Echoes of a Stressful Past: Abiotic Stress Memory in Crop Plants towards Enhanced Adaptation
Abstract
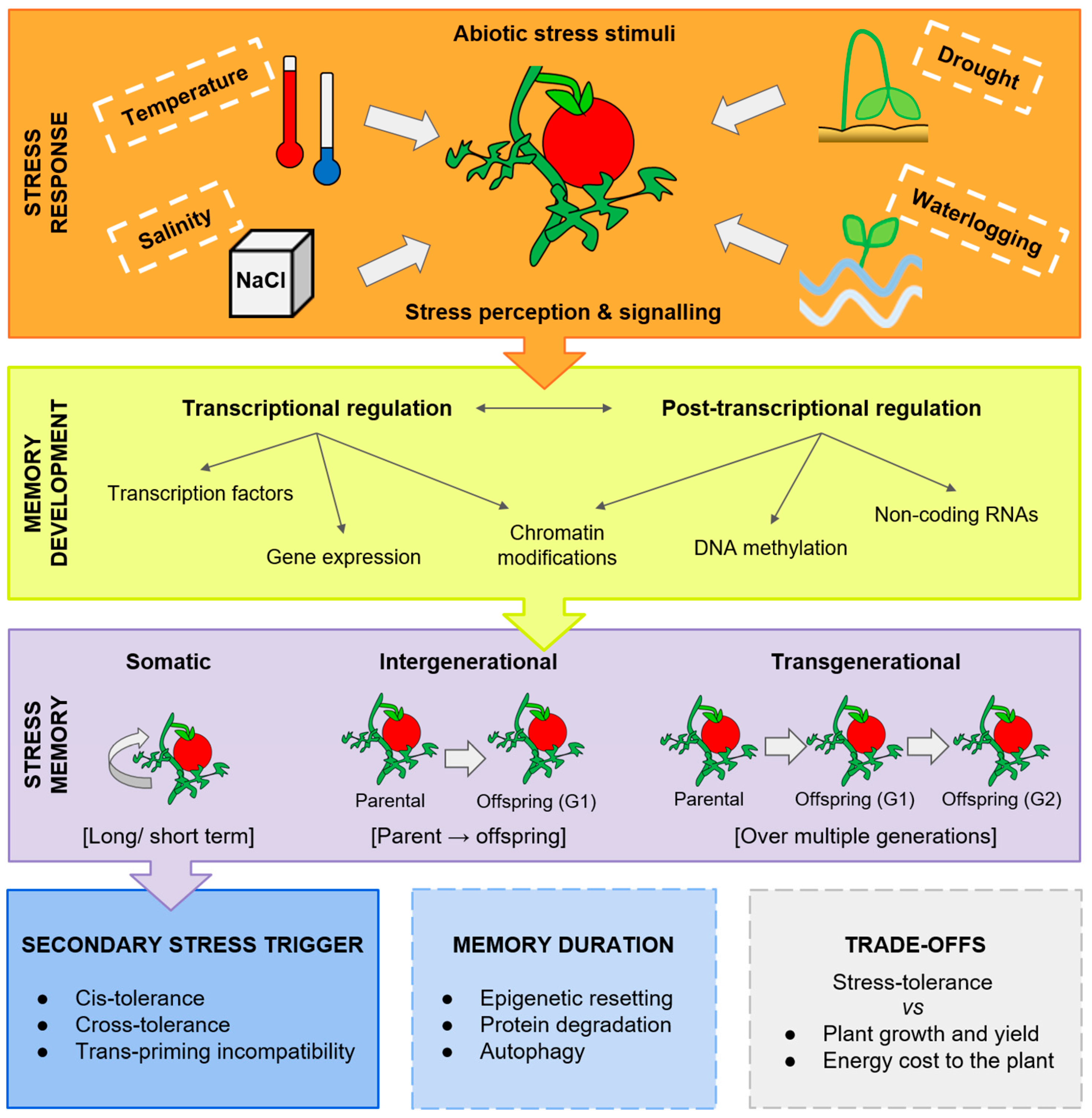
:1. Introduction
2. Development of Abiotic Stress Memory
3. Transgenerational Stress Memory
4. Abiotic Stress-Induced Memory of Crop Plants
4.1. Heat Stress
4.2. Low Temperatures
4.3. Drought
4.4. Waterlogging
4.5. Salinity
4.6. Cross-Tolerance and Stress Memory
5. Stress Memory Trade-Offs
6. The Art of Forgetting
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. IPCC Panel Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global Crop Yield Response to Extreme Heat Stress under Multiple Climate Change Futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant Responses to Flooding Stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Ganopoulos, I.; Tani, E.; Tsaftaris, A. Epigenetics, Epigenomics and Crop Improvement. Adv. Bot. Res. 2018, 86, 287–324. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant Developmental Responses to Climate Change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Arnholdt-Schmitt, B. Stress-Induced Cell Reprogramming. A Role for Global Genome Regulation? Plant Physiol. 2004, 136, 2579–2586. [Google Scholar] [CrossRef]
- Etalo, D.W.; Stulemeijer, I.J.E.; van Esse, H.P.; de Vos, R.C.H.; Bouwmeester, H.J.; Joosten, M.H.A.J. System-Wide Hypersensitive Response-Associated Transcriptome and Metabolome Reprogramming in Tomato. Plant Physiol. 2013, 162, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Toljamo, A.; Blande, D.; Kärenlampi, S.; Kokko, H. Reprogramming of Strawberry (Fragaria vesca) Root Transcriptome in Response to Phytophthora cactorum. PLoS ONE 2016, 11, e0161078. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Markovic, D.; Ninkovic, V.; Martinez, G. Molecular Mechanisms Regulating Priming and Stress Memory. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 247–265. [Google Scholar] [CrossRef]
- Gamir, J.; Sánchez-Bel, P.; Flors, V. Molecular and Physiological Stages of Priming: How Plants Prepare for Environmental Challenges. Plant Cell Rep. 2014, 33, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Priming Mediated Stress and Cross-Stress Tolerance in Plants: Concepts and Opportunities; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817893-5. [Google Scholar]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-Generation Systemic Acquired Resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The “Prime-Ome”: Towards a Holistic Approach to Priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, Maintaining and Modifying DNA Methylation Patterns in Plants and Animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Annacondia, M.L.; Magerøy, M.H.; Martinez, G. Stress Response Regulation by Epigenetic Mechanisms: Changing of the Guards. Physiol. Plant. 2018, 162, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gutzat, R.; Mittelsten Scheid, O. Epigenetic Responses to Stress: Triple Defense? Curr. Opin. Plant Biol. 2012, 15, 568–573. [Google Scholar] [CrossRef]
- Baluska, F.; Gagliano, M.; Witzany, G. (Eds.) Memory and Learning in Plants; Signaling and Communication in Plants; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-75595-3. [Google Scholar]
- Zhang, H.; Lang, Z.; Zhu, J.-K.K. Dynamics and Function of DNA Methylation in Plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.A.; Mosher, R.A. RNA-Directed DNA Methylation: An Epigenetic Pathway of Increasing Complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Liu, C.; Lu, F.; Cui, X.; Cao, X. Histone Methylation in Higher Plants. Annu. Rev. Plant Biol. 2010, 61, 395–420. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Mirouze, M.; Paszkowski, J. Epigenetic Contribution to Stress Adaptation in Plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Ferreira, L.; Maroco, J.; Oliveira, M.M. Abiotic Stress and Induced DNA Hypomethylation Cause Interphase Chromatin Structural Changes in Rice rDNA Loci. Cytogenet. Genome Res. 2011, 132, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H. Senescence, Ageing and Death of the Whole Plant. New Phytol. 2013, 197, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Dinh, H.Q.; Baubec, T.; Rosa, M.; Lettner, N.; Scheid, O.M. Epigenetic Regulation of Repetitive Elements Is Attenuated by Prolonged Heat Stress in Arabidopsis. Plant Cell 2010, 22, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Pandey-Rai, S.; Rai, K.K.; Tiwari, A.; Pandey, N. Molecular and Epigenetic Basis of Heat Stress Responses and Acclimatization in Plants. Nucleus 2023, 66, 69–79. [Google Scholar] [CrossRef]
- Kim, J.-M.; To, T.K.; Ishida, J.; Matsui, A.; Kimura, H.; Seki, M. Transition of Chromatin Status During the Process of Recovery from Drought Stress in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 847–856. [Google Scholar] [CrossRef]
- van Dijk, K.; Ding, Y.; Malkaram, S.; Riethoven, J.J.M.; Liu, R.; Yang, J.; Laczko, P.; Chen, H.; Xia, Y.; Ladunga, I.; et al. Dynamic Changes in Genome-Wide Histone H3 Lysine 4 Methylation Patterns in Response to Dehydration Stress in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 238. [Google Scholar] [CrossRef]
- Ding, B.; Bellizzi, M.R.; Ning, Y.; Meyers, B.C.; Wang, G.-L. HDT701, a Histone H4 Deacetylase, Negatively Regulates Plant Innate Immunity by Modulating Histone H4 Acetylation of Defense-Related Genes in Rice. Plant Cell 2012, 24, 3783–3794. [Google Scholar] [CrossRef]
- Shen, Y.; Conde e Silva, N.; Audonnet, L.; Servet, C.; Wei, W.; Zhou, D.-X. Over-Expression of Histone H3K4 Demethylase Gene JMJ15 Enhances Salt Tolerance in Arabidopsis. Front. Plant Sci. 2014, 5, 290. [Google Scholar] [CrossRef]
- Wibowo, A.; Becker, C.; Marconi, G.; Durr, J.; Price, J.; Hagmann, J.; Papareddy, R.; Putra, H.; Kageyama, J.; Becker, J.; et al. Hyperosmotic Stress Memory in Arabidopsis Is Mediated by Distinct Epigenetically Labile Sites in the Genome and Is Restricted in the Male Germline by Dna Glycosylase Activity. eLife 2016, 5, e13546. [Google Scholar] [CrossRef]
- Lamke, J.; Brzezinka, K.; Altmann, S.; Baurle, I.; Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A Hit-and-Run Heat Shock Factor Governs Sustained Histone Methylation and Transcriptional Stress Memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef]
- Sani, E.; Herzyk, P.; Perrella, G.; Colot, V.; Amtmann, A. Hyperosmotic Priming of Arabidopsis Seedlings Establishes a Long-Term Somatic Memory Accompanied by Specific Changes of the Epigenome. Genome Biol. 2013, 14, R59. [Google Scholar] [CrossRef] [PubMed]
- Galviz, Y.C.F.; Ribeiro, R.V.; Souza, G.M. Yes, Plants Do Have Memory. Theor. Exp. Plant Physiol. 2020, 32, 195–202. [Google Scholar] [CrossRef]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for Crop Improvement in Times of Global Change. Biology 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Springer, N.M.; Schmitz, R.J. Exploiting Induced and Natural Epigenetic Variation for Crop Improvement. Nat. Rev. Genet. 2017, 18, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Turgut-Kara, N.; Arikan, B.; Celik, H. Epigenetic Memory and Priming in Plants. Genetica 2020, 148, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Moschou, P.N. Phenotypic Novelty by CRISPR in Plants. Dev. Biol. 2018, 435, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, P.C.; Sivasubramaniam, K.; Dadlani, M. Seed Dormancy and Regulation of Germination. In Seed Science and Technology: Biology, Production, Quality; Dadlani, M., Yadava, D.K., Eds.; Springer Nature: Singapore, 2023; pp. 39–66. ISBN 978-981-19588-8-5. [Google Scholar]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed Priming Can Enhance and Retain Stress Tolerance in Ensuing Generations by Inducing Epigenetic Changes and Trans-generational Memory. Physiol. Plant. 2023, 175, e13881. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- De Oliveira Sousa, A.R.; Ribas, R.F.; Filho, M.A.C.; Freschi, L.; Ferreira, C.F.; Filho, W.D.S.S.; Pérez-Molina, J.P.; da Silva Gesteira, A. Drought Tolerance Memory Transmission by Citrus Buds. Plant Sci. 2022, 320, 111292. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, A. Transgenerational Stress-Adaption: An Opportunity for Ecological Epigenetics. Plant Cell Rep. 2018, 37, 3–9. [Google Scholar] [CrossRef]
- Sanyal, R.P.; Misra, H.S.; Saini, A. Heat-Stress Priming and Alternative Splicing-Linked Memory. J. Exp. Bot. 2018, 69, 2431–2434. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “Memories” of Plants: Evidence and Possible Mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Avramova, V.; AbdElgawad, H.; Vasileva, I.; Petrova, A.S.; Holek, A.; Mariën, J.; Asard, H.; Beemster, G.T.S. High Antioxidant Activity Facilitates Maintenance of Cell Division in Leaves of Drought Tolerant Maize Hybrids. Front. Plant Sci. 2017, 8, 84. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and Chromatin-Based Mechanisms in Environmental Stress Adaptation and Stress Memory in Plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Raj, S.; Bräutigam, K.; Hamanishi, E.T.; Wilkins, O.; Thomas, B.R.; Schroeder, W.; Mansfield, S.D.; Plant, A.L.; Campbell, M.M. Clone History Shapes Populus Drought Responses. Proc. Natl. Acad. Sci. USA 2011, 108, 12521–12526. [Google Scholar] [CrossRef]
- González, A.P.R.; Dumalasová, V.; Rosenthal, J.; Skuhrovec, J.; Latzel, V. The Role of Transgenerational Effects in Adaptation of Clonal Offspring of White Clover (Trifolium repens) to Drought and Herbivory. Evol. Ecol. 2017, 31, 345–361. [Google Scholar] [CrossRef]
- González, A.P.R.; Chrtek, J.; Dobrev, P.I.; Dumalasová, V.; Fehrer, J.; Mráz, P.; Latzel, V. Stress-Induced Memory Alters Growth of Clonal Offspring of White Clover (Trifolium repens). Am. J. Bot. 2016, 103, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.J.F.; Jansen, J.J.; van Dijk, P.J.; Biere, A. Stress-Induced DNA Methylation Changes and Their Heritability in Asexual Dandelions. New Phytol. 2010, 185, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Morota, G.; Rosa, G.J.M.; Gianola, D. Prediction of Plant Height in Arabidopsis thaliana Using DNA Methylation Data. Genetics 2015, 201, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Avramova, Z. Transcriptional ‘Memory’ of a Stress: Transient Chromatin and Memory (Epigenetic) Marks at Stress-Response Genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Brzezinka, K.; Altmann, S.; Czesnick, H.; Nicolas, P.; Gorka, M.; Benke, E.; Kabelitz, T.; Jähne, F.; Graf, A.; Kappel, C.; et al. Arabidopsis FORGETTER1 Mediates Stress-Induced Chromatin Memory through Nucleosome Remodeling. eLife 2016, 5, e17061. [Google Scholar] [CrossRef] [PubMed]
- Bäurle, I. Can’t Remember to Forget You: Chromatin-Based Priming of Somatic Stress Responses. Semin. Cell Dev. Biol. 2018, 83, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bäurle, I.; Trindade, I. Chromatin Regulation of Somatic Abiotic Stress Memory. J. Exp. Bot. 2020, 71, 5269–5279. [Google Scholar] [CrossRef]
- Mozgova, I.; Mikulski, P.; Pecinka, A.; Farrona, S. Epigenetic Mechanisms of Abiotic Stress Response and Memory in Plants. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants; Alvarez-Venegas, R., De-la-Peña, C., Casas-Mollano, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–64. ISBN 978-3-030-14760-0. [Google Scholar]
- Roberts, M.R.; López Sánchez, A. Plant Epigenetic Mechanisms in Response to Biotic Stress. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants, 2nd ed.; Springer: Cham, Switzerland, 2019; pp. 65–113. ISBN 978-3-030-14760-0. [Google Scholar]
- Liu, Y.; Wang, J.; Liu, B.; Xu, Z. Dynamic Regulation of DNA Methylation and Histone Modifications in Response to Abiotic Stresses in Plants. J. Integr. Plant Biol. 2022, 64, 2252–2274. [Google Scholar] [CrossRef] [PubMed]
- Cocciolone, S.M.; Nettleton, D.; Snook, M.E.; Peterson, T. Transformation of Maize with the P1 Transcription Factor Directs Production of Silk Maysin, a Corn Earworm Resistance Factor, in Concordance with a Hierarchy of Floral Organ Pigmentation. Plant Biotechnol. J. 2005, 3, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, R.S.; Chopra, S. Progressive Loss of DNA Methylation Releases Epigenetic Gene Silencing from a Tandemly Repeated Maize Myb Gene. Genetics 2009, 181, 81–91. [Google Scholar] [CrossRef]
- O’Malley, R.C.; Huang, S.-S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against Environmental Challenges and Proteomics in Plants: Update and Agricultural Perspectives. Front. Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Singh, H.B. Advances in Seed Priming; Springer: Singapore, 2018; ISBN 9789811300325. [Google Scholar]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling Chemical Priming Machinery in Plants: The Role of Reactive Oxygen–Nitrogen–Sulfur Species in Abiotic Stress Tolerance Enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lin, K.H.; Ho, S.L.; Chiang, C.M.; Yang, C.M. Enhancing the Abiotic Stress Tolerance of Plants: From Chemical Treatment to Biotechnological Approaches. Physiol. Plant. 2018, 164, 452–466. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular Priming as an Approach to Induce Tolerance against Abiotic and Oxidative Stresses in Crop Plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef] [PubMed]
- Bilichak, A.; Kovalchuk, I. Transgenerational Response to Stress in Plants and Its Application for Breeding. J. Exp. Bot. 2016, 67, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Seki, M. Epigenetic Memory for Stress Response and Adaptation in Plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Boyko, A.; Kovalchuk, I. Epigenetic Control of Plant Stress Response. Environ. Mol. Mutagen. 2008, 49, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Paszkowski, J. Epigenetic Memory in Plants. EMBO J. 2014, 33, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Daxinger, L.; Whitelaw, E. Transgenerational Epigenetic Inheritance: More Questions than Answers. Genome Res. 2010, 20, 1623–1628. [Google Scholar] [CrossRef]
- Slotkin, R.K.; Martienssen, R. Transposable Elements and the Epigenetic Regulation of the Genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Jacob, Y.; Susaki, D.; LeBlanc, C.; Buendía, D.; Axelsson, E.; Kawashima, T.; Voigt, P.; Boavida, L.; Becker, J.; et al. Targeted Reprogramming of H3K27me3 Resets Epigenetic Memory in Plant Paternal Chromatin. Nat. Cell Biol. 2020, 22, 621–629. [Google Scholar] [CrossRef]
- Perrone, A.; Martinelli, F. Plant Stress Biology in Epigenomic Era. Plant Sci. 2020, 294, 110376. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Kovalchuk, I. Transgenerational Response to Stress in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 995–998. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering Plant Memory: Intersections between Stress Recovery, RNA Turnover, and Epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Kovalchuk, I. Genome Instability and Epigenetic Modification–Heritable Responses to Environmental Stress? Curr. Opin. Plant Biol. 2011, 14, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, O.; Reinders, J.; Čaikovski, M.; Smathajitt, C.; Paszkowski, J. Transgenerational Stability of the Arabidopsis Epigenome Is Coordinated by CG Methylation. Cell 2007, 130, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Kotkar, H.; Giri, A. Plant Epigenetics and the ‘Intelligent’ Priming System to Combat Biotic Stress; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 1, ISBN 978-0-12-817964-2. [Google Scholar]
- McCarrey, J.R. The Epigenome as a Target for Heritable Environmental Disruptions of Cellular Function. Mol. Cell. Endocrinol. 2012, 354, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Molinier, J.; Ries, G.; Zipfel, C.; Hohn, B. Transgeneration Memory of Stress in Plants. Nature 2006, 442, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Bilichak, A.; Ilnystkyy, Y.; Hollunder, J.; Kovalchuk, I. The Progeny of Arabidopsis thaliana Plants Exposed to Salt Exhibit Changes in DNA Methylation, Histone Modifications and Gene Expression. PLoS ONE 2012, 7, e30515. [Google Scholar] [CrossRef] [PubMed]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread Dynamic DNA Methylation in Response to Biotic Stress. Proc. Natl. Acad. Sci. USA 2012, 109, E2183–E2191. [Google Scholar] [CrossRef] [PubMed]
- Grativol, C.; Hemerly, A.S.; Ferreira, P.C.G. Genetic and Epigenetic Regulation of Stress Responses in Natural Plant Populations. Biochim. Biophys. Acta—Gene Regul. Mech. 2012, 1819, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Mittelsten Scheid, O. Stress-Induced Chromatin Changes: A Critical View on Their Heritability. Plant Cell Physiol. 2012, 53, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Eichten, S.R.; Briskine, R.; Song, J.; Li, Q.; Swanson-Wagner, R.; Hermanson, P.J.; Waters, A.J.; Starr, E.; West, P.T.; Tiffin, P.; et al. Epigenetic and Genetic Influences on DNA Methylation Variation in Maize Populations. Plant Cell 2013, 25, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.-T.M.-T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational Epigenetic Inheritance in Plants. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2011, 1809, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, J.; Grossniklaus, U. Selected Aspects of Transgenerational Epigenetic Inheritance and Resetting in Plants. Curr. Opin. Plant Biol. 2011, 14, 195–203. [Google Scholar] [CrossRef]
- Sung, S.; Amasino, R.M. Vernalization in Arabidopsis thaliana Is Mediated by the PHD Finger Protein VIN3. Nature 2004, 427, 159–164. [Google Scholar] [CrossRef]
- Pecinka, A.; Rosa, M.; Schikora, A.; Berlinger, M.; Hirt, H.; Luschnig, C.; Scheid, O.M. Transgenerational Stress Memory Is Not a General Response in Arabidopsis. PLoS ONE 2009, 4, e5202. [Google Scholar] [CrossRef]
- Yadav, N.S.; Titov, V.; Ayemere, I.; Byeon, B.; Ilnytskyy, Y.; Kovalchuk, I. Multigenerational Exposure to Heat Stress Induces Phenotypic Resilience, and Genetic and Epigenetic Variations in Arabidopsis thaliana Offspring. Front. Plant Sci. 2022, 13, 728167. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Fischer, M.; Colot, V.; Bossdorf, O. Epigenetic Variation Creates Potential for Evolution of Plant Phenotypic Plasticity. New Phytol. 2013, 197, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, T.J.M.; Silveira, A.B.; Gilbault, E.; Jiménez-Gómez, J.M.; Martin, A.; Bach, L.; Tisné, S.; Quadrana, L.; Loudet, O.; Colot, V. Mild Drought in the Vegetative Stage Induces Phenotypic, Gene Expression, and DNA Methylation Plasticity in Arabidopsis but No Transgenerational Effects. J. Exp. Bot. 2020, 71, 3588–3602. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.M.; Almeida, L.A.D.H.; Santana-Vieira, D.D.S.; Freschi, L.; Ferreira, C.F.; Soares Filho, W.D.S.; Costa, M.G.C.; Micheli, F.; Coelho Filho, M.A.; Gesteira, A.D.S. Recurrent Water Deficit Causes Epigenetic and Hormonal Changes in Citrus Plants. Sci. Rep. 2017, 7, 13684. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Chae, S.; Oh, N.-I.; Nguyen, N.H.; Cheong, J.-J. Recurrent Drought Conditions Enhance the Induction of Drought Stress Memory Genes in Glycine max L. Front. Genet. 2020, 11, 576086. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, K.; Sun, Y.; Chen, B.; Pan, Z.; WANG, Z.; Pang, B.; He, S.; Miao, Y.; Du, X. Physiological and Transcriptional Analyses Reveal Formation of Memory under Recurring Drought Stresses in Gossypium Hirsutum. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought Priming Improves Subsequent More Severe Drought in a Drought-Sensitive Cultivar of Olive Cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, H.; Wang, L.; Liu, H.; Huo, H.; Zhang, C.; Liu, A.; Zhu, A.; Hu, J.; Lin, Y.; et al. Physiological and Transcriptome Analyses Reveal Short-Term Responses and Formation of Memory under Drought Stress in Rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Auler, P.A.; Nogueira do Amaral, M.; Bolacel Braga, E.J.; Maserti, B. Drought Stress Memory in Rice Guard Cells: Proteome Changes and Genomic Stability of DNA. Plant Physiol. Biochem. 2021, 169, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Gu, Q.; Duan, L.; Liu, G.; Yuan, P.; Li, H.; Wu, Z.; Liu, W.; Huang, P.; Liu, L. Genome-Wide Bisulphite Sequencing Uncovered the Contribution of DNA Methylation to Rice Short-Term Drought Memory Formation. J. Plant Growth Regul. 2022, 41, 2903–2917. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Yi, J.; Yang, Y.; Lei, C.; Gong, M. Transcriptome Response to Drought, Rehydration and Re-Dehydration in Potato. Int. J. Mol. Sci. 2020, 21, 159. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving Potato Drought Tolerance through the Induction of Long-Term Water Stress Memory. Plant Sci. 2015, 238, 26–32. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Antioxidant Response of Wheat Roots to Drought Acclimation. Protoplasma 2010, 245, 153–163. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved Tolerance to Drought Stress after Anthesis Due to Priming before Anthesis in Wheat (Triticum aestivum L.) Var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef]
- Yue, H.; Zhang, H.; Su, N.; Sun, X.; Zhao, Q.; Weining, S.; Nie, X.; Yue, W. Integrate Small RNA and Degradome Sequencing to Reveal Drought Memory Response in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 5917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Tankari, M.; Wang, C.; Ma, H.; Li, X.; Li, L.; Soothar, R.K.; Cui, N.; Zaman-Allah, M.; Hao, W.; Liu, F.; et al. Drought Priming Improved Water Status, Photosynthesis and Water Productivity of Cowpea during Post-Anthesis Drought Stress. Agric. Water Manag. 2021, 245, 106565. [Google Scholar] [CrossRef]
- Pagay, V.; Furlan, T.S.; Kidman, C.M.; Nagahatenna, D. Long-Term Drought Adaptation of Unirrigated Grapevines (Vitis vinifera L.). Theor. Exp. Plant Physiol. 2022, 34, 215–225. [Google Scholar] [CrossRef]
- Racette, K.; Rowland, D.; Tillman, B.; Erickson, J.; Munoz, P.; Vermerris, W. Transgenerational Stress Memory in Seed and Seedling Vigor of Peanut (Arachis hypogaea L.) Varies by Genotype. Environ. Exp. Bot. 2019, 162, 541–549. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Siecińska, J.; Śmiech, M.; Nosalewicz, M.; Wiącek, D.; Pecio, A.; Wach, D. Transgenerational Effects of Temporal Drought Stress on Spring Barley Morphology and Functioning. Environ. Exp. Bot. 2016, 131, 120–127. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Li, M.; Lou, Q.; Xia, H.; Wang, P.; Li, T.; Liu, H.; Luo, L. Transgenerational Variations in DNA Methylation Induced by Drought Stress in Two Rice Varieties with Distinguished Difference to Drought Resistance. PLoS ONE 2013, 8, e80253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational Epimutations Induced by Multi-Generation Drought Imposition Mediate Rice Plant’s Adaptation to Drought Condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental Drought-Priming Enhances Tolerance to Post-Anthesis Drought in Offspring of Wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Kambona, C.M.; Koua, P.A.; Léon, J.; Ballvora, A. Intergenerational and Transgenerational Effects of Drought Stress on Winter Wheat (Triticum aestivum L.). Physiol. Plant. 2023, 175, e13951. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A.; Shahid, M. Terminal Drought and Seed Priming Improves Drought Tolerance in Wheat. Physiol. Mol. Biol. Plants 2018, 24, 845–856. [Google Scholar] [CrossRef]
- Wang, X.; Xin, C.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Heat Priming Induces Trans-Generational Tolerance to High Temperature Stress in Wheat. Front. Plant Sci. 2016, 7, 501. [Google Scholar] [CrossRef]
- Bilichak, A.; Ilnytskyy, Y.; Wóycicki, R.; Kepeshchuk, N.; Fogen, D.; Kovalchuk, I. The Elucidation of Stress Memory Inheritance in Brassica Rapa Plants. Front. Plant Sci. 2015, 6, 5. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.-L.; Jiang, D. Priming: A Promising Strategy for Crop Production in Response to Future Climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat Priming during Early Reproductive Stages Enhances Thermo-Tolerance to Post-Anthesis Heat Stress via Improving Photosynthesis and Plant Productivity in Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple Heat Priming Enhances Thermo-Tolerance to a Later High Temperature Stress via Improving Subcellular Antioxidant Activities in Wheat Seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Q.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and Transcriptional Analyses of Induced Post-Anthesis Thermo-Tolerance by Heat-Shock Pretreatment on Germinating Seeds of Winter Wheat. Environ. Exp. Bot. 2016, 131, 181–189. [Google Scholar] [CrossRef]
- Xin, C.; Wang, X.; Cai, J.; Zhou, Q.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Changes of Transcriptome and Proteome Are Associated with the Enhanced Post-Anthesis High Temperature Tolerance Induced by Pre-Anthesis Heat Priming in Wheat. Plant Growth Regul. 2016, 79, 135–145. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef]
- Lu, J.; Nawaz, M.A.; Wei, N.; Cheng, F.; Bie, Z. Suboptimal Temperature Acclimation Enhances Chilling Tolerance by Improving Photosynthetic Adaptability and Osmoregulation Ability in Watermelon. Hortic. Plant J. 2020, 6, 49–60. [Google Scholar] [CrossRef]
- Imin, N.; Kerim, T.; Rolfe, B.G.; Weinman, J.J. Effect of Early Cold Stress on the Maturation of Rice Anthers. Proteomics 2004, 4, 1873–1882. [Google Scholar] [CrossRef]
- Streb, P.; Aubert, S.; Gout, E.; Feierabend, J.; Bligny, R. Cross Tolerance to Heavy-Metal and Cold-Induced Photoinhibiton in Leaves of Pisum sativum Acclimated to Low Temperature. Physiol. Mol. Biol. Plants 2008, 14, 185–193. [Google Scholar] [CrossRef]
- Tanou, G.; Minas, I.S.; Scossa, F.; Belghazi, M.; Xanthopoulou, A.; Ganopoulos, I.; Madesis, P.; Fernie, A.; Molassiotis, A. Exploring Priming Responses Involved in Peach Fruit Acclimation to Cold Stress. Sci. Rep. 2017, 7, 11358. [Google Scholar] [CrossRef] [PubMed]
- Palta, J.P.; Whitaker, B.D.; Weiss, L.S. Plasma Membrane Lipids Associated with Genetic Variability in Freezing Tolerance and Cold Acclimation of Solanum Species. Plant Physiol. 1993, 103, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Sȩkara, A.; Baczek-Kwinta, R.; Gawȩda, M.; Kalisz, A.; Pokluda, R.; Jezdinský, A. Sequential Abiotic Stress Applied to Juvenile Eggplant Modifies the Seedlings Parameters, Plant Ontogeny and Yield. Hortic. Sci. 2016, 43, 149–157. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Cold Priming Drives the Sub-Cellular Antioxidant Systems to Protect Photosynthetic Electron Transport against Subsequent Low Temperature Stress in Winter Wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, X.; Wang, Z.; Sun, Z.; Zhu, X.; Liu, S.; Song, F.; Liu, F.; Wang, Y. Cold Priming Induced Tolerance to Subsequent Low Temperature Stress Is Enhanced by Melatonin Application during Recovery in Wheat. Molecules 2018, 23, 1091. [Google Scholar] [CrossRef]
- Kubala, S.; Garnczarska, M.; Wojtyla, Ł.; Clippe, A.; Kosmala, A.; Żmieńko, A.; Lutts, S.; Quinet, M. Deciphering Priming-Induced Improvement of Rapeseed (Brassica napus L.) Germination through an Integrated Transcriptomic and Proteomic Approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus Tolerance to High Saline Conditions by Seed Priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Souguir, M.; Hannachi, C. Determination of an Optimal Priming Duration and Concentration Protocol for Pepper Seeds (Capsicum annuum L.). Acta Agric. Slov. 2014, 103, 213–221. [Google Scholar] [CrossRef]
- Khan, H.A.; Ayub, C.M.; Pervez, M.A.; Bilal, R.M.; Shahid, M.A.; Ziaf, K. Effect of Seed Priming with NaCl on Salinity Tolerance of Hot Pepper (Capsicum annuum L.) at Seedling Stage. Soil. Environ. 2009, 28, 81–87. [Google Scholar]
- Sun, L.; Song, G.; Guo, W.; Wang, W.; Zhao, H.; Gao, T.; Lv, Q.; Yang, X.; Xu, F.; Dong, Y.; et al. Dynamic Changes in Genome-Wide Histone3 Lysine27 Trimethylation and Gene Expression of Soybean Roots in Response to Salt Stress. Front. Plant Sci. 2019, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.-S.; Wang, Q.; Huang, M.; Wong, F.-L.; Liu, A.; Ng, M.-S.; Li, K.-P.; Sze, C.-C.; Li, M.-W.; Lam, H.-M. Priming-Induced Alterations in Histone Modifications Modulate Transcriptional Responses in Soybean under Salt Stress. Plant J. 2022, 109, 1575–1590. [Google Scholar] [CrossRef]
- You, X.; Nasrullah; Wang, D.; Mei, Y.; Bi, J.; Liu, S.; Xu, W.; Wang, N.N. N7-SSPP Fusion Gene Improves Salt Stress Tolerance in Transgenic Arabidopsis and Soybean through ROS Scavenging. Plant Cell Environ. 2022, 45, 2794–2809. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wei, W.; Tao, J.-J.; Lu, X.; Bian, X.-H.; Hu, Y.; Cheng, T.; Yin, C.-C.; Zhang, W.-K.; Chen, S.-Y.; et al. Nuclear Factor Y Subunit GmNFYA Competes with GmHDA13 for Interaction with GmFVE to Positively Regulate Salt Tolerance in Soybean. Plant Biotechnol. J. 2021, 19, 2362–2379. [Google Scholar] [CrossRef]
- Biswas, S.; Paul, A.; Biswas, A.K. Potential of Seed Halopriming in Mitigating NaCl-Induced Adversities on Nitrogen Metabolism in Legume Crops. Legume Res. 2022, 45, 73–81. [Google Scholar] [CrossRef]
- Paul, A.; Biswas, S.; Banerjee, R.; Mukherjee, A.; Biswas, A.K. Halopriming Imparts Salt Tolerance by Reducing Oxidative, Osmotic Stress and DNA Damage in Five Different Legume Varieties. Legume Res. 2021, 41, 490–499. [Google Scholar] [CrossRef]
- Hu, T.; Jin, Y.; Li, H.; Amombo, E.; Fu, J. Stress Memory Induced Transcriptional and Metabolic Changes of Perennial Ryegrass (Lolium perenne) in Response to Salt Stress. Physiol. Plant. 2016, 156, 54–69. [Google Scholar] [CrossRef]
- Bharti, P.; Mahajan, M.; Vishwakarma, A.K.; Bhardwaj, J.; Yadav, S.K. AtROS1 Overexpression Provides Evidence for Epigenetic Regulation of Genes Encoding Enzymes of Flavonoid Biosynthesis and Antioxidant Pathways during Salt Stress in Transgenic Tobacco. J. Exp. Bot. 2015, 66, 5959–5969. [Google Scholar] [CrossRef]
- Hidayah, A.; Nisak, R.R.; Susanto, F.A.; Nuringtyas, T.R.; Yamaguchi, N.; Purwestri, Y.A. Seed Halopriming Improves Salinity Tolerance of Some Rice Cultivars During Seedling Stage. Bot. Stud. 2022, 63, 24. [Google Scholar] [CrossRef]
- De Souza, M.O.D.; Pelacani, C.R.; Willems, L.A.J.; Castro, R.D.D.; Hilhorst, H.W.M.; Ligterink, W. Effect of Osmopriming on Germination and Initial Growth of Physalis angulata L. under Salt Stress and on Expression of Associated Genes. An. Acad. Bras. Ciênc. 2016, 88, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, S.F.; Almutairi, Z.M. Effect of Priming With Gibberellin On Germination and Expression of Ga Biosynthesis Gene Of Tomato (Solanum lycopersicum L.) Seedlings Under Salt Stress. Int. J. Bio-Technol. Res. 2018, 8, 11–22. [Google Scholar] [CrossRef]
- Cayuela, E.; Estañ, M.T.; Parra, M.; Caro, M.; Bolarin, M.C. NaCl Pre-Treatment at the Seedling Stage Enhances Fruit Yield of Tomato Plants Irrigated with Salt Water. Plant Soil. 2001, 230, 231–238. [Google Scholar] [CrossRef]
- Alzahrani, O.; Abouseadaa, H.; Abdelmoneim, T.; Alshehri, M.; El-Mogy, M.; El-Beltagi, H.; Omar, M.A. Agronomical, Physiological and Molecular Evaluation Reveals Superior Salt-Tolerance in Bread Wheat through Salt-Induced Priming Approach. Not. Bot. Horti Agrobot. 2021, 49, 12310. [Google Scholar] [CrossRef]
- Janda, T.; Darko, É.; Shehata, S.; Kovács, V.; Pál, M.; Szalai, G. Salt Acclimation Processes in Wheat. Plant Physiol. Biochem. 2016, 101, 68–75. [Google Scholar] [CrossRef]
- Marconi, G.; Pace, R.; Traini, A.; Raggi, L.; Lutts, S.; Chiusano, M.; Guiducci, M.; Falcinelli, M.; Benincasa, P.; Albertini, E. Use of MSAP Markers to Analyse the Effects of Salt Stress on DNA Methylation in Rapeseed (Brassica napus Var. Oleifera). PLoS ONE 2013, 8, e75597. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Ye, W.; Wang, H.; Wang, J.; Fang, B. Study on DNA Cytosine Methylation of Cotton (Gossypium hirsutum L.) Genome and Its Implication for Salt Tolerance. Agric. Sci. China 2010, 9, 783–791. [Google Scholar] [CrossRef]
- Kęska, K.; Szcześniak, M.W.; Makałowska, I.; Czernicka, M. Long-Term Waterlogging as Factor Contributing to Hypoxia Stress Tolerance Enhancement in Cucumber: Comparative Transcriptome Analysis of Waterlogging Sensitive and Tolerant Accessions. Genes 2021, 12, 189. [Google Scholar] [CrossRef]
- Tsuji, H.; Saika, H.; Tsutsumi, N.; Hirai, A.; Nakazono, M. Dynamic and Reversible Changes in Histone H3-Lys4 Methylation and H3 Acetylation Occurring at Submergence-Inducible Genes in Rice. Plant Cell Physiol. 2006, 47, 995–1003. [Google Scholar] [CrossRef]
- Li, C.; Jiang, D.; Wollenweber, B.; Li, Y.; Dai, T.; Cao, W. Waterlogging Pretreatment during Vegetative Growth Improves Tolerance to Waterlogging after Anthesis in Wheat. Plant Sci. 2011, 180, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and Proteomic Mechanisms of Waterlogging Priming Improves Tolerance to Waterlogging Stress in Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
- Onyekachi, O.G.; Boniface, O.O.; Gemlack, N.F.; Namessan, N. The Effect of Climate Change on Abiotic Plant Stress: A Review. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding Rnas, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Nishad, A.; Nandi, A.K. Recent Advances in Plant Thermomemory. Plant Cell Rep. 2021, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.-P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The Heat Shock Factor Family from Triticum aestivum in Response to Heat and Other Major Abiotic Stresses and Their Role in Regulation of Heat Shock Protein Genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Tomás, D.; Brazão, J.; Viegas, W.; Silva, M. Differential Effects of High-Temperature Stress on Nuclear Topology and Transcription of Repetitive Noncoding and Coding Rye Sequences. Cytogenet. Genome Res. 2013, 139, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Gevaert, K.; De Smet, I. Feeling the Heat: Searching for Plant Thermosensors. Trends Plant Sci. 2019, 24, 210–219. [Google Scholar] [CrossRef]
- Mishkind, M.; Vermeer, J.E.M.; Darwish, E.; Munnik, T. Heat Stress Activates Phospholipase D and Triggers PIP2 Accumulation at the Plasma Membrane and Nucleus. Plant J. 2009, 60, 10–21. [Google Scholar] [CrossRef]
- Danilova, M.N.; Kudryakova, N.V.; Andreeva, A.A.; Doroshenko, A.S.; Pojidaeva, E.S.; Kusnetsov, V.V. Differential Impact of Heat Stress on the Expression of Chloroplast-Encoded Genes. Plant Physiol. Biochem. 2018, 129, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.V.; Siddique, K.H.M.; Nayyar, H. Plant Growth-Regulating Molecules as Thermoprotectants: Functional Relevance and Prospects for Improving Heat Tolerance in Food Crops. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef]
- Gong, M.; Chen, B.O.; Li, Z.G.; Guo, L.H. Heat-Shock-Induced Cross Adaptation to Heat, Chilling, Drought and Salt Stress in Maize Seedlings and Involvement of H2O2. J. Plant Physiol. 2001, 158, 1125–1130. [Google Scholar] [CrossRef]
- Mendanha, T.; Rosenqvist, E.; Hyldgaard, B.; Ottosen, C.O. Heat Priming Effects on Anthesis Heat Stress in Wheat Cultivars (Triticum aestivum L.) with Contrasting Tolerance to Heat Stress. Plant Physiol. Biochem. 2018, 132, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Entrambasaguas, L.; Ruocco, M.; Verhoeven, K.J.F.; Procaccini, G.; Marín-Guirao, L. Gene Body DNA Methylation in Seagrasses: Inter- and Intraspecific Differences and Interaction with Transcriptome Plasticity under Heat Stress. Sci. Rep. 2021, 11, 14343. [Google Scholar] [CrossRef] [PubMed]
- Korotko, U.; Chwiałkowska, K.; Sańko-Sawczenko, I.; Kwasniewski, M. DNA Demethylation in Response to Heat Stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 1555. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, L.; Li, J.; He, Z. Genetic and Epigenetic Control of Plant Heat Responses. Front. Plant Sci. 2015, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Oberkofler, V.; Pratx, L.; Bäurle, I. Epigenetic Regulation of Abiotic Stress Memory: Maintaining the Good Things While They Last. Curr. Opin. Plant Biol. 2021, 61, 102007. [Google Scholar] [CrossRef] [PubMed]
- Daiana, A.D.S.; Raquel, L.D.M.D.R.; Joao, G.R.G.; Sergio, A.M.C.; Alisson, F.C. Effect of Heat Stress on Common Bean under Natural Growing Conditions in Three Locations in Different Climate Zones in the State of So Paulo, Brazil. J. Plant Breed. Crop Sci. 2018, 10, 134–145. [Google Scholar] [CrossRef]
- Kim, K.D.; El Baidouri, M.; Abernathy, B.; Iwata-Otsubo, A.; Chavarro, C.; Gonzales, M.; Libault, M.; Grimwood, J.; Jackson, S.A. A Comparative Epigenomic Analysis of Polyploidy-Derived Genes in Soybean and Common Bean. Plant Physiol. 2015, 168, 1433–1447. [Google Scholar] [CrossRef]
- Crampton, M.; Sripathi, V.R.; Hossain, K.; Kalavacharla, V. Analyses of Methylomes Derived from Meso-American Common Bean (Phaseolus vulgaris L.) Using MeDIP-Seq and Whole Genome Sodium Bisulfite-Sequencing. Front. Plant Sci. 2016, 7, 447. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T. Plant Proteome Responses to Salinity Stress-Comparison of Glycophytes and Halophytes. Funct. Plant Biol. 2013, 40, 775–786. [Google Scholar] [CrossRef]
- Adhikari, L.; Baral, R.; Paudel, D.; Min, D.; Makaju, S.O.; Poudel, H.P.; Acharya, J.P.; Missaoui, A.M. Cold Stress in Plants: Strategies to Improve Cold Tolerance in Forage Species. Plant Stress. 2022, 4, 100081. [Google Scholar] [CrossRef]
- Antoniou-Kourounioti, R.L.; Zhao, Y.; Dean, C.; Howard, M. Feeling Every Bit of Winter—Distributed Temperature Sensitivity in Vernalization. Front. Plant Sci. 2021, 12, 628726. [Google Scholar] [CrossRef] [PubMed]
- Bhadouriya, S.L.; Mehrotra, S.; Basantani, M.K.; Loake, G.J.; Mehrotra, R. Role of Chromatin Architecture in Plant Stress Responses: An Update. Front. Plant Sci. 2021, 11, 603380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Cold-Stress Responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Park, J.; Lim, C.J.; Shen, M.; Park, H.J.; Cha, J.-Y.; Iniesto, E.; Rubio, V.; Mengiste, T.; Zhu, J.-K.; Bressan, R.A.; et al. Epigenetic Switch from Repressive to Permissive Chromatin in Response to Cold Stress. Proc. Natl. Acad. Sci. USA 2018, 115, E5400–E5409. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhao, L.; Li, J.; He, S.; Zhou, K.; Yang, F.; Huang, M.; Jiang, L.; Li, L. Trichostatin A Selectively Suppresses the Cold-Induced Transcription of the ZmDREB1 Gene in Maize. PLoS ONE 2011, 6, e22132. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, F.; Huang, L.; Sun, Q.; Li, C.; Zhao, Y.; Zhou, D.-X. Rice Histone Deacetylase Genes Display Specific Expression Patterns and Developmental Functions. Biochem. Biophys. Res. Commun. 2009, 388, 266–271. [Google Scholar] [CrossRef]
- Fu, W.; Wu, K.; Duan, J. Sequence and Expression Analysis of Histone Deacetylases in Rice. Biochem. Biophys. Res. Commun. 2007, 356, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of Rice DREB1/CBF-Type Transcription Factors Involved in Cold-Responsive Gene Expression in Transgenic Rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef]
- Roy, D.; Paul, A.; Roy, A.; Ghosh, R.; Ganguly, P.; Chaudhuri, S. Differential Acetylation of Histone H3 at the Regulatory Region of OsDREB1b Promoter Facilitates Chromatin Remodelling and Transcription Activation during Cold Stress. PLoS ONE 2014, 9, e0100343. [Google Scholar] [CrossRef]
- Sicilia, A.; Scialò, E.; Puglisi, I.; Lo Piero, A.R. Anthocyanin Biosynthesis and DNA Methylation Dynamics in Sweet Orange Fruit [Citrus Sinensis L. (Osbeck)] under Cold Stress. J. Agric. Food Chem. 2020, 68, 7024–7031. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Giri, S.K.; Singh, G.; Gill, R.; Kumar, A. Epigenetic Regulation of Heat and Cold Stress Responses in Crop Plants. Plant Gene 2022, 29, 100351. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, Photoinhibition and Low Temperature Acclimation in Cold Tolerant Plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kang, G.; Guo, T. Proteomic Analysis of Spring Freeze-Stress Responsive Proteins in Leaves of Bread Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 63, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Satake, T.; Hayase, H. Male Sterility Caused by Cooling Treatment at the Young Microspore Stage in Rice Plants: V. Estimations of Pollen Developmental Stage and the Most Sensitive Stage to Coolness. Jpn. J. Crop Sci. 1970, 39, 468–473. [Google Scholar] [CrossRef]
- Ito, H. Plant Models of Transgenerational Epigenetic Inheritance; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-405944-3. [Google Scholar]
- Iba, K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Hossain, M.A.; Li, Z.G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or Cold Priming-Induced Cross-Tolerance to Abiotic Stresses in Plants: Key Regulators and Possible Mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- Jan, N.; Majeed, U.; Andrabi, K.I.; John, R. Cold Stress Modulates Osmolytes and Antioxidant System in Calendula Officinalis. Acta Physiol. Plant. 2018, 40, 73. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of Plant Root System Architecture: Implications for Crop Advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Li, M.; López, R.; Cano, F.J.; Oleksyn, J.; Atkin, O.K.; Pita, P.; Aranda, I.; Gil, L. Drought-Induced Shoot Dieback Starts with Massive Root Xylem Embolism and Variable Depletion of Nonstructural Carbohydrates in Seedlings of Two Tree Species. New Phytol. 2017, 213, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Schaffer, B.; Rowland, D. Water-Deficit Priming of Papaya Reduces High-Light Stress through Oxidation Avoidance Rather than Anti-Oxidant Activity. Environ. Exp. Bot. 2018, 156, 106–119. [Google Scholar] [CrossRef]
- Ding, Y.; Avramova, Z.; Fromm, M. The Arabidopsis Trithorax-like Factor ATX1 Functions in Dehydration Stress Responses via ABA-Dependent and ABA-Independent Pathways. Plant J. 2011, 66, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in Photosynthesis and Antioxidant Enzyme Activity in Winter Wheat Subjected to Post-Anthesis Water-Logging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of Waterlogging Tolerance in Wheat—A Review of Root and Shoot Physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Lukić, N.; Schurr, F.M.; Trifković, T.; Kukavica, B.; Walter, J. Transgenerational Stress Memory in Plants Is Mediated by Upregulation of the Antioxidative System. Environ. Exp. Bot. 2023, 205, 105129. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed Priming as a Cost Effective Technique for Developing Plants with Cross Tolerance to Salinity Stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Xu, W.; Ahmed, N.; Yu, A.; Wang, Z.; Liu, A. Changes and Associations of Genomic Transcription and Histone Methylation with Salt Stress in Castor Bean. Plant Cell Physiol. 2020, 61, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z.; Islam, S.; Ahmad, H. Salinity Imposed Stress on Principal Cereal Crops and Employing Seed Priming as a Sustainable Management Approach. Acta Ecol. Sin. 2019, 39, 280–283. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of Seed Priming Strategies for Enhancing Salinity Tolerance in Plants: An Overview of the Progress and Achievements. Plant Stress. 2023, 9, 100186. [Google Scholar] [CrossRef]
- Singroha, G.; Kumar, S.; Gupta, O.P.; Singh, G.P.; Sharma, P. Uncovering the Epigenetic Marks Involved in Mediating Salt Stress Tolerance in Plants. Front. Genet. 2022, 13, 811732. [Google Scholar] [CrossRef] [PubMed]
- Suter, L.; Widmer, A. Environmental Heat and Salt Stress Induce Transgenerational Phenotypic Changes in Arabidopsis thaliana. PLoS ONE 2013, 8, e60364. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Du, M.; Tian, H.; Wang, B. Exposure to High Salinity During Seed Development Markedly Enhances Seedling Emergence and Fitness of the Progeny of the Extreme Halophyte Suaeda Salsa. Front. Plant Sci. 2020, 11, 1291. [Google Scholar] [CrossRef]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally Responsive Genome-Wide Accumulation of de Novo Arabidopsis thaliana Mutations and Epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Qiao, K.; Fan, S.; Ma, Q. Genome-Wide Analysis of JMJ-C Histone Demethylase Family Involved in Salt-Tolerance in Gossypium hirsutum L. Plant Physiol. Biochem. 2021, 158, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Gao, H.; Zhou, Y.; Jing, Y.; Li, S.; Yan, Z.; Xu, K.; Zhou, F.; Zhang, W.; Yang, X.; et al. Unfolding Molecular Switches for Salt Stress Resilience in Soybean: Recent Advances and Prospects for Salt-Tolerant Smart Plant Production. Front. Plant Sci. 2023, 14, 1162014. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Kumar, R.R.; Praveen, S. Plant Transcriptional Regulation in Modulating Cross-Tolerance to Stress; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817893-5. [Google Scholar]
- Mahajan, S.; Tuteja, N. Cold, Salinity and Drought Stresses: An Overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Liu, S.; Zhu, X.; Song, F.; Liu, F. Induction of Cross Tolerance by Cold Priming and Acclimation in Plants: Physiological, Biochemical and Molecular Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817893-5. [Google Scholar]
- Turkan, I. ROS and RNS: Key Signalling Molecules in Plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional Profiling of Arabidopsis Heat Shock Proteins and Transcription Factors Reveals Extensive Overlap between Heat and Non-Heat Stress Response Pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.G.; Casalongué, C.A.; Simontacchi, M.; Marquez-Garcia, B.; Foyer, C.H. Interactions between Hormone and Redox Signalling Pathways in the Control of Growth and Cross Tolerance to Stress. Environ. Exp. Bot. 2013, 94, 73–88. [Google Scholar] [CrossRef]
- Faralli, M.; Lektemur, C.; Rosellini, D.; Gürel, F. Effects of Heat Shock and Salinity on Barley Growth and Stress-Related Gene Transcription. Biol. Plant. 2015, 59, 537–546. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological Stress Memory and Cross Stress Tolerance in Plants in the Face of Climate Extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, M. Genome-Wide Association Studies for Improving Agronomic Traits in Foxtail Millet. In The Foxtail Millet Genome; Prasad, M., Ed.; Compendium of Plant, Genomes; Springer International Publishing: Cham, Switzerland, 2017; pp. 63–75. ISBN 978-3-319-65617-5. [Google Scholar]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and Memory of Stress Responses in Organisms Lacking a Nervous System. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Bittner, A.; Prescher, A.; van Buer, J. Preparing Plants for Improved Cold Tolerance by Priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Duan, W.; Huang, F.; Hou, X. Cold Acclimation Alters DNA Methylation Patterns and Confers Tolerance to Heat and Increases Growth Rate in Brassica Rapa. J. Exp. Bot. 2017, 68, 1213–1224. [Google Scholar] [CrossRef]
- Singha, A.; Soothar, R.K.; Wang, C.; Marín, E.E.T.; Tankari, M.; Hao, W.; Wang, Y. Drought Priming Alleviated Salinity Stress and Improved Water Use Efficiency of Wheat Plants. Plant Growth Regul. 2022, 96, 357–368. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhong, J.; Zhou, Q.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Drought Priming Induces Thermo-Tolerance to Post-Anthesis High-Temperature in Offspring of Winter Wheat. Environ. Exp. Bot. 2016, 127, 26–36. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought Priming at Vegetative Growth Stages Improves Tolerance to Drought and Heat Stresses Occurring during Grain Filling in Spring Wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Abid, M.; Shao, Y.; Liu, S.; Wang, F.; Gao, J.; Jiang, D.; Tian, Z.; Dai, T. Pre-Drought Priming Sustains Grain Development under Post-Anthesis Drought Stress by Regulating the Growth Hormones in Winter Wheat (Triticum aestivum L.). Planta 2017, 246, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Physiological, Proteomic and Transcriptional Responses of Wheat to Combination of Drought or Waterlogging with Late Spring Low Temperature. Funct. Plant Biol. 2014, 41, 690–703. [Google Scholar] [CrossRef]
- Li, X.; Topbjerg, H.B.; Jiang, D.; Liu, F. Drought Priming at Vegetative Stage Improves the Antioxidant Capacity and Photosynthesis Performance of Wheat Exposed to a Short-Term Low Temperature Stress at Jointing Stage. Plant Soil. 2015, 393, 307–318. [Google Scholar] [CrossRef]
- Gupta, S.K.; Verma, K.; Kumar, R.; Sarkar, B.; Mantha, A.K.; Kumar, S. Priming Alleviates High Temperature Induced Oxidative DNA Damage and Repair Using Apurinic/Apyrimidinic Endonuclease (Ape1L) Homologue in Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2020, 156, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Babajamali, A.; Gholami, M.; Baninasab, B. Drought Preconditioning Improves Freezing Tolerance in Drought-Tolerant and -Intolerant Grape Cultivars. Theor. Exp. Plant Physiol. 2022, 34, 395–407. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C.; Leitão, A.E.; Fortunato, A.S.; Pais, I.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; et al. Stress Cross-Response of the Antioxidative System Promoted by Superimposed Drought and Cold Conditions in Coffea Spp. PLoS ONE 2018, 13, e198694. [Google Scholar] [CrossRef]
- Rossatto, T.; Souza, G.M.; Do Amaral, M.N.; Auler, P.A.; Pérez-Alonso, M.-M.; Pollmann, S.; Braga, E.J.B. Cross-Stress Memory: Salt Priming at Vegetative Growth Stages Improves Tolerance to Drought Stress during Grain-Filling in Rice Plants. Environ. Exp. Bot. 2023, 206, 105187. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Chen, H.; Huang, J.; Zhang, Y.; Qi, M.; Liu, Y.; Li, T. Photosynthetic Response Mechanism of Soil Salinity-Induced Cross-Tolerance to Subsequent Drought Stress in Tomato Plants. Plants 2020, 9, 363. [Google Scholar] [CrossRef]
- Yang, X.; Zou, F.; Zhang, Y.; Shi, J.; Qi, M.; Liu, Y.; Li, T. NaCl Pretreatment Enhances the Low Temperature Tolerance of Tomato Through Photosynthetic Acclimation. Front. Plant Sci. 2022, 13, 891697. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Bolarín, M.C.; Asíns, M.J.; Moreno, V. Increasing Salt Tolerance in the Tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Methenni, K.; Taamalli, W.; Hessini, K.; Ben Youssef, N. Cross-Priming Approach Induced Beneficial Metabolic Adjustments and Repair Processes during Subsequent Drought in Olive. Water 2022, 14, 4050. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mostofa, M.; Fujita, M. Cross Protection by Cold-Shock to Salinity and Drought Stress-Induced Oxidative Stress in Mustard (Brassica campestris L.) Seedlings. Mol. Plant Breed. 2013, 4, 50–70. [Google Scholar] [CrossRef]
- Yasuda, H. Cross-Tolerance to Thermal Stresses and Its Application to the Development of Cold Tolerant Rice. Jpn. Agric. Res. Q. JARQ 2017, 51, 99–105. [Google Scholar] [CrossRef]
- Bittner, A.; van Buer, J.; Baier, M. Cold Priming Uncouples Light- and Cold-Regulation of Gene Expression in Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Akimoto-Tomiyama, C.; Tanabe, S.; Kajiwara, H.; Minami, E.; Ochiai, H. Loss of Chloroplast-Localized Protein Phosphatase 2Cs in Arabidopsis thaliana Leads to Enhancement of Plant Immunity and Resistance to Xanthomonas campestris Pv. campestris Infection. Mol. Plant Pathol. 2018, 19, 1184–1195. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic Stress Globally Downregulates Photosynthesis Genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Bao, F.; Zhang, X.; Yang, S. A Gain-of-Function Mutation in the Arabidopsis Disease Resistance Gene RPP4 Confers Sensitivity to Low Temperature. Plant Physiol. 2010, 154, 796–809. [Google Scholar] [CrossRef]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Enhancing Crop Resilience to Combined Abiotic and Biotic Stress through the Dissection of Physiological and Molecular Crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Balasubramanian, S.; Hu, T.T.; Traw, M.B.; Horton, M.; Epple, P.; Kuhns, C.; Sureshkumar, S.; Schwartz, C.; Lanz, C.; et al. Natural Allelic Variation Underlying a Major Fitness Trade-off in Arabidopsis thaliana. Nature 2010, 465, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic Regulation of Stress Responses in Plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Vandenbroucke, K.; Clauw, P.; Maleux, K.; De Meyer, B.; Dhondt, S.; Pucci, A.; Gonzalez, N.; Hoeberichts, F.; Tognetti, V.B.; et al. Survival and Growth of Arabidopsis Plants given Limited Water Are Not Equal. Nat. Biotechnol. 2011, 29, 212–214. [Google Scholar] [CrossRef]
- de Lima Pereira, J.W.; Albuquerque, M.B.; Melo Filho, P.A.; Mansur Custódio Nogueira, R.J.; de Lima, L.M.; Santos, R.C. Assessment of Drought Tolerance of Peanut Cultivars Based on Physiological and Yield Traits in a Semiarid Environment. Agric. Water Manag. 2016, 166, 70–76. [Google Scholar] [CrossRef]
- Songsri, P.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Patanothai, A.; Holbrook, C.C. Root Distribution of Drought-Resistant Peanut Genotypes in Response to Drought. J. Agron. Crop Sci. 2008, 194, 92–103. [Google Scholar] [CrossRef]
- Byrd, S.A.; Rowland, D.L.; Bennett, J.; Zotarelli, L.; Wright, D.; Alva, A.; Nordgaard, J. Reductions in a Commercial Potato Irrigation Schedule during Tuber Bulking in Florida: Physiological, Yield, and Quality Effects. J. Crop Improv. 2014, 28, 660–679. [Google Scholar] [CrossRef]
- Vincent, C.; Rowland, D.; Schaffer, B. Primed Acclimation of Papaya Increases Short-Term Water Use But Does Not Confer Long-Term Drought Tolerance. HortScience 2017, 52, 441–449. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Larsen, D.H.; Zhu, X.; Song, F.; Liu, F. Drought Priming at Vegetative Growth Stage Enhances Nitrogen-Use Efficiency under Post-Anthesis Drought and Heat Stress in Wheat. J. Agron. Crop Sci. 2017, 203, 29–40. [Google Scholar] [CrossRef]
- Vincent, C.; Rowland, D.; Schaffer, B.; Bassil, E.; Racette, K.; Zurweller, B. Primed Acclimation: A Physiological Process Offers a Strategy for More Resilient and Irrigation-Efficient Crop Production. Plant Sci. 2020, 295, 110240. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Akram, N.A. Improving Salinity Tolerance of Plants through Conventional Breeding and Genetic Engineering: An Analytical Comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef]
- Cohen, S.P.; Leach, J.E. High Temperature-Induced Plant Disease Susceptibility: More than the Sum of Its Parts. Curr. Opin. Plant Biol. 2020, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Saka, N.; Mizukami, Y.; Koga, H.; Yamanouchi, U.; Yoshioka, Y.; Hayashi, N.; Ebana, K.; Mizobuchi, R.; Yano, M. Gene Pyramiding Enhances Durable Blast Disease Resistance in Rice. Sci. Rep. 2015, 5, 7773. [Google Scholar] [CrossRef]
- Liu, H.; Lämke, J.; Lin, S.; Hung, M.-J.; Liu, K.-M.; Charng, Y.; Bäurle, I. Distinct Heat Shock Factors and Chromatin Modifications Mediate the Organ-Autonomous Transcriptional Memory of Heat Stress. Plant J. 2018, 95, 401–413. [Google Scholar] [CrossRef]
- Stief, A.; Brzezinka, K.; Lämke, J.; Bäurle, I. Epigenetic Responses to Heat Stress at Different Time Scales and the Involvement of Small RNAs. Plant Signal. Behav. 2014, 9, e970430. [Google Scholar] [CrossRef]
- Song, J.; Angel, A.; Howard, M.; Dean, C. Vernalization—A Cold-Induced Epigenetic Switch. J. Cell Sci. 2012, 125, 3723–3731. [Google Scholar] [CrossRef]
- Hepworth, C.; Caine, R.S.; Harrison, E.L.; Sloan, J.; Gray, J.E. Stomatal Development: Focusing on the Grasses. Curr. Opin. Plant Biol. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Le Gac, A.-L.; Lafon-Placette, C.; Chauveau, D.; Segura, V.; Delaunay, A.; Fichot, R.; Marron, N.; Le Jan, I.; Berthelot, A.; Bodineau, G.; et al. Winter-Dormant Shoot Apical Meristem in Poplar Trees Shows Environmental Epigenetic Memory. J. Exp. Bot. 2018, 69, 4821–4837. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schmülling, T. Stress Priming, Memory, and Signalling in Plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- He, Y.; Li, Z. Epigenetic Environmental Memories in Plants: Establishment, Maintenance, and Reprogramming. Trends Genet. 2018, 34, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein Degradation—An Alternative Respiratory Substrate for Stressed Plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T.; Baluška, F.; Bozhkov, P.V.; Elander, P.H.; Fernie, A.R.; Galili, G.; Hassan, A.; Hofius, D.; Isono, E.; Le Bars, R.; et al. Autophagy-Related Approaches for Improving Nutrient Use Efficiency and Crop Yield Protection. J. Exp. Bot. 2018, 69, 1335–1353. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The Plastid Metalloprotease FtsH6 and Small Heat Shock Protein HSP21 Jointly Regulate Thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Thirumalaikumar, V.P.; Kamranfar, I.; Marmagne, A.; Masclaux-Daubresse, C.; Balazadeh, S. A Regulatory Role of Autophagy for Resetting the Memory of Heat Stress in Plants. Plant Cell Environ. 2019, 42, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Thirumalaikumar, V.P.; Kamranfar, I.; Schulz, K.; Mueller-Roeber, B.; Sampathkumar, A.; Balazadeh, S. Autophagy Complements Metalloprotease FtsH6 in Degrading Plastid Heat Shock Protein HSP21 during Heat Stress Recovery. J. Exp. Bot. 2021, 72, 7498–7513. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Gorka, M.; Schulz, K.; Masclaux-Daubresse, C.; Sampathkumar, A.; Skirycz, A.; Vierstra, R.D.; Balazadeh, S. Selective Autophagy Regulates Heat Stress Memory in Arabidopsis by NBR1-Mediated Targeting of HSP90.1 and ROF1. Autophagy 2021, 17, 2184–2199. [Google Scholar] [CrossRef] [PubMed]

| Stress | Type of Stress Memory | Plant Species | Stress Memory | References |
|---|---|---|---|---|
| Drought | Somatic | Citrus scion/rootstock combinations | DNA methylation patterns with an increase in ABA levels | [100] |
| Drought | Somatic | Citrus scion/rootstock combinations | Modification of methylation status and gene expression with the use of drought-primed scions | [46] |
| Drought | Somatic | Glycine max | Increased expression of drought response genes or dehydration memory genes encoding transcription factors, protein phosphatase 2Cs, and late embryogenesis rich proteins | [101] |
| Drought | Somatic | Gossypium hirsutum L. | Histone modifications | [102] |
| Drought | Somatic | Olea europaea L. | Higher photosynthetic efficiency, higher proline and sugar contents, as well as more active antioxidant machinery | [103] |
| Drought | Somatic | Oryza sativa | DNA methylation, lncRNAs, and abscisic acid (ABA) regulatory pathways induce drought-responsive genes | [104] |
| Drought | Somatic | Oryza sativa | Global DNA methylation changes regulate stress memory gene expression and transposons | [105,106] |
| Drought | Somatic | Solanum tuberosum L. | Increased expression of genes related to biosynthesis and signal transduction | [107] |
| Drought | Somatic | Solanum tumberosum | Increased antioxidant activity | [108] |
| Drought | Somatic | Triticum aestivum L. | Activation of antioxidant defence and redox homeostasis mechanisms | [109,110] |
| Drought | Somatic | Triticum aestivum L. | miRNAs induced osmoregulation | [111] |
| Drought | Somatic | Triticum aestivum L. | Phytohormones ABA and JA induced the activity of detoxifying enzymes | [112] |
| Drought | Somatic | Vigna unguiculata | Improved water status, water productivity of biomass index, photosynthesis, and plant hormones | [113] |
| Drought | Somatic | Vitis vinifera L. | Increased water status, leaf gas exchange, and berry size | [114] |
| Drought | Transgenerational | Arachis hypogea L. | Drought-resistance mechanisms, exemplified by characteristics such as enhanced rooting, seed weight, and germination efficiency | [115] |
| Drought | Transgenerational | Hordeum vulgare | Enhanced root development | [116] |
| Drought | Transgenerational | Oryza sativa | Decreasing energy dissipation, increasing ATP energy provision, reducing oxidative damage in GC | [117] |
| Drought | Transgenerational | Oryza sativa | Alteration in DNA methylation levels in guard cells, modulation of proteins involved in pathways for coping with oxidative stress and maintaining GC, enhanced photosynthesis and metabolism, improved gas exchange | [118] |
| Drought | Transgenerational | Triticum aestivum L. | Improved grain yield, preservation of photosynthetic activity and induced osmolyte production | [119] |
| Drought | Transgenerational | Triticum aestivum L. | Increased plant height, above-ground biomass, number of grains per plant, grain weight per plant, and water potential, improved osmolyte accumulation and reduced lipid peroxidation | [120,121] |
| Heat | Intergenerational | Triticum aestivum L. | Thermo-tolerance manifested as higher yield, improved photosynthesis, enhanced antioxidant activity, energy production, and reduced cell damage, upregulation of the lysine-specific histone demethylase 1 (LSD1) | [122] |
| Heat | Intergenerational | Brassica rapa L. | Changes in small RNA profiles in pollen grain | [123] |
| Heat | Somatic | Triticum aestivum L. | Increased metabolites and antioxidant defence mechanisms | [124,125] |
| Heat | Somatic | Triticum aestivum L. | HSPs redox homeostasis genes and downregulation of lipid metabolism genes involved in membrane rigidity | [126,127,128] |
| Heat | Transgenerational | Phaseolus vulgaris L. | Increased expression of 22 genes related to biological processes involved in the heat stress response (activation of HSPs, abiotic stress signalling, germination and seedling development, flowering time, protein thermo-stability, molecular chaperones, and cell-wall integrity) | [129] |
| Low temperatures | Somatic | Citrullus lanatus (Thunb.) Matsum & Nakai | Osmoregulation, decrease in electrolyte leakage and MDA accumulation, activation of photoprotective mechanisms, increase in Rubisco activase (CIRCA) and in gene expression of the Benson–Calvin cycle | [130] |
| Low temperatures | Somatic | Oryza sativa | Altered protein content and induction of selective protein degradation in the anthers | [131] |
| Low temperatures | Somatic | Pisum sativum | Increased enzyme activities in the Calvin cycle, higher resistance to photoinhibition of PSII, a more oxidised electron transport chain, less oxidative damage, and less impaired metabolite synthesis | [132] |
| Low temperatures | Somatic | Prunus persica L. | Accumulation of proteins related to energy metabolism | [133] |
| Low temperatures | Somatic | Solanum comersonii Poir. | Reduction in linoleic acid and sterol phospholipid ratios | [134] |
| Low temperatures | Somatic | Solanum melongena L. | Enhanced morphological and physiological parameters, increased pigment content and chlorophyll fluorescence parameters and enhanced max. quantum yield of PSII (Fv/Fm) and performance index (PI) | [135] |
| Low temperatures | Somatic | Triticum aestivum L. | Activation of the sub-cellular antioxidant systems, reduction in oxidative burst in photosynthetic apparatus | [136] |
| Low temperatures | Somatic | Triticum aestivum L. | Increased photosynthetic rate and stomatal conductance, enhanced antioxidant enzyme activities, and altered stress-related gene expressions | [137] |
| Salinity | Somatic | Brassica napus | Seed priming induced changes in transcriptome (mainly in MYB, DREB and NAC genes) and proteome (eIF4A, eIF3 subunit K, eIF6, eEF1) corresponding to translation initiation, elongation factors, seed storage proteins (SSPs) and management of oxidative stress. Higher expression of genes and proteins involved in water transport, cell wall modification, cytoskeletal organization, and cell division was linked to the advanced germination of primed seeds | [138] |
| Salinity | Somatic | Brassica napus | Higher genotype-dependent growth rates, stabilization of cell membranes integrity, increased chlorophyll content | [139] |
| Salinity | Somatic | Capsicum annuum L. | Seed-halopriming improved total germination, germination index, germination speed, vigour index, plumule and radicle length, and dry weight of the seedlings | [140,141] |
| Salinity | Somatic | Glycine max | Alterations in the transcriptional landscape of salt stress responsive genes through methylation and acetylation | [142,143,144,145] |
| Salinity | Somatic | Leguminous species | Seed-halopriming elevated activities of nitrate assimilatory enzymes resulting in improved nitrate uptake, reduced ammonium accumulation and glutamate dehydrogenase activity. The efficacy of halopriming was more effective in salt sensitive cultivars | [146] |
| Salinity | Somatic | Leguminous species | Improved catalase activity, higher water contents, lower accumulation of ROS, MDA and proline, reduced DNA damage, and enhanced growth | [147] |
| Salinity | Somatic | Lolium perenne L. | Reduced accumulation of Na+, BPSP, and sucrose synthase showed a high level of transcriptional memory | [148] |
| Salinity | Somatic | Nicotiana tabacum | Reduced level of DNA methylation in the promoter and coding regions of flavonoid biosynthesis and antioxidant genes | [149] |
| Salinity | Somatic | Oryza sativa | Seed-halopriming increased expression of ion homoeostasis genes | [150] |
| Salinity | Somatic | Physalis angulata L. | Seed osmopriming increased transcript levels of salt stress responsive genes (GST, TXN and APX) | [151] |
| Salinity | Somatic | Solanum lycopersicum | Seed-halopriming induced the upregulation of Gibberellic Acid (GA) biosynthesis genes, while improving germination and NaCl tolerance | [152] |
| Salinity | Somatic | Solanum lycopersicum | Greater partitioning of biomass to roots, higher growth rate, yield, maintenance of K+ selectivity in the developing leaves, priming-induced adaptation capacity is growth stage- and stress priming level dependent | [153] |
| Salinity | Somatic | Triticum aestivum L. | Seed-halopriming increased expression of salt responsive genes related to improved biosynthesis of photosynthetic pigments and decreased levels of oxidative stress markers | [154] |
| Salinity | Somatic | Triticum aestivum L. | Enhanced osmotic and antioxidant potential | [155] |
| Salinity | Transgenerational | Brassica napus | Demethylation promotes the expression of stress-related genes and induces salt resistance in these species | [156] |
| Salinity | Transgenerational | Gossypium hirsutum | Demethylation promotes the expression of stress-related genes and induces salt resistance in these species | [157] |
| Waterlogging | Somatic | Cucumis sativus L. | Investment in adventitious roots and up-regulated expression of genes related to the activation of amino acid metabolism, plant hormone biosynthesis, and glycolysis pathway | [158] |
| Waterlogging | Somatic | Oryza sativa | Alteration in expression and chromatin level of flooding-responsive genes | [159] |
| Waterlogging | Somatic | Triticum aestivum L. | Increased activities of antioxidant enzymes and photosynthetic capacity, higher chlorophyll content, and light usage efficiency | [160] |
| Waterlogging | Somatic | Triticum aestivum L. | Enzymatic and non-enzymatic processes involved in ascorbic acid-glutathione (ASA-GSH) cycle, increased plant biomass, maintenance of root growth, induction of ethylene biosynthesis and formation of aerenchyma in roots | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagiotis, G.; Madesis, P.; Stavridou, E. Echoes of a Stressful Past: Abiotic Stress Memory in Crop Plants towards Enhanced Adaptation. Agriculture 2023, 13, 2090. https://doi.org/10.3390/agriculture13112090
Lagiotis G, Madesis P, Stavridou E. Echoes of a Stressful Past: Abiotic Stress Memory in Crop Plants towards Enhanced Adaptation. Agriculture. 2023; 13(11):2090. https://doi.org/10.3390/agriculture13112090
Chicago/Turabian StyleLagiotis, Georgios, Panagiotis Madesis, and Evangelia Stavridou. 2023. "Echoes of a Stressful Past: Abiotic Stress Memory in Crop Plants towards Enhanced Adaptation" Agriculture 13, no. 11: 2090. https://doi.org/10.3390/agriculture13112090







