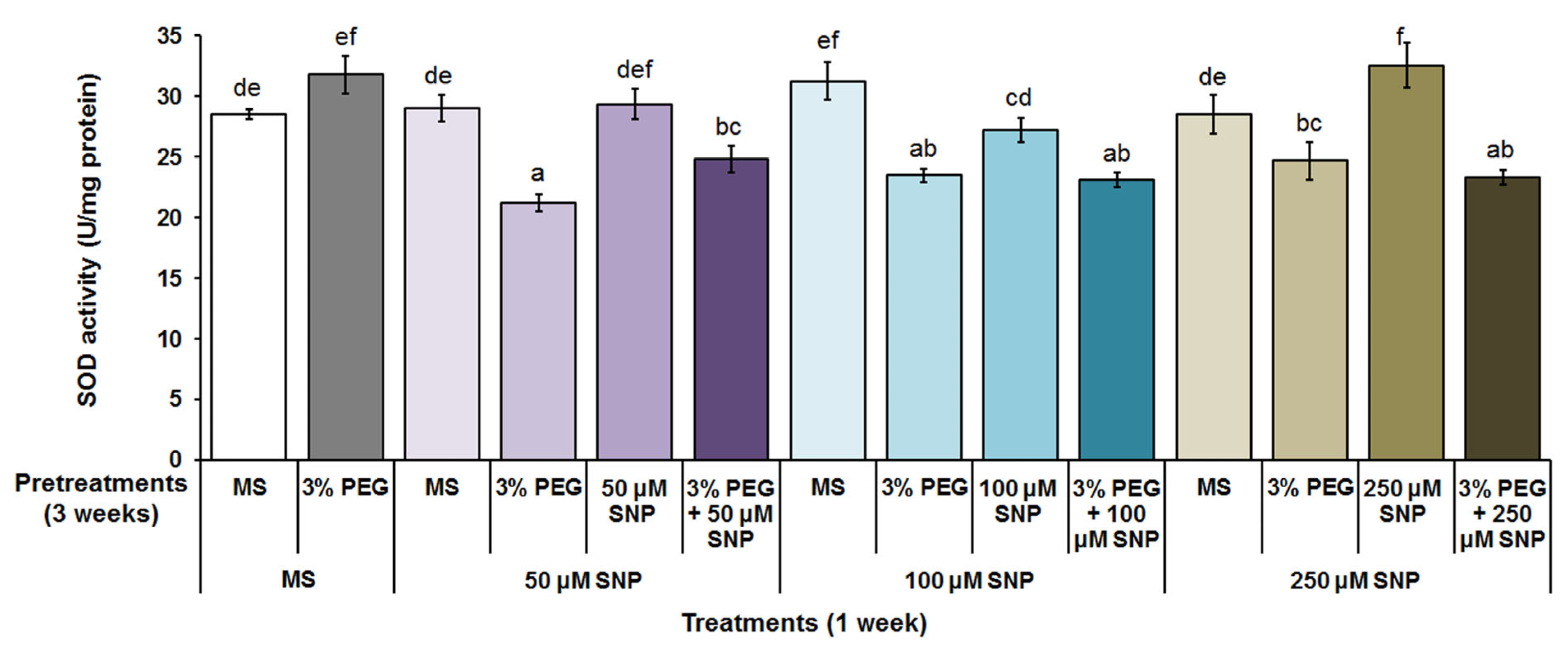
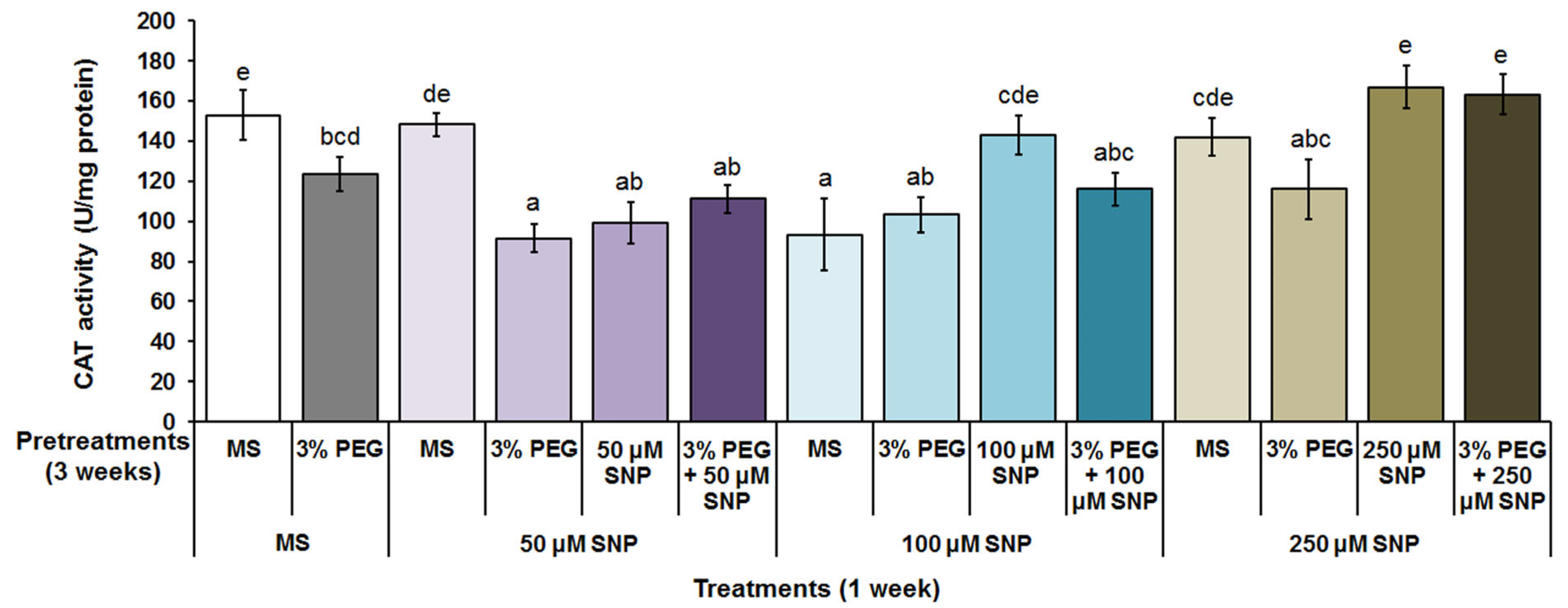
3.4. Superoxide Dismutase, Catalase and Peroxidase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Ayub, M.; Ayub, A.; Rasool, A. A review of morphological, physiological and biochemical responses of plants under drought stress conditions. Imp. J. Interdis Res. 2016, 2, 1600–1606. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and development responses of crop plants under drought stress: A review. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Zamaguchi-Shizonaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–22. [Google Scholar] [CrossRef]
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Interact. 2015, 10, 202–210. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio./Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitino, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between •OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges-A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Dvorak, P.; Krasylenko, Y.; Zeiner, A.; Samaj, J.; Takac, T. Signaling toward ROS-scavenging enzymes in plants. Front. Plant Sci. 2020, 11, 2178. [Google Scholar] [CrossRef]
- Cevahir, G.; Aytamka, E.; Erol, Ç. The role of nitric oxide in plants. Biotechnol. Biotechnol. Equip. 2007, 2, 13–17. [Google Scholar] [CrossRef]
- Santisree, P.; Adimulam, S.S.; Sharma, K.; Bhatnagar-Mathur, P.; Sharma, K.K. Insights into the Nitric Oxide Mediated Stress Tolerance in Plants. In Plant Signaling Molecules; Khan, M.I.R., Sudhakar Reddy, P., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 385–406. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalsky, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef]
- Chakraborty, N.; Acharya, K. “NO way”! Says the plant to abiotic stress. Plant Genome 2017, 11, 99–105. [Google Scholar] [CrossRef]
- Jangid, K.K.; Dwivedi, P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant 2017, 39, 73. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind. Crop. Prod. 2020, 158, 113034. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Tauseef, S.; Farid, G.; Khalid, I.; Mehmood, K. Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.B.; Knox, E.B.; Huysmans, S.; Smets, E.F.; Merckx, V.S. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: Result of a global climate change. Mol. Phylogenetics Evol. 2009, 52, 806–824. [Google Scholar] [CrossRef]
- Antonić, D.; Milošević, S.; Cingel, A.; Lojić, M.; Trifunović-Momčilov, M.; Petrić, M.; Subotić, A.; Simonović, A. Effects of exogenous salicylic acid on Impatiens walleriana L grown in vitro under polyethylene glycol-imposed drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Pires Jr, E.O.; Caleja, C.; Garcia, C.C.; Ferreira, I.C.; Barros, L. Current status of genus Impatiens: Bioactive compounds and natural pigments with health benefits. Trends Food Sci. Technol. 2021, 117, 106–124. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and metabolomic analysis of seedling-stage soybean responses to PEG-simulated drought stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Clemente, R.M.; Gόmez-Canedas, A. In vitro Tissue Culture, a Tool for the Study and Breeding of Plants Subjected to Abiotic Stress Conditions. In Recent Advances in Plant in Vitro Culture; Leva, A., Rinaldi, L.M.R., Eds.; IntechOpen: Rijeka, Croatia, 2007; pp. 91–98. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Amir, M.; Bano, H.; Chater, C.C.; Jellason, N.P. Clarity on frequently asked questions about drought measurements in plant physiology. Sci. Afr. 2020, 8, e00405. [Google Scholar] [CrossRef]
- Burnett, S.; van Iersel, M.; Thomas, P. PEG-8000 alters morphology and nutrient concentration of hydroponic Impatiens. HortScience 2005, 40, 1768–1772. [Google Scholar] [CrossRef]
- Liao, X.L.; Wen, G.Q.; Liu, Q.L.; Li, X.Y.; Wu, M.X.; Oan, Y.Z. The drought-stress response of a drought resistant Impatiens processed in PEG-6000 solution in a simulation test. arXiv 2016, arXiv:1611.04425. [Google Scholar] [CrossRef]
- Lau, S.E.; Hadman, V.F.; Pua, T.L.; Saidi, N.B.; Tan, B.C. Plant nitric oxide signaling under drought stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. PROTOCOL: Extraction and Determination of Proline. Prometheus, Protocols in Ecological & Environmental Science. 2011. Available online: https://prometheusprotocols.net/function/tissue-chemistry/primary-metabolites/extraction-and-determination-of-proline/ (accessed on 13 January 2021).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolation Chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kim, J.K.; Noh, J.H.; Lee, S.E.; Choi, J.S.; Suh, H.S.; Chung, H.Y.; Song, Y.O.; Choi, W.C. The first total synthesis of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB) and its antioxidant activity. Bull. Korean Chem. Soc. 2002, 23, 661–666. [Google Scholar] [CrossRef]
- Ðurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Cingel, A.; Vujičić, M.; Milošević, S. Morpho-physiological and molecular evaluation of drought and recovery in Impatiens walleriana grown ex vitro. Plants 2020, 9, 1559. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridowich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Kukavica, B.; Veljović-Jovanović, S. Senescence-related changes in antioxidant status of ginkgo and birch leaves during autumn yellowing. Physiol. Plant. 2004, 122, 321–327. [Google Scholar] [CrossRef]
- Milošević, S.; Simonović, A.; Cingel, A.; Jevremović, S.; Todorović, S.; Filipović, B.; Subotić, A. Response of antioxidative enzymes to long-term Tomato spotted wilt virus infection and virus elimination by meristem-tip culture in two Impatiens species. Physiol. Mol. Plant Pathol. 2012, 79, 79–88. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Cui, Z.H.; Bi, W.L.; Hao, X.Y.; Xu, Y.; Li, P.M.; Walker, M.A.; Wang, Q.C. Responses of in vitro-grown plantlets (Vitis vinifera) to grapevine leafroll-associated virus-3 and PEG-induced drought stress. Front. Physiol. 2016, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, C.A.; Gómez-Merino, F.C.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. In vitro screening of sugarcane cultivars (Saccharum spp. hybrids) for tolerance to polyethylene glycol-induced water stress. Agronomy 2021, 11, 598. [Google Scholar] [CrossRef]
- Martínez-Santos, E.; Cruz-Cruz, C.A.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. In vitro response of vanilla (Vanilla planifolia Jacks. ex Andrews) to PEG-induced osmotic stress. Sci. Rep. 2021, 11, 22611. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R. Sodium nitroprusside: Its beneficial role in drought stress tolerance of “Mexican lime” (Citrus aurantifolia (Christ.) Swingle) under in vitro conditions. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 155–168. [Google Scholar] [CrossRef]
- Meena, M.; Kumari, D.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Jiroutova, P.; Toman, J.; Dobrovolna, D.; Drbohlavova, L. Physiological responses of apple and cherry in vitro culture under different levels of drought stress. Agonomy 2020, 10, 1689. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Mitre, V. Response of the five highbush blueberry cultivars to in vitro induced drought stress by polyethylene glycol. Agronomy 2022, 12, 732. [Google Scholar] [CrossRef]
- García-Valenzuela, X.; García-Moya, E.; Rascón-Cruz, Q.; Herrera-Estrella, L.; Aguado-Santacruz, G.A. Chlorophyll accumulation is enhanced by osmotic stress in graminaceous Chlorophyllic cells. J. Plant Physiol. 2005, 162, 650–661. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, S.; Gómez-Merino, F.C.; Trejo-Téllez, L.I.; Tavitas-Fuentes, L.; Hernández-Aragón, L. Osmotic stress affects growth, content of Chlorophyll, abscisic acid, Na+, and K+, and expression of novel NAC genes in contrasting rice cultivars. Biol. Plant. 2018, 62, 307–317. [Google Scholar] [CrossRef]
- Khodabin, G.; Tahmasebi-Sarvestani, Z.; Rad, A.H.S.; Modarres-Sanavy, S.A.M. Effect of drought stress on certain morphological and physiological characteristics of a resistant and a sensitive canola cultivar. Chem. Biodivers. 2017, 17, e190039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Yang, C.; Du, S.; Yang, W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S. Afr. J. Bot. 2016, 105, 279–287. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, X.; Wen, T.; Liu, M.; Yang, M.; Chen, X. Implications of terminal oxidase function in regulation of salicylic acid on soybean seedling photosynthetic performance under water stress. Plant Physiol. Biochem. 2017, 112, 19–28. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; MohseniFard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and molecular aspects of two Thymus species differently sensitive to drought stress. BioTech 2022, 11, 8. [Google Scholar] [CrossRef]
- Karimi, A.; Tabari, M.; Javanmard, Z.; Bader, M.K.F. Drought effects on morpho-physiological and biochemical traits in persian oak and black poplar seedlings. Forests 2022, 13, 399. [Google Scholar] [CrossRef]
- Cechin, I.; Cardoso, G.S.; Fumis, T.F.; Corniani, N. Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Plant Prot. 2015, 74, 200–206. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Jothimani, K.; Arulbalachandra, D. Physiological and biochemical studies of black gram (Vigna mungo (L.) Hepper) under polyethylene glycol induced drought stress. Biocatal. Agric. Biotech. 2020, 29, 101777. [Google Scholar] [CrossRef]
- Jia, P.; Melnyk, A.; Li, L.; Kong, X.; Dai, H.; Zhang, Z.; Butenko, S. Effects of drought and rehydration on the growth and physiological characteristics of mustard seedlings. J. Cent. Eur. Agric. 2021, 22, 836–847. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J. Plant Interact. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Sahay, S.; Khan, E.; Gupta, M. Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants. Nitric Oxide 2019, 89, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, M.; Khan, M.K.; Pandey, A.; Ozdemir, C.; Avsaroglu, Z.Z.; Elbasan, F.; Omay, A.H.; Gezgin, S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. 3 Biotech 2020, 10, 494. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Filippou, P.; Diamantidis, G.; Vasilakakis, M.; Fotopoulos, V.; Molassiotis, A. Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol. Biochem. 2013, 38, 118–126. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Islam, M.J.; Uddin, M.J.; Anwar Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.S. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhao, F.; Yang, J.X.; Yang, H.Q. Nitric oxide alleviates lipid peroxidation induced by osmotic stress during senescence of detached leaves of Malus hupehensis Rehd. J. Hortic. Sci. Biotechnol. 2010, 85, 367–373. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhadi, N.; Nikpour-Rashidabad, N. Responses of in vitro-cultured Allium hirtifolium to exogenous sodium nitroprusside under PEG-imposed drought stress. Plant Cell Tissue Organ Cult. 2018, 133, 237–248. [Google Scholar] [CrossRef]
- Sekita, M.C.; Dias, D.C.F.S.; Pinheiro, D.T.; da Silva, A.L.; Matos, A.C.B.; da Silva, L.J. Nitric oxide in physiological potential and biochemical mechanisms of pea seeds under water deficit. J. Seed Sci. 2018, 44, e202244016. [Google Scholar] [CrossRef]
- Santisree, P.; Bhatnagar-Mathur, P.; Sharma, K.K. NO to drought multifunctional role of nitric oxide in plant drought: Do we have all the answers? Plant Sci. 2015, 239, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Effect of sodium niroprusside, putrescine and glycine betaine on alleviation of drought stress in cotton plant. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 1252–1265. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Khan, R.T.; Gerdezi, S.D.A.; Habib, T.; Abbas, S.R. DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity of tomato genotypes against peg simulated drought stress. Pak. J. Bot. 2019, 51, 1249–1253. [Google Scholar] [CrossRef]
- Siswoyo, T.A.; Arum, L.S.; Sanjaya, B.R.L.; Aisyah, Z.S. The growth responses and antioxidant capabilities of melinjo (Gnetum gnemon L.) in different durations of drought stress. Ann. Agric. Sci. 2021, 66, 81–86. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-stimulated drought stress improves the attributes of in vitro growth, steviol glycosides production, and antioxidant activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Sardoei, A.S.; Khalili, H. Nitric oxide signaling pathway in medicinal plants. Cell. Mol. Biomed. Rep. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Razzaq, A.; Bibi, Y.; Khan, S.U.; Abbasi, K.S.; Sher, A.; Mehmood, A.; Ahmed, W.; Mahmood, I.; Manaf, A.; et al. Water stress effects on biochemical traits and antioxidant activities of wheat (Triticum aestivum L.) under in vitro conditions. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 283–290. [Google Scholar] [CrossRef]
- Laus, M.N.; De Santis, M.A.; Flagella, Z.; Socci, M. Changes in antioxidant defence system in durum wheat under hyperosmotic stress: A concise overview. Plants 2022, 11, 98. [Google Scholar] [CrossRef]
- Li, C.; Wan, Y.; Shang, X.; Fang, S. Responses of microstructure, ultrastructure and antioxidant enzyme activity to PEG-induced drought stress in Cyclocarya paliurus seedlings. Forest 2022, 13, 836. [Google Scholar] [CrossRef]
- Murthy, S.M.; Devaraj, V.R.; Anitha, P.; Tejavathi, D.H. Studies on the activities of antioxidant enzymes under induced drought stress in in vivo and in vitro plants of Macrotyloma uniflorum (Lam.) Verdc. Recent Res. Sci. Technol. 2012, 4, 34–37. [Google Scholar]
- Silva, E.N.; Silveira, J.A.G.; Aragão, R.M.; Vieira, C.F.; Carvalho, F.E.L. Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol. Plant. 2019, 41, 4. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2020, 172, 809–819. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, X.; Long, Y.; Ji, X. Transcriptional analysis reveals sodium nitroprusside affects alfalfain response to PEG-induced osmotic stress at germination stage. Protoplasma 2020, 257, 1345–1358. [Google Scholar] [CrossRef]
- Sheikhaliyan, M.; Sohrabi, Y.; Hossainpanahi, F.; ShiraniRad, A. Effect of sodium nitroprusside on physiological traits and grain yield of oilseed rape (Brassica napus L.) under different irrigation regimes. Gesunde Pflanz. 2022, 74, 111–123. [Google Scholar] [CrossRef]
- Hatamzadeh, A.; Molaahmad Nalousi, A.; Ghasemnezhad, M.; Biglouei, M.H. The potential of nitric oxide for reducing oxidative damage induced by drought stress in two turfgrass species, creeping bentgrass and tall fescue. Grass Forage Sci. 2015, 70, 538–548. [Google Scholar] [CrossRef]
- Aalam, L.; Sedghi, M.; Sofalian, O. Sodium nitroprusside and salicylic acid decrease antioxidant enzymes activity in soybean. Iran. J. Plant Physiol. 2019, 10, 3073–3077. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurić, M.; Trifunović-Momčilov, M.; Milošević, S.; Marković, M.; Radulović, O.; Subotić, A.; Uzelac, B. Does Sodium Nitroprusside Alleviate Water Deficit Stress in Impatiens walleriana Shoots Grown In Vitro? Agriculture 2023, 13, 1903. https://doi.org/10.3390/agriculture13101903
Đurić M, Trifunović-Momčilov M, Milošević S, Marković M, Radulović O, Subotić A, Uzelac B. Does Sodium Nitroprusside Alleviate Water Deficit Stress in Impatiens walleriana Shoots Grown In Vitro? Agriculture. 2023; 13(10):1903. https://doi.org/10.3390/agriculture13101903
Chicago/Turabian StyleĐurić, Marija, Milana Trifunović-Momčilov, Snežana Milošević, Marija Marković, Olga Radulović, Angelina Subotić, and Branka Uzelac. 2023. "Does Sodium Nitroprusside Alleviate Water Deficit Stress in Impatiens walleriana Shoots Grown In Vitro?" Agriculture 13, no. 10: 1903. https://doi.org/10.3390/agriculture13101903







