Growth of Lettuce in Hydroponics Fed with Aerobic- and Anaerobic–Aerobic-Treated Domestic Wastewater
Abstract
:1. Introduction
2. Methods
2.1. Experimental Hydroponic Set-Up
2.2. Treatments
2.2.1. Hoagland Solution (HS)
2.2.2. Conventional Aerobic-Treated Wastewater (CE)
2.2.3. Ozonised CE (CEO)
2.2.4. Anaerobically Pre-Treated and Nitrified Wastewater (AN)
2.2.5. Biological Activated Carbon-Filtered AN Water (ANC)
2.3. Experimental Operation
2.4. Sampling
2.5. Climate Measurements
2.6. Determination of Leaf Chlorophyll
2.7. Water and Plant Analytics
2.8. Data Analysis
3. Results
3.1. First Experiment
3.1.1. Greenhouse Climate and Water Temperatures
3.1.2. Treatment Solutions
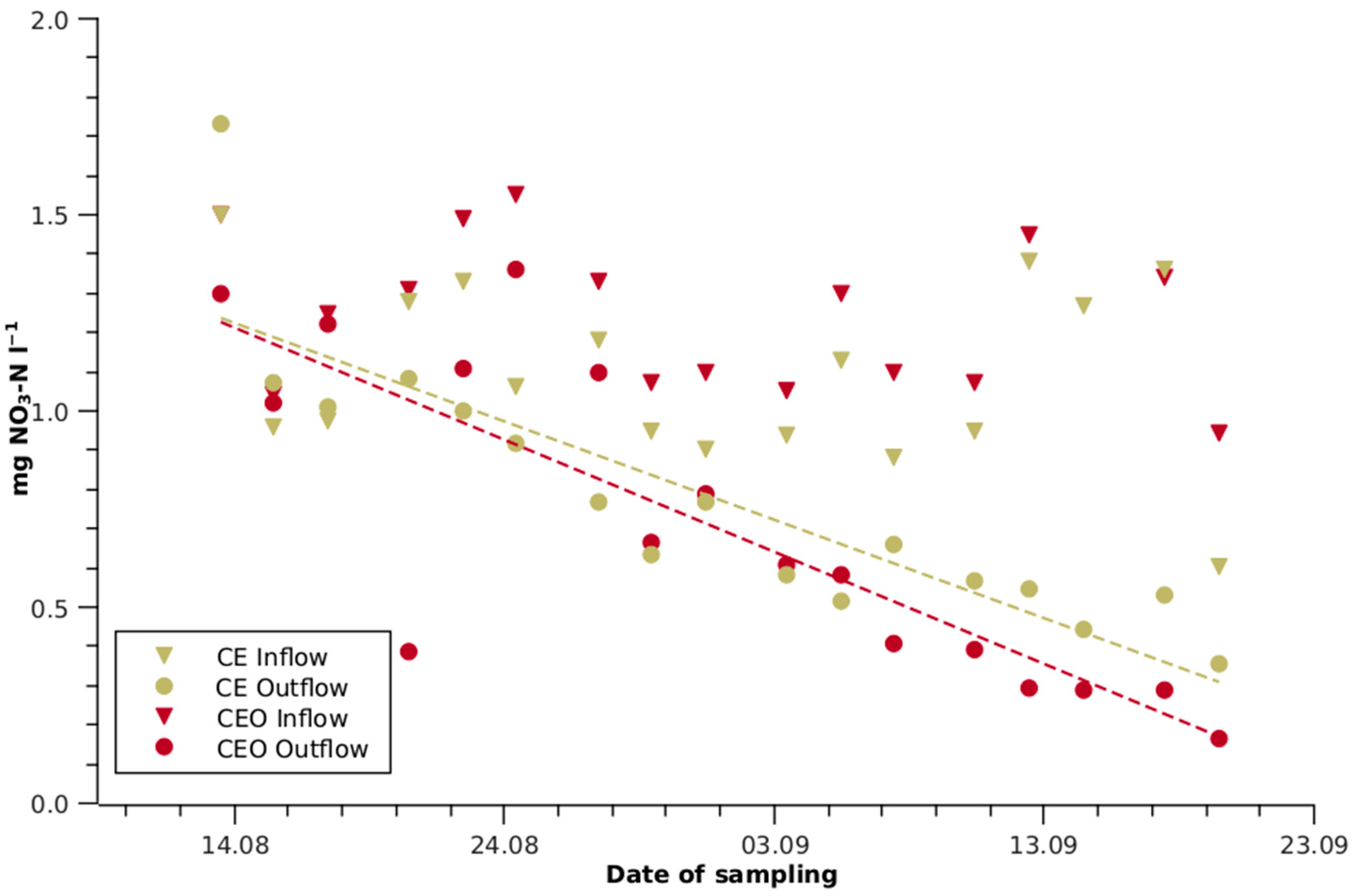
Nutrient Contents
pH
Electrical Conductivity (EC)
Dissolved Oxygen (DO)
3.1.3. Plant Development
Biomass
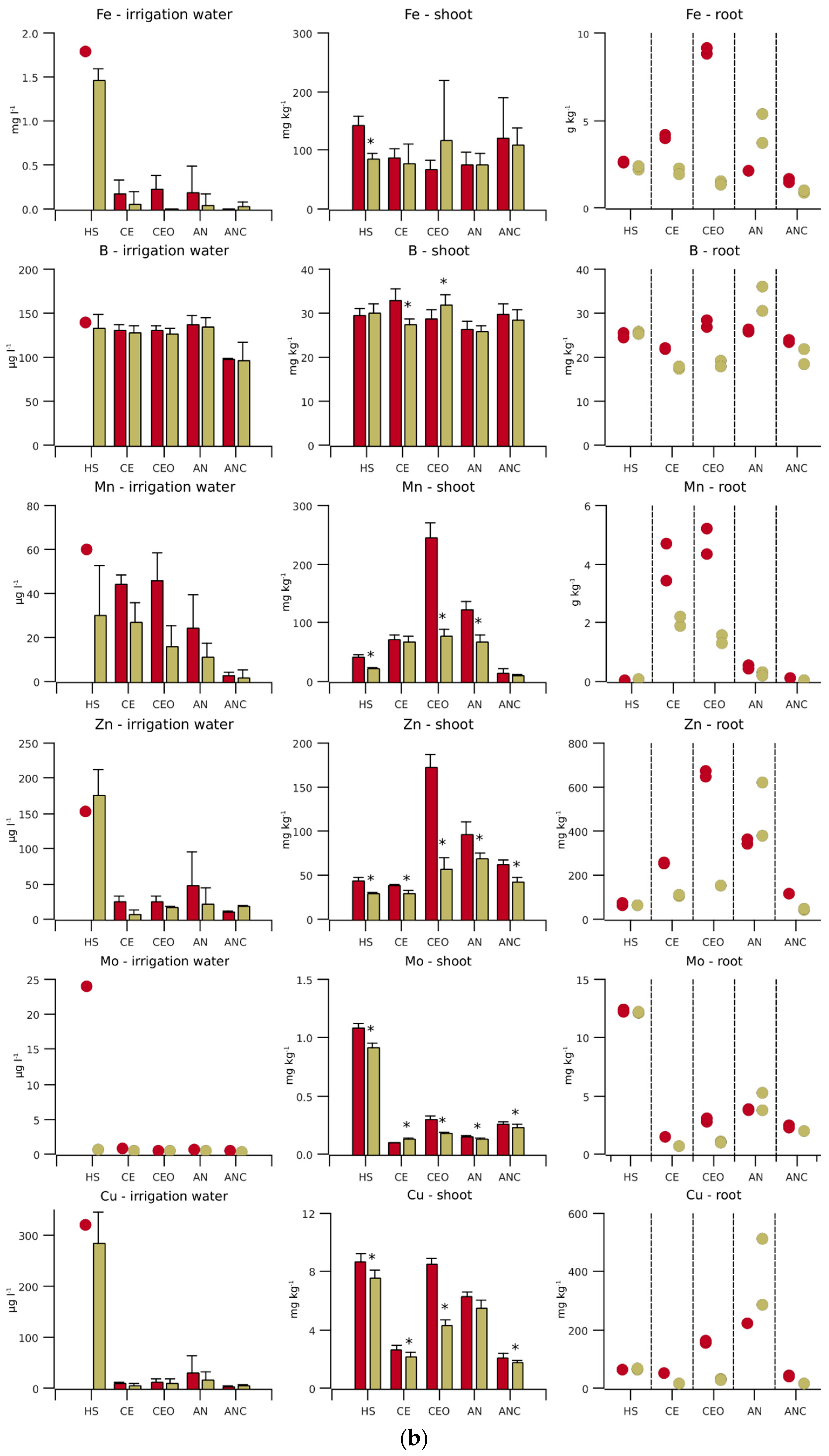
Shoot and Root Nutrient Content
Relative Chlorophyll Concentration
3.1.4. Second Experiment
4. Discussion
4.1. Suitability of Unamended Aerobic- and Anaerobic–Aerobic-Treated Domestic Wastewater for Lettuce Production
Nutrient Uptake of Lettuce from Aerobically and Anaerobically–Aerobically Treated Domestic Wastewater
4.2. Greenhouse Climate and Water Temperatures
4.3. Effect of Ozonation at Low Temperatures
5. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.T.; Scherzer, A.; Tramberend, S.; Nava, L.F.; Wada, Y.; Eisner, S.; Flörke, M. Water Futures and Solution—Fast Track Initiative (Final Report); International Institute for Applied Systems Analysis: Laxenburg, Austria, 2016. [Google Scholar]
- Adrover, M.; Moyà, G.; Vadell, J. Use of Hydroponics Culture to Assess Nutrient Supply by Treated Wastewater. J. Environ. Manag. 2013, 127, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Boyden, B.H.; Rababah, A.A. Recycling Nutrients from Municipal Wastewater. Desalination 1996, 106, 241–246. [Google Scholar] [CrossRef]
- Vaillant, N.; Monnet, F.; Sallanon, H.; Coudret, A.; Hitmi, A. Treatment of Domestic Wastewater by an Hydroponic NFT System. Chemosphere 2003, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bawiec, A. Efficiency of Nitrogen and Phosphorus Compounds Removal in Hydroponic Wastewater Treatment Plant. Environ. Technol. 2019, 40, 2062–2072. [Google Scholar] [CrossRef]
- Norström, A. Treatment of Domestic Wastewater Using Microbiological Processes and Hydroponics in Sweden; KTH Royal Institute of Technology in Stockholm: Stockholm, Sweden, 2005. [Google Scholar]
- Maruo, T.; Takagaki, M.; Shinohara, Y. Critical Nutrient Concentrations for Absorption of Some Vegetables. Acta Hortic. 2004, 644, 493–499. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Williams, E.; Smith, R.F. Establishing Lettuce Leaf Nutrient Optimum Ranges Through DRIS Analysis. HortScience 2007, 42, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Sosa, A.; Padilla, J.; Ortiz, J.; Etchevers, J.D. Biomass Accumulation and Its Relationship with the Demand and Concentration of Nitrogen, Phosphorus, and Potassium in Lettuce. Commun. Soil Sci. Plant Anal. 2012, 43, 121–133. [Google Scholar] [CrossRef]
- Bliedung, A.; Dockhorn, T.; Germer, J.; Mayerl, C.; Mohr, M. Experiences of Running a Hydroponic System in a Pilot Scale for Resource-Efficient Water Reuse. J. Water Reuse Desalination 2020, 10, 347–362. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005; ISBN 978-0-87893-172-9. [Google Scholar]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of Granular Activated Carbon Adsorption and Deep-Bed Filtration as a Single Advanced Wastewater Treatment Step for Organic Micropollutant and Phosphorus Removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of Emerging Micropollutants from Wastewater by Activated Carbon Adsorption: Experimental Study of Different Activated Carbons and Factors Influencing the Adsorption of Micropollutants in Wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Carruthers, T.J.B.; Longstaff, B.J.; Dennison, W.C.; Abal, E.G.; Aioi, K. Chapter 19—Measurement of Light Penetration in Relation to Seagrass. In Global Seagrass Research Methods; Short, F.T., Coles, R.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 369–392. ISBN 978-0-444-50891-1. [Google Scholar]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of Air Temperature on Tipburn Incidence of Butterhead and Leaf Lettuce in a Plant Factory. In Transplant Production in the 21st Century: Proceedings of the International Symposium on Transplant Production in Closed System for Solving the Global Issues on Environmental Conservation, Food, Resources and Energy; Kubota, C., Chun, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. ISBN 978-94-015-9371-7. [Google Scholar]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J.; CEA Staff. Hydroponic Lettuce Handbook; Cornell Controlled Environment Agriculture: Ithaca, NY, USA, 1996; pp. 504–509. [Google Scholar]
- Bergmann, W. Nutritional Disorders of Plants: Development, Visual and Analytical Diagnosis; Gustav Fischer: Jena, Germany, 1992; ISBN 978-1-56081-357-6. [Google Scholar]
- Eysinga, J.P.N.L.R.V.; Smilde, K.W. Nutritional Disorders in Glasshouse Lettuce; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1971; ISBN 978-90-220-0349-7. [Google Scholar]
- Kelly, S.D.; Bateman, A.S. Comparison of Mineral Concentrations in Commercially Grown Organic and Conventional Crops—Tomatoes (Lycopersicon esculentum) and Lettuces (Lactuca sativa). Food Chem. 2010, 119, 738–745. [Google Scholar] [CrossRef]
- Baslam, M.; Morales, F.; Garmendia, I.; Goicoechea, N. Nutritional Quality of Outer and Inner Leaves of Green and Red Pigmented Lettuces (Lactuca sativa L.) Consumed as Salads. Sci. Hortic. 2013, 151, 103–111. [Google Scholar] [CrossRef]
- Urbano, V.R.; Mendonça, T.G.; Bastos, R.G.; Souza, C.F. Effects of Treated Wastewater Irrigation on Soil Properties and Lettuce Yield. Agric. Water Manag. 2017, 181, 108–115. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L. Effects of Light-Diffusing Plastic Film on Lettuce Production and Quality Attributes. Span. J. Agric. Res. 2017, 15, 0801. [Google Scholar] [CrossRef] [Green Version]
- Msilini, N.; Attia, H.; Rabhi, M.; Karray, N.; Lachaâl, M.; Ouerghi, Z. Responses of Two Lettuce Cultivars to Iron Deficiency. Exp. Agric. 2012, 48, 523–535. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient Deprivation Eustress Elicits Differential Secondary Metabolites in Red and Green-Pigmented Butterhead Lettuce Grown in a Closed Soilless System. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- Van Geluwe, S.; Braeken, L.; Van der Bruggen, B. Ozone Oxidation for the Alleviation of Membrane Fouling by Natural Organic Matter: A Review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qian, G.; Ye, L.; Hu, X.; Yu, X.; Lyu, W. Research on the Enhancement of Biological Nitrogen Removal at Low Temperatures from Ammonium-Rich Wastewater by the Bio-Electrocoagulation Technology in Lab-Scale Systems, Pilot-Scale Systems and a Full-Scale Industrial Wastewater Treatment Plant. Water Res. 2018, 140, 77–89. [Google Scholar] [CrossRef]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated Wastewater Reuse for Irrigation: Pros and Cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- Turner, E.; Gian, R. Corrosion Control Techniques. In Internal Corrosion Control in Water Distribution Systems; Manual of Water Supply Practices; American Water Works Association: Denver, CO, USA, 2011; ISBN 978-1-58321-790-0. [Google Scholar]
- da Silva Oliveira, A.; Bocio, A.; Trevilato, T.M.B.; Takayanagui, A.M.M.; Domingo, J.L.; Segura-Muñoz, S.I. Heavy Metals in Untreated/Treated Urban Effluent and Sludge from a Biological Wastewater Treatment Plant. Environ. Sci. Pollut. Res. Int. 2007, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C.; Grieve, C.M. Tolerance of Vegetable Crops to Salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Andriolo, J.L.; da Luz, G.L.; Witter, M.H.; Godoi, R.d.S.; Barros, G.T.; Bortolotto, O.C. Growth and Yield of Lettuce Plants under Salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef] [Green Version]
- Cramer, G.R.; Spurr, A.R. Responses of Lettuce to Salinity. I. Effects of NaCl and Na2SO4 on Growth. J. Plant Nutr. 1986, 9, 115–130. [Google Scholar] [CrossRef]
- Goto, E.; Both, A.J.; Albright, L.D.; Langhans, R.W.; Leed, A.R. Effect of Dissolved Oxygen Concentration on Lettuce Growth in Floating Hydroponics. Acta Hortic. 1996, 440, 205–210. [Google Scholar] [CrossRef]
- Atkin, O.K.; Edwards, E.J.; Loveys, B.R. Response of Root Respiration to Changes in Temperature and Its Relevance to Global Warming. New Phytol. 2000, 147, 141–154. [Google Scholar] [CrossRef]
- Goto, K.; Yabuta, S.; Tamaru, S.; Ssenyonga, P.; Emanuel, B.; Katsuhama, N.; Sakagami, J.-I. Root Hypoxia Causes Oxidative Damage on Photosynthetic Apparatus and Interacts with Light Stress to Trigger Abscission of Lower Position Leaves in Capsicum. Sci. Hortic. 2022, 305, 111337. [Google Scholar] [CrossRef]
- Broadley, M.R.; Escobar-Gutiérrez, A.J.; Burns, A.; Burns, I.G. Nitrogen-Limited Growth of Lettuce Is Associated with Lower Stomatal Conductance. New Phytol. 2001, 152, 97–106. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Kovács, B.; Puskás-Preszner, A.; Huzsvai, L.; Lévai, L.; Bódi, É. Effect of Molybdenum Treatment on Molybdenum Concentration and Nitrate Reduction in Maize Seedlings. Plant Physiol. Biochem. 2015, 96, 38–44. [Google Scholar] [CrossRef] [Green Version]
- van den Ende, J.; Boertje, G.A. Molybdenum Deficiency in Young Lettuce and Tomato Plants. Acta Hortic. 1972, 26, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Plant, W. Molybdenum Deficiency in Lettuce. Nature 1952, 169, 803. [Google Scholar] [CrossRef]
- Steiner, F.; Zoz, T.; Zuffo, A.M.; Machado, P.P.; Zoz, J.; Zoz, A. Foliar Application of Molybdenum Enhanced Quality and Yield of Crispleaf Lettuce (Lactuca Sativa L., Cv. Grand Rapids). Acta Agronómica 2018, 67, 73–78. [Google Scholar] [CrossRef]
- Bloom, A.J.; Sukrapanna, S.S.; Warner, R.L. Root Respiration Associated with Ammonium and Nitrate Absorption and Assimilation by Barley. Plant Physiol. 1992, 99, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, G.; Yamashita, Y.; Nakabayashi, K. Effect of Supersaturation of Dissolved Oxygen on the Growth of Tomato Plants and Nutrient Uptake in Hydroponic Culture. Shokubutsu Kojo Gakkaishi 2001, 13, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Pacumbaba, R.O.; Beyl, C.A. Changes in Hyperspectral Reflectance Signatures of Lettuce Leaves in Response to Macronutrient Deficiencies. Adv. Space Res. 2011, 48, 32–42. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of Mineral Nutritional Status on Shoot-Root Partitioning of Photoassimilates and Cycling of Mineral Nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Wallace, A.; Patel, P.M.; Berry, W.L.; Lunt, O.R. Reclaimed Sewage Water: A Hydroponic Growth Medium for Plants. Resour. Recover. Conserv. 1978, 3, 191–199. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M.L. Iron Uptake and Transport in Plants: The Good, the Bad, and the Ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharbi, F.; Rejeb, S.; Ghorbal, M.H.; Morel, J.L. Plant Response to Copper Toxicity as Affected by Plant Species and Soil Type. J. Plant Nutr. 2005, 28, 379–392. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water Air Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]
- Thompson, H.C.; Langhans, R.W.; Both, A.-J.; Albright, L.D. Shoot and Root Temperature Effects on Lettuce Growth in a Floating Hydroponic System. J. Am. Soc. Hortic. Sci. 1998, 123, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Fuentes, J.A.; Shackel, K.; Heinrich Lieth, J.; Albornoz, F.; Benavides-Mendoza, A.; Evans, R.Y. Diurnal Root Zone Temperature Variations Affect Strawberry Water Relations, Growth, and Fruit Quality. Sci. Hortic. 2016, 203, 169–177. [Google Scholar] [CrossRef]
- Tibbitts, T.W.; Bottenberg, G. Growth of Lettuce under Controlled Humidity Levels. J. Am. Soc. Hortic. Sci. 1976, 101, 70–73. [Google Scholar] [CrossRef]
- Weiguo, F.; Pingping, L.; Yanyou, W.; Jianjian, T. Effects of Different Light Intensities on Anti-Oxidative Enzyme Activity, Quality and Biomass in Lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Lee, S.K.; Dodd, I.C. Limitations to Photosynthesis of Lettuce Grown under Tropical Conditions: Alleviation by Root-zone Cooling. J. Exp. Bot. 2001, 52, 1323–1330. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, Photosynthesis, and Nutrient Uptake at Different Light Intensities and Temperatures in Lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Kitano, M.; Eguchi, H. Growth of Lettuce Plants (Lactuca sativa L.) under Control of Dissolved O2 Concentration in Hydroponics. BioTronics 1997, 26, 39–45. [Google Scholar]
- Lee, J.W.; Lee, B.S.; Kang, J.G.; Bae, J.H.; Ku, Y.G.; Gorinstein, S.; Lee, J.H. Effect of Root Zone Aeration on the Growth and Bioactivity of Cucumber Plants Cultured in Perlite Substrate. Biologia 2014, 69, 610–617. [Google Scholar] [CrossRef]
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of Ozone in Water: A Review. Ozone Sci. Eng. 2012, 34, 233–242. [Google Scholar] [CrossRef]
- Mori, N.; Watanabe, H. Effects of Oxidative Stress on the Growth of Leaf Lettuce upon H2O2 Treatment. Eco-Eng. 2017, 29, 31–38. [Google Scholar] [CrossRef]
- Graham, T.; Zhang, P.; Woyzbun, E.; Dixon, M. Response of Hydroponic Tomato to Daily Applications of Aqueous Ozone via Drip Irrigation. Sci. Hortic. 2011, 129, 464–471. [Google Scholar] [CrossRef]
- Machuca, A.; Odio, A.; Tapia, M.L.; Silveira, A.C.; Escalona, V. Ozone Applied to the Nutrient Solution and Its Impact on Red Chard Baby Leaves Yield. In Proceedings of the VIII International Postharvest Symposium: Enhancing Supply Chain and Consumer Benefits-Ethical and Technological Issues 1194, Cartagena Murcia, Spain, 21–24 June 2016; pp. 173–180. [Google Scholar]
- Najarian, M.; Mohammadi-Ghehsareh, A.; Fallahzade, J. Interactive Effects of Drought Stress and Ozonated Water on Growth and Yield of Cucumber (Cucumis sativus L.). J. Environ. Sci. Technol. 2015, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Kaneko, K.; Yoshii, M.; Isobe, T.; Park, J.-S.; Kurata, K.; Fujiwara, K. Nutrient Solution Prepared with Ozonated Water Does Not Damage Early Growth of Hydroponically Grown Tomatoes. Ozone Sci. Eng. 2009, 31, 21–27. [Google Scholar] [CrossRef]



| Dissolved Oxygen (mg L−1) | ||||
|---|---|---|---|---|
| 1st Experiment | 2nd Experiment | |||
| Inflow | Outflow | Inflow | Outflow | |
| CE | 6.2 | 5.4 a | 8.7 | 8.1 a |
| CEO | 16.1 | 12.6 c | 19.5 | 11.0 c,# |
| AN | 6.8 | 5.5 b | 9.8 | 7.5 ns |
| ANC | 8.3 | 6.9 c | 9.9 | 9.1 b |
| Dry Matter | N | P | K | Ca | Mg | S | B | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient Content | mg kg−1 | ||||||||||
| 1 Min | 45,000 | 7855 | 49,805 | 10,721 | 3619 | 2000 | 24 | 100 | 20 | 30 | 7 |
| 1 Max | 60,000 | 13,092 | 99,610 | 21,441 | 9048 | 4000 | 40 | 600 | 200 | 330 | 17 |
| 2 Min | 45,000 | 4500 | 42,000 | 12,000 | 3500 | 25 | 30 | 30 | 7 | ||
| 2 Max | 55,000 | 7000 | 60,000 | 21,000 | 6000 | 60 | 100 | 80 | 15 | ||
| 3 | 40,000 | 50,000 | 2800 | 220 | 50 | 55 | 8 | ||||
| 4 | 35,417 | 1733 | 67,933 | 12,400 | 3786 | 2133 | 81 | 120 | 48 | 2 | |
| 5 | 41,300 | 4530 | 48,600 | 9710 | 2730 | 2730 | 20 | 485 | 83 | 10 | 10 |
| 6 | 44,340 | 5203 | 79,820 | 18,020 | 4728 | 3300 | 95 | 22 | 49 | 10 | |
| Target | 45,000 | 6300 | 62,000 | 15,000 | 3500 | 2700 | 35 | 120 | 80 | 80 | 10 |
| Nutrient supply with 565 l treated wastewater in % of the demand for the production of 680 g biomass | |||||||||||
| CE | −55 | 762 | 420 | 6926 | 4235 | 16,577 | 5314 | 1941 | 816 | 600 | 1119 |
| CEO | −45 | 756 | 420 | 6945 | 4227 | 16,593 | 5314 | 2621 | 870 | 643 | 1407 |
| AN | 1468 | 2087 | 466 | 6926 | 4480 | 13,087 | 5314 | 2117 | 400 | 1308 | 4334 |
| ANC | 1456 | 1919 | 447 | 6473 | 4461 | 13,413 | 3940 | −100 | −50 | 169 | 126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germer, J.; Brandt, C.; Rasche, F.; Dockhorn, T.; Bliedung, A. Growth of Lettuce in Hydroponics Fed with Aerobic- and Anaerobic–Aerobic-Treated Domestic Wastewater. Agriculture 2023, 13, 1529. https://doi.org/10.3390/agriculture13081529
Germer J, Brandt C, Rasche F, Dockhorn T, Bliedung A. Growth of Lettuce in Hydroponics Fed with Aerobic- and Anaerobic–Aerobic-Treated Domestic Wastewater. Agriculture. 2023; 13(8):1529. https://doi.org/10.3390/agriculture13081529
Chicago/Turabian StyleGermer, Jörn, Christian Brandt, Frank Rasche, Thomas Dockhorn, and Alexa Bliedung. 2023. "Growth of Lettuce in Hydroponics Fed with Aerobic- and Anaerobic–Aerobic-Treated Domestic Wastewater" Agriculture 13, no. 8: 1529. https://doi.org/10.3390/agriculture13081529





