Shining a Light on Symbiosis: N-Fixing Bacteria Boost Legume Growth under Varied Light Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setting
2.2. Calculation of δ15N
2.3. Enzyme Activities Measurements
2.4. Statistical Analysis
3. Results
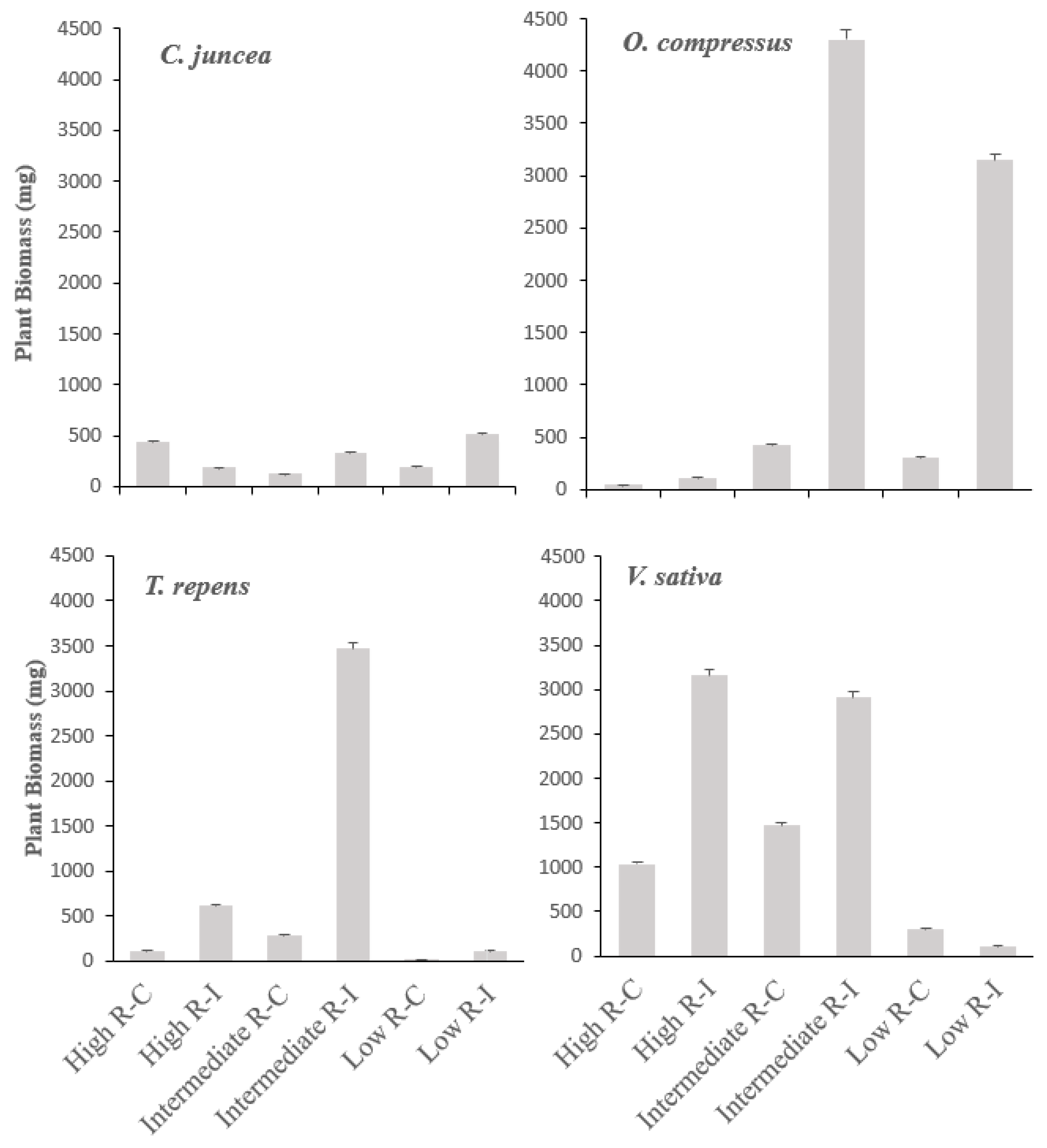
3.1. Biomass Production
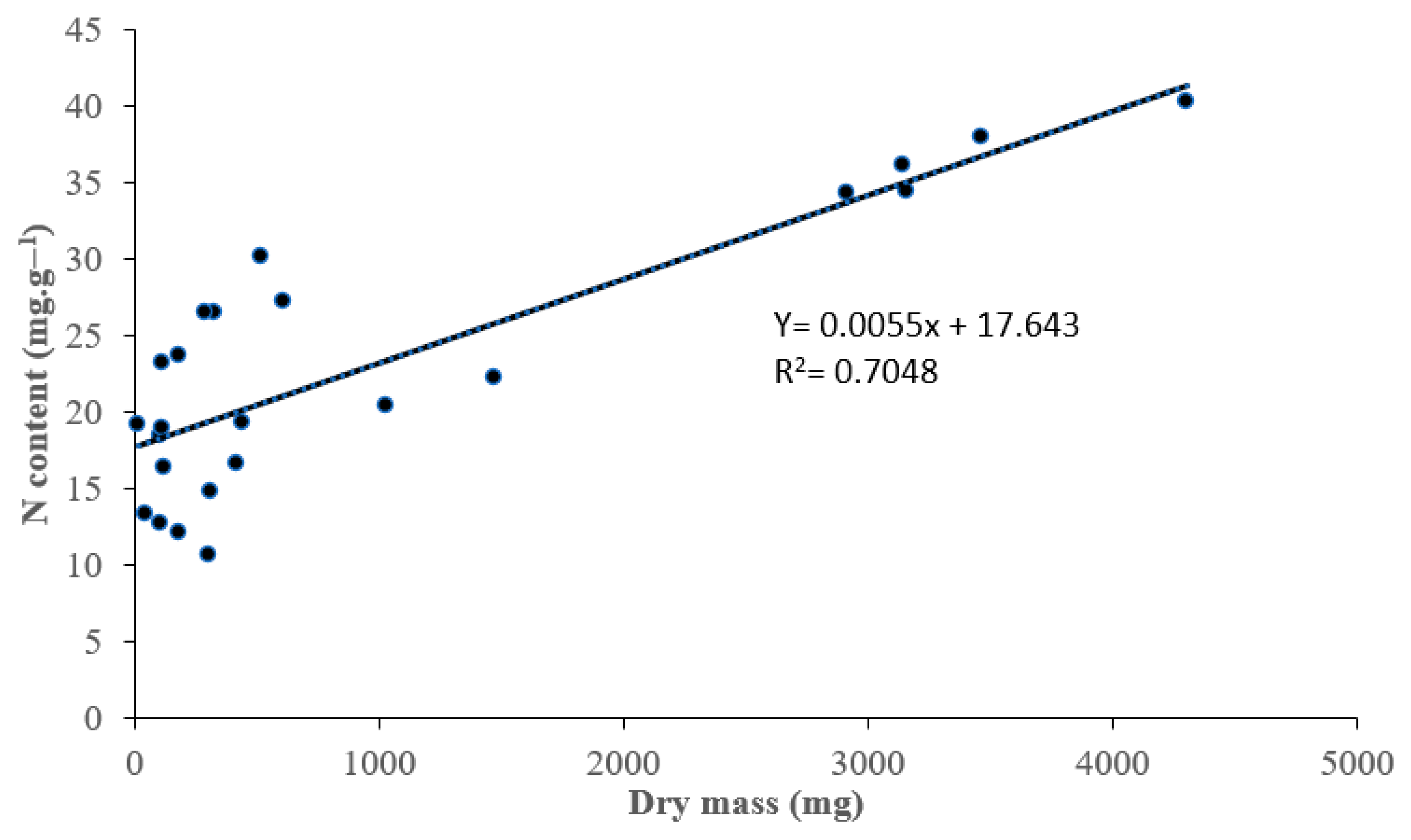
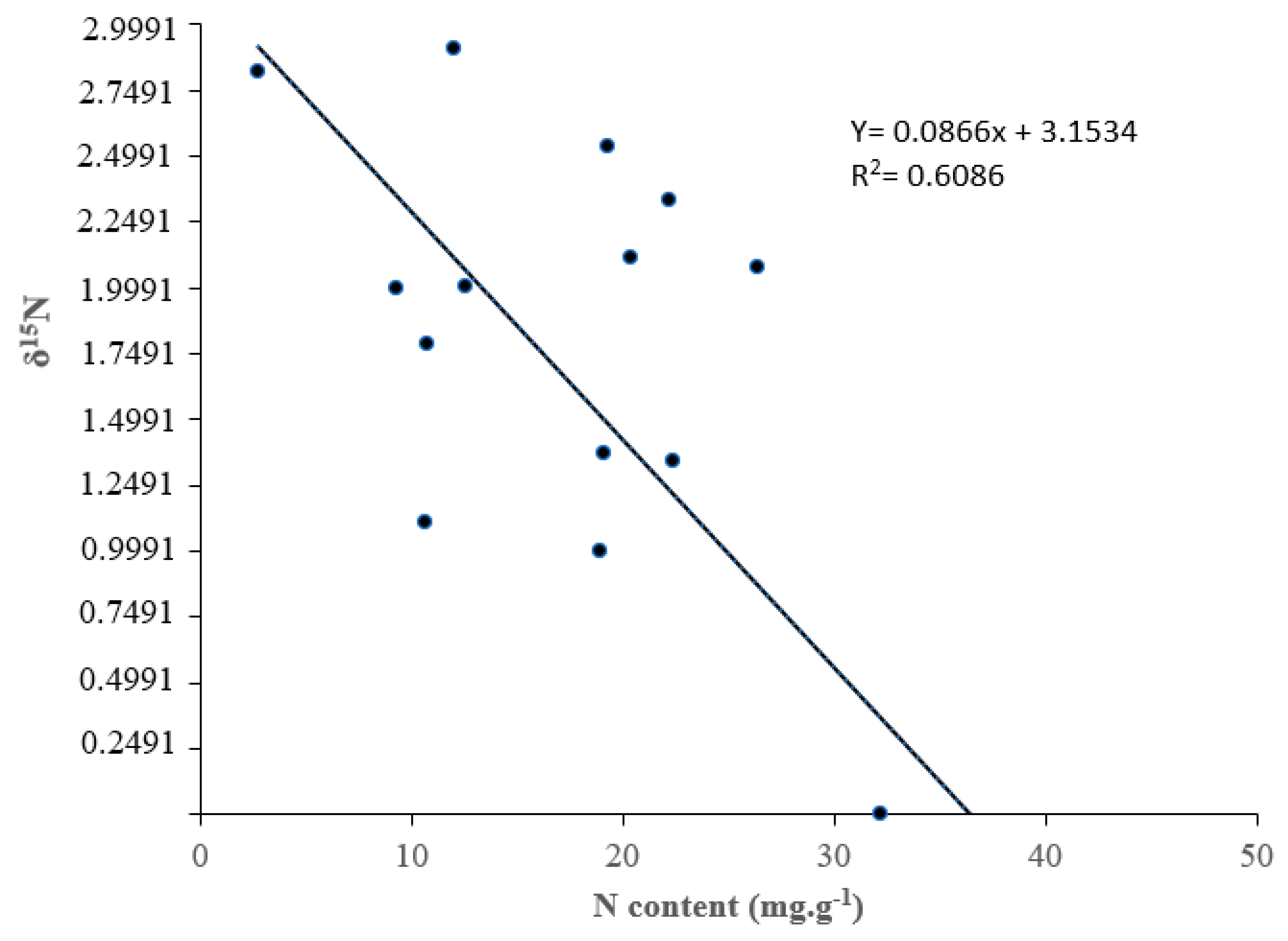
3.2. Nitrogen Concentrantions and Rhizobia Symbiotic Efficiency
3.3. Enzymatic Activities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloepper, J.W.; Ryu, C.M.J. Sustainable agriculture: The emerging role of rhizosphere bacteria. Nat. Rev. Microbiol. 2006, 4, 866–879. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 858p. [Google Scholar]
- Gazolla-Neto, A.; Aumonde, T.Z.; Pedó, T.; Olsen, D. Ação de Níveis de Luminosidade Sobre o Crescimento de Plantas de Maria-Pretinha (Solanum americanum Mill.); Revista Brasileira de Biociências: Porto Alegre, Brazil, 2013; Volume 11. [Google Scholar]
- De Lara-Del Rey, I.A.; Pérez-Fernández, M.A. Regulatory Effect of Light and Rhizobial Inoculation on the Root Architecture and Plant Performance of Pasture Legumes. Agronomy 2023, 13, 2058. [Google Scholar] [CrossRef]
- Domínguez-Martín, M.A.; Sauer, P.V.; Kirst, H.; Sutter, M.; Bína, D.; Greber, B.J.; Nogales, E.; Polívka, T.; Kerfeld, C.A. Structures of a Phycobilisome in Light-Harvesting and Photoprotected States. Nature 2022, 609, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Alsanius, B.; Karlsson, M.; Rosberg, A.; Dorais, M.; Naznin, M.; Khalil, S.; Bergstrand, K.-J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae 2019, 5, 41. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon Input by Plants into the Soil Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root Exudates Mediated Interactions Belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource limitation in plants an economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B.; Castro-Díez, P. Tri-trophic interactions in the context of climate change: Effects of psyllid herbivory on nitrogen acquisition and resource allocation in a fast-growing herb. Oikos 2008, 117, 1865–1877. [Google Scholar]
- Lambers, H.; Raven, J.; Shaver, G.; Smith, S. Plant Nutrient-Acquisition Strategies Change with Soil Age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced Root Exudation Induces Microbial Feedbacks to N Cycling in a Pine Forest under Long-term CO2 Fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Bainard, L.D.; Klironomos, J.N.; Hart, M.M. Differential Effect of Sample Preservation Methods on Plant and Arbuscular Mycorrhizal Fungal DNA. J. Microbiol. Methods 2010, 82, 124–130. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-Microbe-Soil Interactions in the Rhizosphere: An Evolutionary Perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Smith, A. Adaptation of Plants to High-Light Environments. Plant Ecol. 2019, 214, 215–231. [Google Scholar]
- Brown, C. Shade as a Driver of Plant Adaptations to Low-Light Environments. Environ. Biol. 2020, 145, 1611–1621. [Google Scholar]
- Lachica, M.; Aguilar, A.; Yánez, J. Análisis foliar. Métodos utilizados en la Estación Experimental del Zaidín CSIC (II). An. Edaf. Agrobiol. 1973, 32, 1033–1047. [Google Scholar]
- Bouat, A.; Crouzet, C. Notes techniques sur un appareil semiautomatique de clorage de l’azote et de certains composes volatiles. Ann. Agric. 1965, 16, 107–118. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-fixation in field settings: Estimations based on natural 15N abundance. Funct. Plant Biol. 1986, 13, 699–756. [Google Scholar]
- Langelaan, J.G.; Troelstra, S.R. Growth, Chemical Composition, and Nitrate Reductase Activity of Rumex Species in Relation to Form and Level of N Supply. Plant Soil 1992, 145, 215–229. [Google Scholar] [CrossRef]
- Streeter, J.G. Acid and alkaline phosphatase activities in roots of legumes. Plant Physiol. 1985, 77, 610–614. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- IBM SPSS Software. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 1 November 2023).
- Liu, Y.; Liu, M.; Liu, S.; Wang, X.; Li, X. Light intensity and rhizobial inoculation affect root morphology and exudation in soybean (Glycine max L.). J. Plant Physiol. 2021, 259, 153333. [Google Scholar]
- Li, Y.; Sun, W.; Zhang, X.; Li, F.; Wang, Y. Light intensity-dependent effects of rhizobial inoculation on growth, photosynthesis, and nitrogen metabolism in soybean (Glycine max L.). Front. Plant Sci. 2022, 13, 873180. [Google Scholar]
- Cui, J.; Zhao, J.; Wang, Y.; Liu, S.; Li, J.; Zhang, F. Light intensity and rhizobial inoculation affect the growth, photosynthesis, and nitrogen metabolism of alfalfa (Medicago sativa L.). J. Integr. Agric. 2022, 21, 617–628. [Google Scholar]
- Chen, Y.; Wang, Z.; Zhang, Z.; Sun, Y.; Li, X. Light intensity affects the rhizosphere microbiome composition and nitrogen fixation in soybean-rhizobia symbiosis. Front. Microbiol. 2021, 12, 746538. [Google Scholar]
- Wang, C.; Han, J.; Sun, J.; Gao, Q. Light intensity affects the growth, physiological traits, and root exudates of soybean plants (Glycine max L.) inoculated with rhizobia. Plant Physiol. Biochem. 2020, 152, 129–141. [Google Scholar]
- Zhao, J.; Cui, J.; Chen, J.; Liu, S.; Zhang, F. Rhizobial inoculation enhances the tolerance of alfalfa (Medicago sativa L.) to salt stress under different light conditions. Front. Plant Sci. 2023, 14, 1063596. [Google Scholar]
- Hussain, H.A.; Farooq, M.; Ashraf, M.; Khan, M.I.R. Physiological and molecular mechanisms of drought tolerance in legumes: A review. Agronomy 2020, 10, 695. [Google Scholar]
- Khan, A.; Ali, S.; Hameed, A.; Farooq, M.; Iqbal, N. Role of nitrate reductase activity in drought tolerance of legumes: A review. Plants 2022, 11, 348. [Google Scholar]
- Wang, C.; Zhang, H.; Zhang, W.; Wang, L.; Zhang, Y.; Li, G.; Yang, J. Nitrate reductase and its regulation in plants under abiotic stress. Front. Plant Sci. 2022, 13, 858372. [Google Scholar]
- Hu, J.; Lu, L.; Chen, X.; Li, Z. Acid phosphatase activity and physiological responses of alfalfa (Medicago sativa L.) to salt stress and arbuscular mycorrhizal fungus inoculation. Environ. Sci. Pollut. Res. 2022, 29, 43338–43353. [Google Scholar]
- Güsewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Bloom, A.J. Phosphate absorption: A major component of nutrient acquisition for some wild plant communities. Oecologia 1981, 51, 11–21. [Google Scholar]
- Míguez-Montero, M.A.; Valentine, A.; Pérez-Fernández, M.A. Regulatory Effect of Phosphorus and Nitrogen on Nodulation and Plant Performance of Leguminous Shrubs. AoB Plants 2019, 12, plz047. [Google Scholar] [CrossRef]
- Meng, X.; Li, H.; Wang, Z.; Ma, Z.; Wang, J.; Wang, X.; Li, X. Rhizobial inoculation enhances the tolerance of soybean (Glycine max L.) to high light stress through modulating the antioxidant system and nitrogen metabolism. Front. Plant Sci. 2021, 12, 657643. [Google Scholar]
- Baxter, I.; Dilkes, B.J. The plant mining strategy theory and its implications for plant evolution and nutrient cycling. J. Ecol. 2018, 106, 1696–1704. [Google Scholar]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Chapin, F.S. The Mineral Nutrition of Wild Plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; He, S.H.; Liu, C.Y.; Wang, X.J.; Wang, C.K.; Jian, T.M.; Chen, F.Q.; Zhang, G.L. Light-regulated NO production in plants: The role of NADPH oxidase and nitrate reductase. Photosynth. Res. 2014, 118, 7–19. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Fernández, M.; De Lara-Del Rey, I.A.; Magadlela, A. Shining a Light on Symbiosis: N-Fixing Bacteria Boost Legume Growth under Varied Light Conditions. Agriculture 2024, 14, 164. https://doi.org/10.3390/agriculture14020164
Pérez-Fernández M, De Lara-Del Rey IA, Magadlela A. Shining a Light on Symbiosis: N-Fixing Bacteria Boost Legume Growth under Varied Light Conditions. Agriculture. 2024; 14(2):164. https://doi.org/10.3390/agriculture14020164
Chicago/Turabian StylePérez-Fernández, María, Irene Ariadna De Lara-Del Rey, and Anathi Magadlela. 2024. "Shining a Light on Symbiosis: N-Fixing Bacteria Boost Legume Growth under Varied Light Conditions" Agriculture 14, no. 2: 164. https://doi.org/10.3390/agriculture14020164
APA StylePérez-Fernández, M., De Lara-Del Rey, I. A., & Magadlela, A. (2024). Shining a Light on Symbiosis: N-Fixing Bacteria Boost Legume Growth under Varied Light Conditions. Agriculture, 14(2), 164. https://doi.org/10.3390/agriculture14020164






