Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed and Grain Germination
2.2. Analysis of Polar Metabolites
2.3. Targeted Analysis of Verbascose
2.4. Statistical Analysis
3. Results
3.1. Polar Metabolites in Dry Seeds
3.2. Seeds Germination
3.3. Changes in Polar Metabolites during Seed Imbibition and Germination
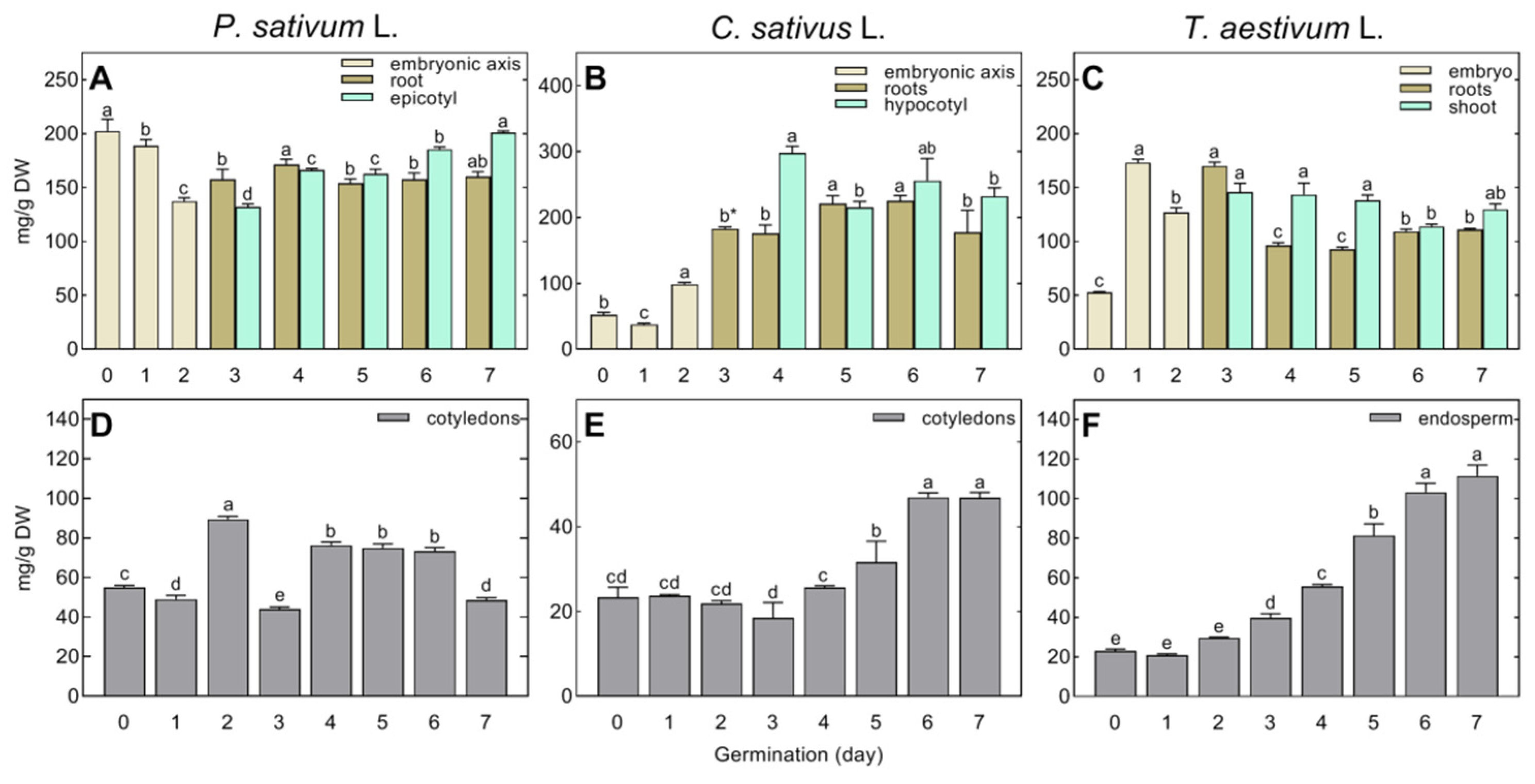
3.4. Seedling Growth and Development
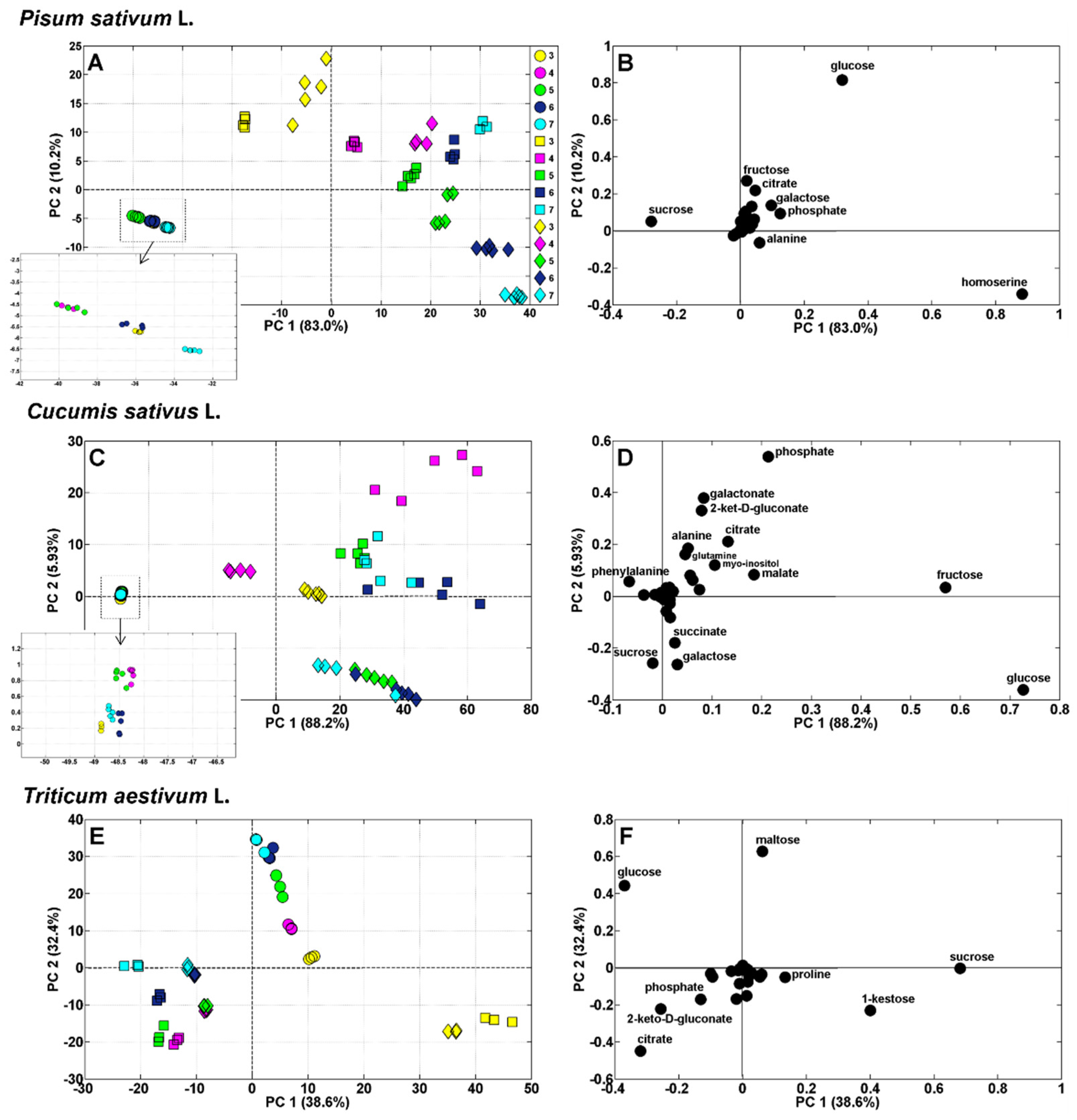
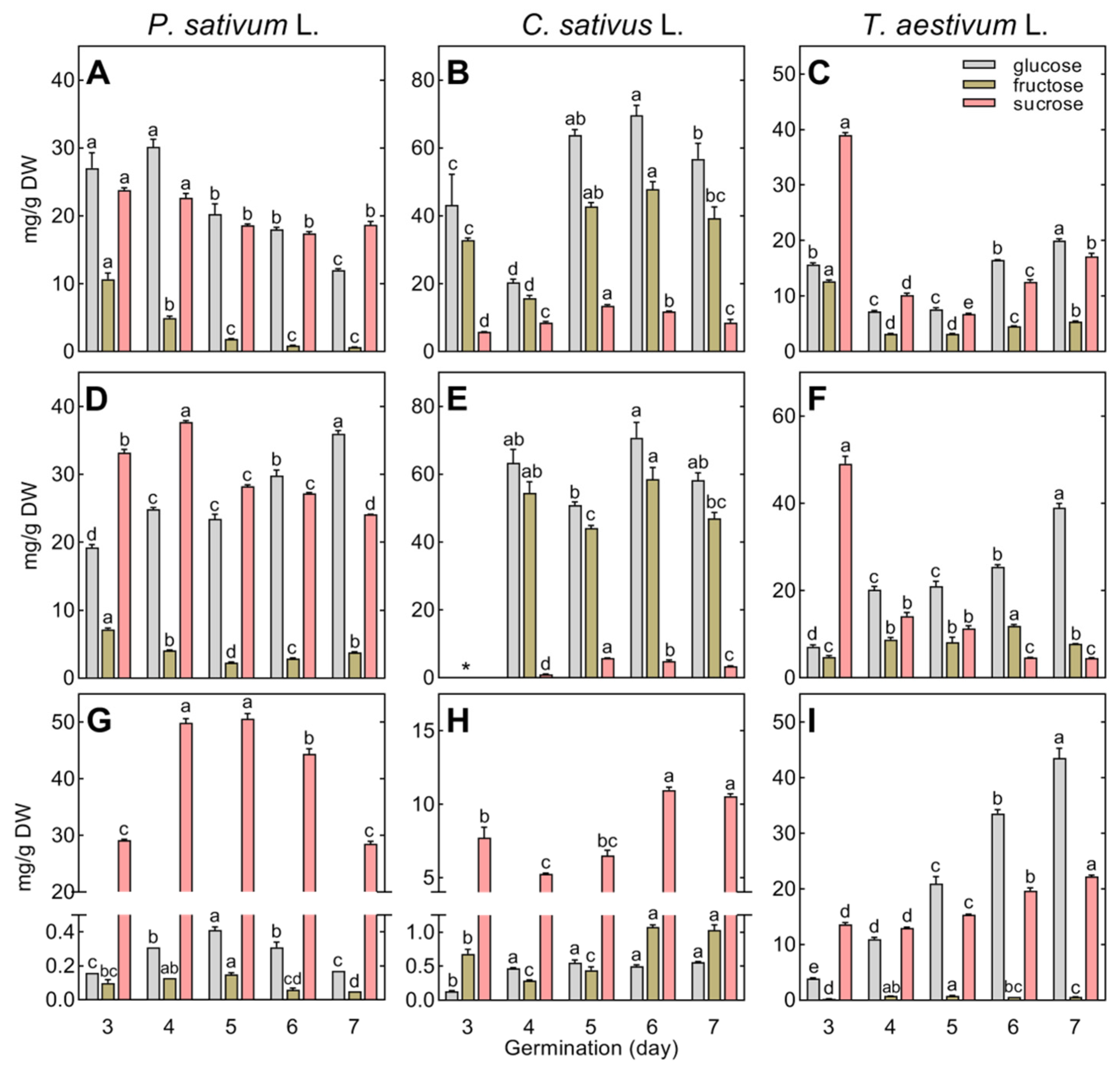
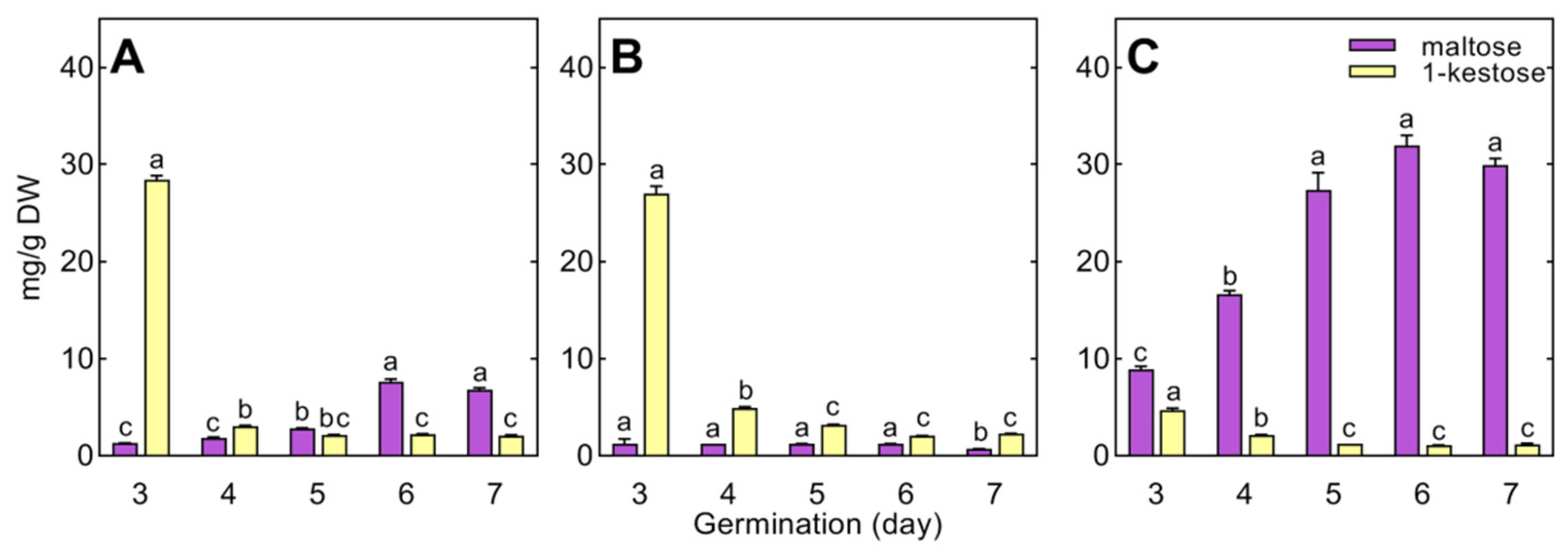
3.5. Changes in Polar Metabolites during Seedling Growth
3.5.1. Soluble Carbohydrates
3.5.2. Amino Acids
3.5.3. Organic Acids
3.5.4. Remaining Compounds
4. Discussion
4.1. Profile of Polar Metabolites in Dry Seeds
4.2. Changes in the Polar Metabolites Profile during Seed Germination
4.3. Changes in the Polar Metabolite Profiles of Growing Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed dormancy and germination–emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Han, C.; Yang, P. Studies on the molecular mechanisms of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Silva, A.T.; Ribone, P.A.; Chan, R.L.; Ligterink, W.; Hilhorst, H.W.M. A predictive coexpression network identifies novel genes controlling the seed-to-seedling phase transition in Arabidopsis thaliana. Plant Physiol. 2016, 170, 2218–2231. [Google Scholar] [CrossRef]
- North, H.; Baud, S.; Debeaujon, I.; Dubos, C.; Dubreucq, B.; Grappin, P.; Jullien, M.; Lepiniec, L.; Marion-Poll, A.; Miquel, M.; et al. Arabidopsis seed secrets unravelled after a decade of genetic and omics-driven research. Plant J. 2010, 61, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Catusse, J.; Job, C.; Job, D. Transcriptome- and proteome-wide analyses of seed germination. Comptes Rendus Biol. 2008, 331, 815–822. [Google Scholar] [CrossRef]
- Allen, E.; Moing, A.; Ebbels, T.M.D.; Maucourt, M.; Tomos, A.D.; Rolin, D.; Hooks, M.A. Correlation network analysis reveals a sequential reorganization of metabolic and transcriptional states during germination and gene-metabolite relationships in developing seedlings of Arabidopsis. BMC Syst. Biol. 2010, 4, 62. [Google Scholar] [CrossRef]
- Silva, A.T.; Ligterink, W.; Hilhorst, H.W.M. Metabolite profiling and associated gene expression reveal two metabolic shifts during the seed-to-seedling transition in Arabidopsis thaliana. Plant Mol. Biol. 2017, 95, 481–496. [Google Scholar] [CrossRef]
- Weckwerth, W. Metabolomics in systems biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef]
- Sreenivasulu, N. Systems biology of seeds: Deciphering the molecular mechanisms of seed storage, dormancy and onset of germination. Plant Cell Rep. 2017, 36, 633–635. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Ramalingam, A.; Kudapa, H.; Pazhamala, L.T.; Weckwerth, W.; Varshney, R.K. Proteomics and metabolomics: Two emerging areas for legume improvement. Front. Plant Sci. 2015, 6, 1116. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Tamura, T.; Kamei, A.; Ueda, R.; Arai, S.; Mura, K. Characterization of the quality of imbibed soybean at an early stage of pre-germination for the development of a new protein food item. Biosci. Biotechnol. Biochem. 2014, 78, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Liu, S.J.; Song, S.H.; Wang, R.X.; Wang, W.Q.; Song, S.Q. Proteomics analysis reveals distinct involvement of embryo and endosperm proteins during seed germination in dormant and non-dormant rice seeds. Plant Physiol. Biochem. 2016, 103, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M.; et al. Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008, 146, 1738–1758. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and role of starch, protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef]
- Kelly, A.A.; Quettier, A.L.; Shaw, E.; Eastmond, P.J. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol. 2011, 157, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of seed protein reserves. Physiol. Plant 2012, 145, 140–153. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Transitory and storage starch metabolism: Two sides of the same coin? Curr. Opin. Biotechnol. 2015, 32, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, P.J.; Germain, V.; Lange, P.R.; Bryce, J.H.; Smith, S.M.; Graham, I.A. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. USA 2000, 97, 5669–5674. [Google Scholar] [CrossRef] [PubMed]
- To, J.P.C.; Reiter, W.D.; Gibson, S.I. Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biol. 2002, 2, 4. [Google Scholar] [CrossRef]
- Erbaş, S.; Tonguç, M.; Karakurt, Y.; Şanli, A. Mobilization of seed reserves during germination and early seedling growth of two sunflower cultivars. J. Appl. Bot. Food Qual. 2016, 89, 217–222. [Google Scholar]
- Han, C.; Yin, X.; He, D.; Yang, P. Analysis of proteome profile in germinating soybean seed, and its comparison with rice showing the styles of reserves mobilization in different crops. PLoS ONE 2013, 8, e56947. [Google Scholar] [CrossRef]
- Allwood, J.W.; Ellis, D.I.; Goodcare, R. Metabolomic technologies and their application to the study of plant and plant-host interactions. Physiol. Plant 2008, 132, 117–135. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Obata, T.; Matthes, A.; Koszior, S.; Lehmann, M.; Araújo, W.L.; Bock, R.; Sweetlove, L.J.; Fernie, A.R. Alternation of mitochondrial protein complexes in relation to metabolic regulation under short-term oxidative stress in Arabidopsis seedlings. Phytochemistry 2011, 71, 1081–1091. [Google Scholar] [CrossRef]
- Frank, T.; Scholz, B.; Peter, S.; Engel, K.H. Metabolite profiling of barley: Influence of the malting process. Food Chem. 2011, 124, 948–957. [Google Scholar] [CrossRef]
- Howell, K.A.; Narsai, R.; Carroll, A.; Ivanova, A.; Lohse, M.; Usadel, B.; Millar, A.H.; Whelan, J. Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol. 2009, 149, 961–980. [Google Scholar] [CrossRef] [PubMed]
- Na Jom, K.; Frank, T.; Engel, K.H. A metabolite profiling approach to follow the sprouting process of mung beans (Vigna radiata). Metabolomics 2011, 7, 102–117. [Google Scholar] [CrossRef]
- Na Jom, K.; Chanput, W.; Ngampongsai, S. Effect of genetic and climatic variability on the metabolic profiles of black gram (Vigna mungo L.) seeds and sprouts. J. Sci. Food Agric. 2015, 95, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Gomez Roldan, M.V.; Engel, B.; de Vos, R.C.H.; Vereijken, P.; Astola, L.; Groenenboom, M.; van de Geest, H.; Bovy, A.; Molenaar, J.; van Eeuwijk, F.; et al. Metabolomics reveals organ-specific metabolic rearrangements during early tomato seedling development. Metabolomics 2014, 10, 958–974. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Willems, L.A.J.; Mudde, E.; Fernandez, L.G.; de Castro, R.D.; Ligterink, W.; Hilhorst, H.W.M. Metabolite profiling of the oilseed crop Ricinus communis during early seed imbibition reveals a specific metabolic signature in response to temperature. Ind. Crops Prod. 2015, 67, 305–309. [Google Scholar] [CrossRef]
- Gu, E.J.; Kim, D.W.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Kim, B.M.; Cho, Y.; Lee, H.J.; Kim, H.J. Mass-based metabolomic analysis of soybean sprouts during germination. Food Chem. 2017, 217, 311–319. [Google Scholar] [CrossRef]
- He, M.; Zhu, C.; Dong, K.; Zhang, T.; Cheng, Z.; Li, J.; Yan, Y. Comparative proteome analysis of embryo and endosperm reveals central differential expression proteins involved in wheat seed germination. BMC Plant Biol. 2015, 15, 97. [Google Scholar] [CrossRef]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Shu, X.L.; Frank, T.; Shu, Q.Y.; Engel, K.R. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Swarcewicz, B.; Sawikowska, A.; Marczak, Ł.; Łuczak, M.; Ciesiołka, D.; Krystkowiak, K.; Kuczyńska, A.; Piślewska-Bednarek, M.; Krajewski, P.; Stobiecki, M. Effect of drought stress on metabolite contents in barley recombinant inbred line population revealed by untargeted GC-MS profiling. Acta Physiol. Plant. 2017, 39, 158. [Google Scholar] [CrossRef]
- Virtanen, A.I.; Berg, A.; Kari, S. Formation of homoserine in germinating pea seeds. Acta Chem. Scand. 1953, 7, 1423–1424. [Google Scholar] [CrossRef]
- Melcher, I.M. Homoserine synthesis in dark-grown and light-grown seedlings of Pisum sativum. New Phytol. 1985, 100, 157–162. [Google Scholar] [CrossRef]
- Lin, T.P.; Yen, W.L.; Chien, C.T. Disappearance of desiccation tolerance of imbibed crop seeds is not associated with the decline of oligosaccharides. J. Exp. Bot. 1998, 49, 1203–1212. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, Z. Carbohydrate metabolism of cucurbits. In Handbook of Cucurbits Growth, Cultural Practices, and Physiology; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Valluru, R.; Van Den Ende, W. Plant fructans in stress environments: Emerging concepts and future prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Górecki, R.J.; Zalewski, K.; Hedley, C.L. Sorbitol accumulation during natural and accelerated ageing of pea (Pisum sativum L.) seeds. Acta Physiol. Plant. 2007, 29, 527–534. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Górecki, R.J. Raffinose in seedlings of winter vetch (Vicia villosa Roth.) under osmotic stress and followed by recovery. Acta Physiol. Plant. 2011, 33, 725–733. [Google Scholar] [CrossRef]
- Sun, X.; Weckwerth, W. COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 2012, 8, 81–93. [Google Scholar] [CrossRef]
- Abozeid, A.; Liu, J.; Ma, Y.; Liu, Y.; Guo, X.; Tang, Z. Seed metabolite profiling of Vicia species from China via GC-MS. Nat. Prod. Res. 2017, 32, 1863–1866. [Google Scholar] [CrossRef] [PubMed]
- Szablińska-Piernik, J.; Lahuta, L.B.; Stałanowska, K.; Horbowicz, M. The imbibition of pea (Pisum sativum L.) seeds in silver nitrate reduces seed germination, seedlings development and their metabolic profile. Plants 2022, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The size-dependent effects of silver nanoparticles on germination, early seedling development and polar metabolite profile of wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef] [PubMed]
- Lahuta, L.B.; Szablińska-Piernik, J.; Głowacka, K.; Stałanowska, K.; Railean-Plugaru, V.; Horbowicz, M.; Pomastowski, P.; Buszewski, B. The effect of bio-synthesized silver nanoparticles on germination, early seedling development, and metabolome of wheat (Triticum aestivum L.). Molecules 2022, 27, 2303. [Google Scholar] [CrossRef]
- Stałanowska, K.; Szablińska-Piernik, J.; Okorski, A.; Lahuta, L.B. Zinc Oxide Nanoparticles affect early seedlings’ growth and polar metabolite profiles of pea (Pisum sativum L.) and wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2023, 24, 14992. [Google Scholar] [CrossRef]
- Kuo, T.M.; van Middlesworth, J.F.; Wolf, W.J. Content of raffinose oligosaccharides and sucrose in various plant seeds. J. Agric. Food Chem. 1988, 36, 32–36. [Google Scholar] [CrossRef]
- Horbowicz, M.; Obendorf, R. Seed desiccation tolerance and storability: Dependence on flatulence-producing oligosaccharides and cycylitols—Revew and survey. Seed Sci. Res. 1994, 4, 385–405. [Google Scholar] [CrossRef]
- Bernal-Lugo, I.; Leopold, A.C. Seed stability during storage: Raffinose content and seed glassy state. Seed Sci. Res. 1985, 5, 75–80. [Google Scholar] [CrossRef]
- Sun, W.Q.; Irving, T.C.; Leopold, A.C. The role of sugars, vitrification and membrane phase transition in seed desiccation tolerance. Physiol. Plant. 1994, 90, 621–628. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Obendorf, R.L.; Górecki, R.J. Soluble carbohydrates in legume seeds. Seed Sci. Res. 2012, 22, 219–242. [Google Scholar] [CrossRef]
- Blöchl, A.; Peterbauer, T.; Richter, A. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J. Plant Physiol. 2007, 164, 1093–1096. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Goszczyńska, J. Inhibition of raffinose family oligosaccharides and galactosyl pinitols breakdown delays germination of winter vetch (Vicia villosa Roth.) seeds. Acta Soc. Bot. Pol. 2009, 78, 203–208. [Google Scholar] [CrossRef]
- Gangl, R.; Tenhaken, R. Raffinose family oligosaccharides act as galactose stores in seeds and are required for rapid germination of Arabidopsis in the dark. Front. Plant Sci. 2016, 7, 1115. [Google Scholar] [CrossRef]
- Livingston III, D.P.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.A.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Protein and amino acid composition of select wild legume species of tribe Fabeae. Food Chem. 2014, 163, 97–102. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Knežević, D.; Mihajlović, D.; Kondić, D. Contents of amino acids in grains of different bread wheat genotypes. Agro-Knowl. J. 2013, 14, 431–439. [Google Scholar] [CrossRef]
- Jacks, T.J.; Hensarling, T.P.; Yatsu, L.Y. Cucurbit Seeds: I. Characterizations and uses of oils and proteins. A review. Econ. Bot. 1972, 26, 135–141. [Google Scholar] [CrossRef]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, J.W.M. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Garnczarska, M.; Zalewski, T.; Bednarski, W.; Ratajczak, L.; Jurga, S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J. Plant Physiol. 2006, 163, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Rathjen, J.R.; Strounina, E.V.; Mares, D.J. Water movement into dormant and non dormant wheat (Triticum aestivum L.) grains. J. Exp. Bot. 2009, 60, 1619–1631. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. SEEDS Physiology of Development and Germination; Springer: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Aisien, A.O. Utilisation of soluble carbohydrates during sorghum germination and seedling growth. J. Inst. Brew. 1982, 88, 164–166. [Google Scholar] [CrossRef]
- Blöchl, A.; Peterbauer, T.; Hofmann, J.; Richter, A. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 2008, 228, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Pang, H.; Wang, Z.; Lu, J.; Wei, Y.; Huang, R. Characterization of an invertase with pH tolerance and truncation of its N-terminal to shift optimum activity toward neutral pH. PLoS ONE 2013, 8, e62306. [Google Scholar] [CrossRef]
- Borek, S.; Kubala, S.; Kubala, S. Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet): I. Mobilization of storage protein. Acta Physiol. Plant. 2012, 34, 701–711. [Google Scholar] [CrossRef]
- Urbano, G.; Lopez-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The role of phytic acid in legumes: Antinutrient or beneficial function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef]
- Geiger, J.H.; Jin, X. The structure and mechanism of myo-inositol-1-phosphate synthase. In Biology of Inositols and Phosphoinositides; Lahiri Majumder, A., Biswas, B.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Frank, A.W. (Ed.) Phytic Acid. In Chemistry of Plant Phosphorus Compounds; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Müntz, K.; Belozersky, M.A.; Dunaevsky, Y.E.; Schleretch, A.; Tiedemann, J. Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef]
- Hanning, I.; Baumgarten, K.; Schott, K.; Heldt, H.W. Oxaloacetate transport into plant mitochondria. Plant Physiol. 1999, 119, 1025–1031. [Google Scholar] [CrossRef]
- Binder, S. Branched-chain amino acid metabolism in Arabidopsis thaliana. Arab. Book 2010, 8, e0137. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Cejudo, F.J. Programmed cell death (PCD): An essential process of cereal seed development and germination. Front. Plant Sci. 2014, 5, 366. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.; Vernon, L.P.; Porath, D.; Arzee, T. Developmental stages of cucumber seedlings. Plant Physiol. 1990, 92, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Starck, Z. Transport i Dystrybucja Substancji Pokarmowych w Roślinach; Wydawnictwo SGGW: Warsaw, Poland, 2003. (In Polish) [Google Scholar]
- Turner, N.C. Turgor maintenance by osmotic adjustment, an adaptive mechanism for coping with plant water deficits. Plant Cell Environ. 2017, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. App Biol. 2006, 150, 1–26. [Google Scholar] [CrossRef]
- Sengupta-Gopalan, C.; Ortega, J.L. An insight into the role and regulation of glutamine synthetase in plants. In Amino Acids in Higher Plants; D’mello, P.F., Ed.; CABI: Wallingford, UK, 2015. [Google Scholar]
- Wu, P.; Tian, J.C.; Walker, C.E.; Wang, F.C. Determination of phytic acid in cereals—A brief review. Int. J. Food Sci. Technol. 2009, 44, 1671–1676. [Google Scholar] [CrossRef]
- Azeke, M.A.; Egielewa, S.J.; Eigbogbo, M.U.; Ihimire, I.G. Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J. Food Sci. Technol. 2011, 48, 724–729. [Google Scholar] [CrossRef]
- Borek, S.; Stefaniak, S.; Nuc, K.; Wojtyla, Ł.; Ratajczak, E.; Sitkiewicz, E.; Malinowska, A.; Świderska, B.; Wleklik, K.; Pietrowska-Borek, M. Sugar starvation disrupts lipid breakdown by inducing autophagy in embryonic axes of lupin (Lupinus spp.) germinating seeds. Int. J. Mol. Sci. 2023, 24, 11773. [Google Scholar] [CrossRef]
- Matsui, K.; Hijiya, K.; Tabuchi, Y.; Kajiwara, T. Cucumber cotyledon lipoxygenase during postgerminative growth. Its expression and action on lipid bodies. Plant Physiol. 1999, 119, 1279–1288. [Google Scholar] [CrossRef]
- Yu, X.; Li, A.; Li, W. How membranes organize during seed germination: Three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis. Plant Cell Environ. 2015, 38, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Sainis, J.K.; Mayne, R.G.; Wallsgrove, R.M.; Lea, P.J.; Miflin, B.J. Localisation and characterisation of homoserine dehydrogenase isolated from barley and pea leaves. Planta 1981, 152, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Amino acids in seeds and seedlings of the genus Lens. Phytochemistry 2001, 58, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.A.; Pharr, D.M.; Madore, M.A. Cucurbits. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablińska-Piernik, J.; Lahuta, L.B. Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat. Agriculture 2023, 13, 2278. https://doi.org/10.3390/agriculture13122278
Szablińska-Piernik J, Lahuta LB. Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat. Agriculture. 2023; 13(12):2278. https://doi.org/10.3390/agriculture13122278
Chicago/Turabian StyleSzablińska-Piernik, Joanna, and Lesław Bernard Lahuta. 2023. "Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat" Agriculture 13, no. 12: 2278. https://doi.org/10.3390/agriculture13122278




