Absorption and Transport of Phosphorus in Nodulated Soybean Plants and Diagnosis of Phosphorus Status Using Xylem Sap Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Changes in the Xylem Sap Flow Rate and Its Chemical Compositions after Shoot Removal
2.3. Effect of Period of PO4 Treatments on the P Absorption Rate and P Concentration in Xylem Sap
2.4. Effect of P Concentrations in Culture Solution on the P Absorption Rate and P Concentration in Xylem Sap and Each Part of Soybean Plants
2.5. Chemical Analysis
2.5.1. Measurement of PO4 Concentration
2.5.2. Measurement of Cation and Anion Concentrations
2.5.3. Measurement of Amide and Ureide Concentrations
2.6. Statistics
3. Results
3.1. Changes in the Xylem Sap Flow Rate and the Concentrations of Nutrients after Shoot Removal
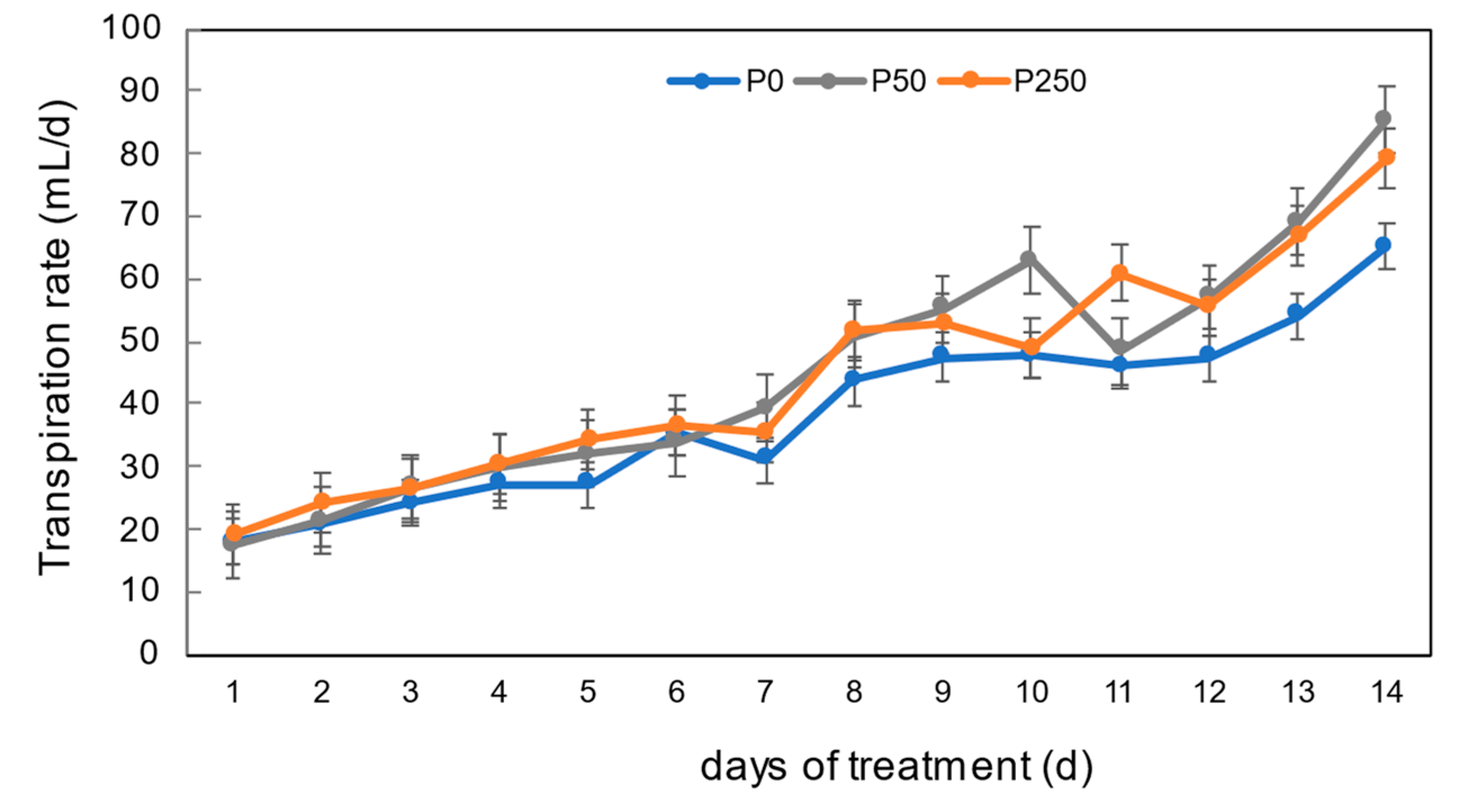
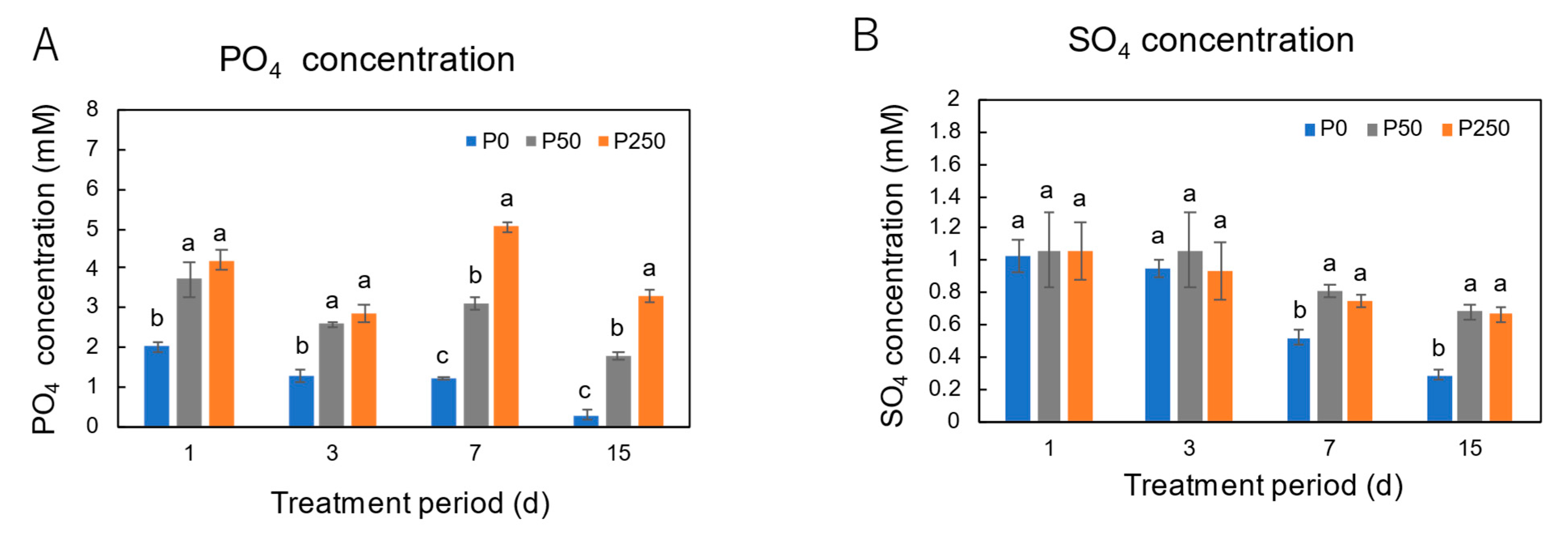
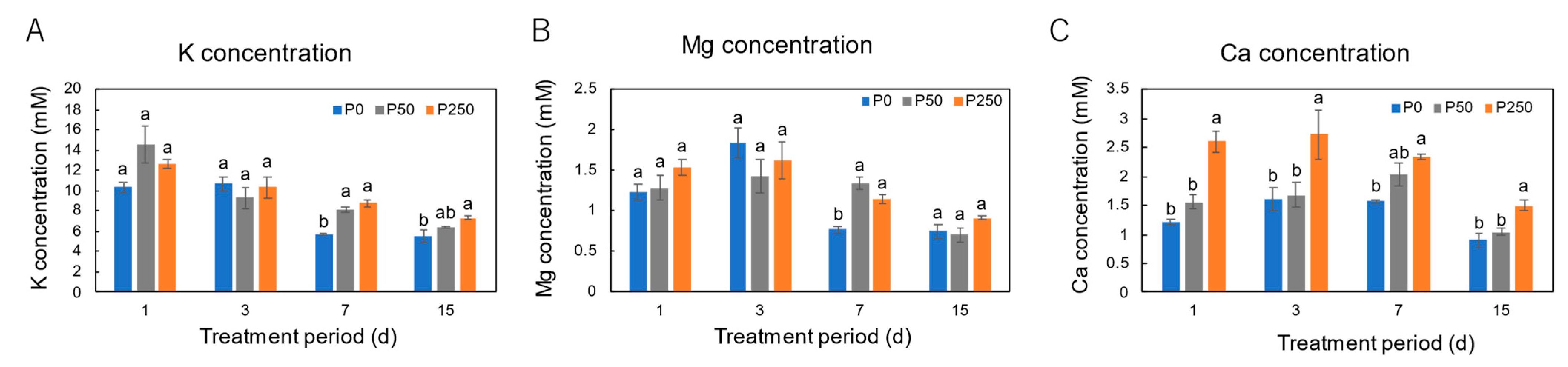
3.2. Effect of Period of P Treatments in Culture Solution on the P and Nutrient Concentrations in Xylem Sap of Soybean Plants Cultivated with an N-Free Culture Solution
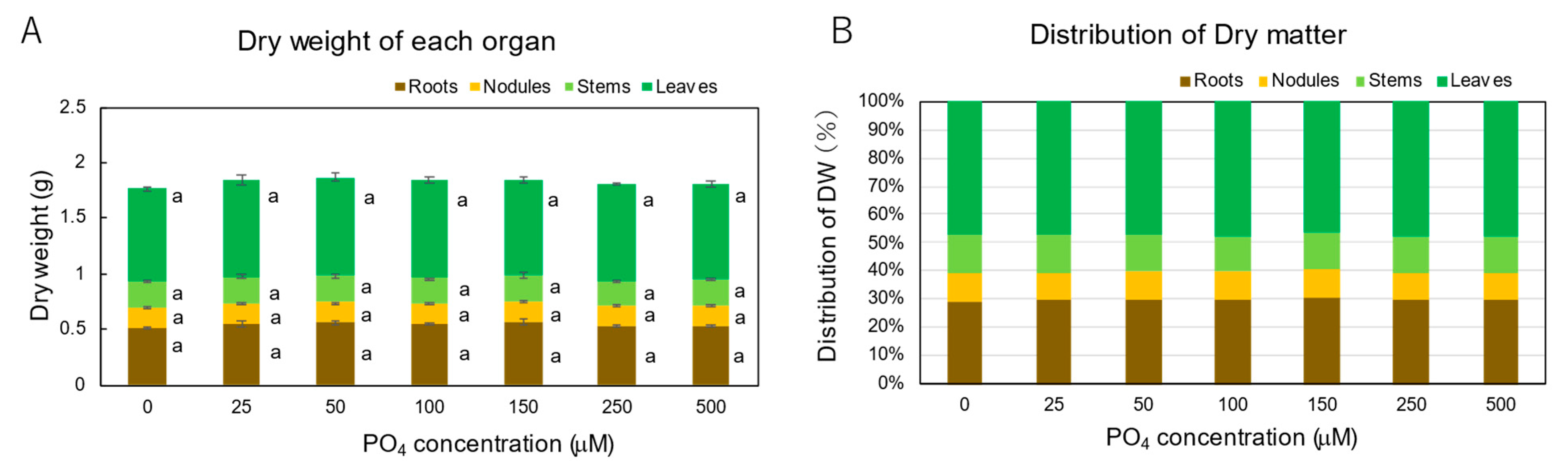
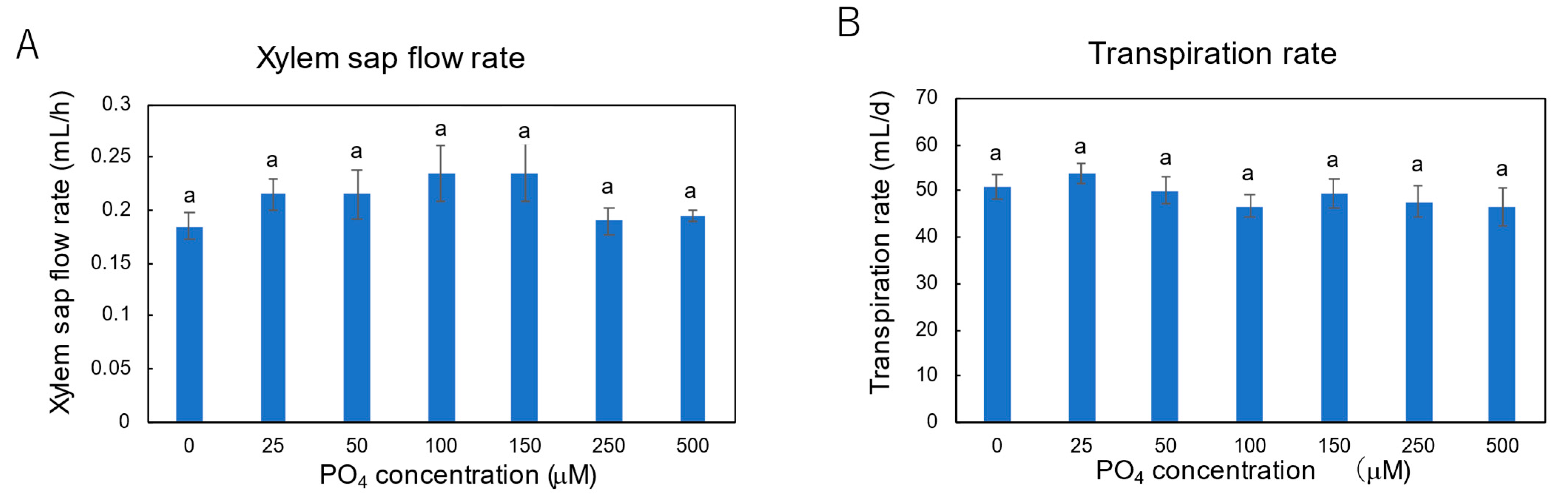
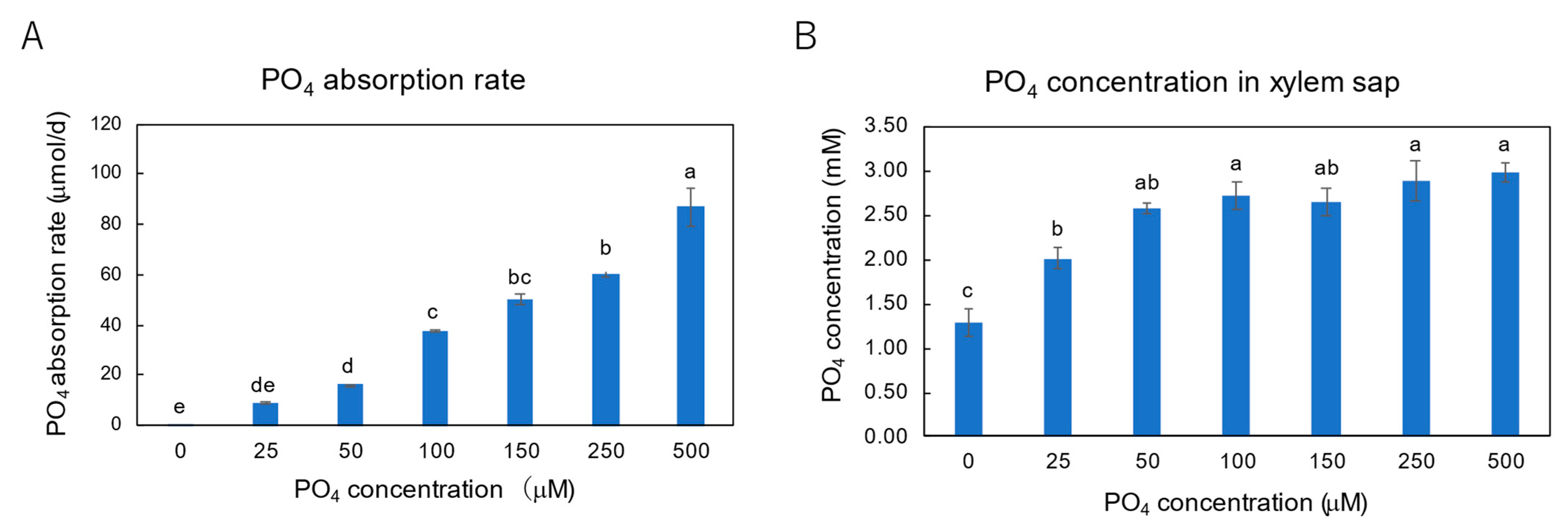
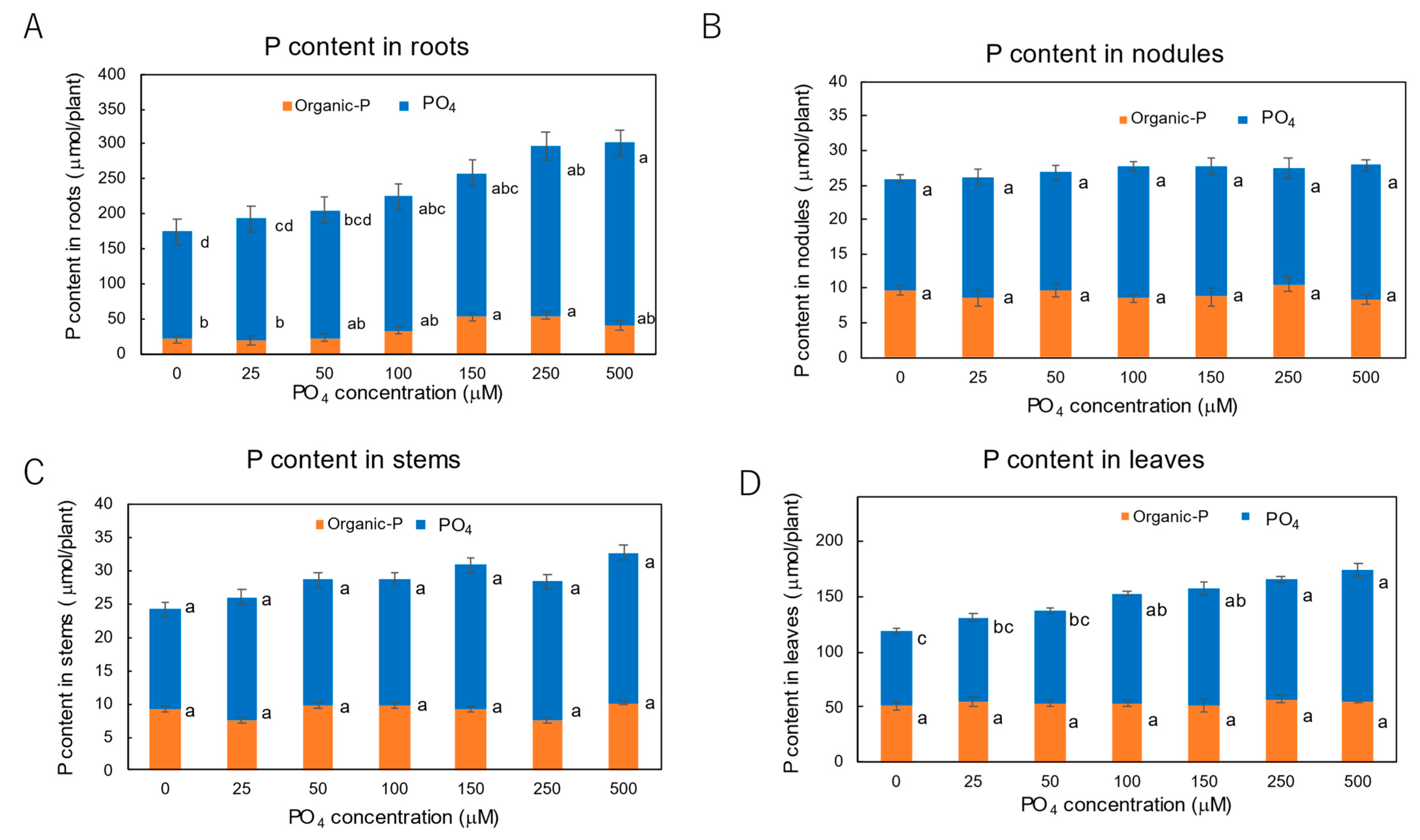
3.3. Effect of P Concentrations in Culture Solution on the P Concentrations in Xylem Sap and Plant Parts of Soybean Plants Cultivated with N-Free Culture Solution
4. Discussion
4.1. Changes in the Xylem Sap Flow Rate and the Concentration of Nutrients after Shoot Removal of the Plants Cultivated with N-Free or NO3 Culture Solution
4.2. Long-Term Effect of Three Levels of P Treatment on the P and Nutrient Concentrations in Xylem Sap of Soybean Plants
4.3. Effects of Seven Levels of P Concentration on the P Concentration in Xylem Sap and in Each Plant Part of Soybean Plants
4.4. Diagnosis of P in Soybean Plants by Xylem Sap Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Marschner, H. Functions of mineral nutrients: Macronutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1986; pp. 229–312. [Google Scholar]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Annu. Plant Rev. 2015, 48, 3–22. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Cassman, K.G.; Whitney, A.S.; Stockinger, K.R. Root growth and dry matter distribution of soybean as affected by phosphorus stress, nodulation, and nitrogen source. Crop Sci. 1980, 20, 239–244. [Google Scholar] [CrossRef]
- Israel, D.W. Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol. 1987, 84, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yuan, H.; Wu, G.; Ma, C.; Gong, Z. Proteome analysis of the soybean nodule phosphorus response mechanism and characterization of stress-induced ribosome structural and protein expression changes. Front. Plant Sci. 2022, 13, 908889. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Chinushi, T.; Nakano, T.; Ohyama, T. Evaluation of N2 fixation activity and N absorption activity by relative ureide methods in field-grown soybean plants with deep placement of coated urea. Soil Sci. Plant Nutr. 1992, 38, 699–708. [Google Scholar] [CrossRef]
- Sakazume, T.; Tanaka, K.; Aida, H.; Ishikawa, S.; Nagumo, Y.; Takahashi, Y.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Estimation of nitrogen fixation rate of soybean (Glycine max(L.) Merr.) by micro-scale relative ureide analysis using root bleeding xylem sap and apoplast fluid in stem. Bull. Facul. Agric. Niigata Univ. 2014, 67, 27–41. [Google Scholar]
- Noguchi, A.; Kageyama, M.; Shinmachi, F.; Schmidhalter, U.; Hasegawa, I. Potential for using plant xylem sap to evaluate inorganic nutrient availability in soil. I. Influence of inorganic nutrients present in the rhizosphere on those in the xylem sap of Luffa cylindrica Roem. Soil Sci. Plant Nutr. 2005, 51, 333–341. [Google Scholar] [CrossRef]
- Noguchi, A.; Kageyama, M.; Shinmachi, F.; Schmidhalter, U.; Hasegawa, I. Potential for using plant xylem sap to evaluate inorganic nutrient availability in soil. II. Comparison between the xylem sap method and the extraction method. Soil Sci. Plant Nutr. 2005, 51, 343–350. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, Q.; Lyu, X.; Li, S.; Gong, Z.; Dong, S.; Yan, C.; Ma, C. Regulation of phosphorus supply on nodulation and nitrogen fixation in soybean plants with dual-root systems. Agronomy 2021, 11, 2354. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Y.; Chen, L.; Liang, R.; Gu, M.; Xu, G.; Zhao, J.; Walk, T.; Liao, H. Functional characterization of 14 Pht1 family genes in yeast and their expressions in response to nutrient starvation in soybean. PLoS ONE 2012, 7, e47726. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, L.; Zhou, L.; Li, X.; Chen, Z.; Sun, L.; Wang, W.; Lin, Z.; Zhao, J.; Yamaji, N.; et al. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2016, 221, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Diagnosis of deficiency and toxicity of mineral nutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1986; pp. 461–479. [Google Scholar]
- Bennett, W.F. Nutrient Deficiencies & Toxicities in Crop Plants; APS Press—The American Phytopathological Society: St. Paul, MN, USA, 1993. [Google Scholar]
- Roppongi, K. Studies on nutritional diagnosis in fruit vegetables. 1 The diagnosis of nitrogen nutrition in cucumber on nitrate density of petiole juice. Bull. Saitama Hortic. Exp. Stn. 1991, 18, 1–15. [Google Scholar]
- Yamazaki, H.; Roppongi, K. The diagnosis of cucumber based on inorganic phosphorus concentration in petiole sap. Jpn. J. Soil Sci. Plant Nutr. 2006, 77, 691–694. [Google Scholar]
- Yoneyama, T.; Yamazaki, H.; Yoshimitsu, S.; Fujiwara, S.; Takebe, M. Minor element concentrations on the leaf fluids and xylem sap for various crops. Jpn. J. Soil Sci. Plant Nutr. 1997, 68, 508–513. [Google Scholar]
- Kato, S.; Suzuki, S. Nutritional diagnosis of hydroponically grown eggplants based on the nitrate ion concentration in petiolar sap. Sci. Rep. Fac. Agric. Meijo Univ. 2018, 55, 7–14. [Google Scholar]
- Noodén, L.D.; Mauk, C.S. Changes in the mineral composition of soybean xylem sap during monocarpic senescence and alterations by depodding. Physiol. Plant. 1987, 70, 735–742. [Google Scholar] [CrossRef]
- Rao, I.M.; Terry, M. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet. I. Changes in growth, gas exchange, and Calvin cycle enzymes. Plant Physiol. 1989, 90, 814–819. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, M.; Zhang, Z.; Lu, X.; Liang, C.; Tian, J. Phosphate (Pi) starvation up-regulate GmCSN5A/B participates in anthocyanin synthesis in soybean (Glycine max) dependent on Pi availability. Int. J. Mol. Sci. 2021, 22, 12348. [Google Scholar] [CrossRef] [PubMed]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef]
- Sinclair, J.B. Soybeans. In Nutrient Deficiencies & Toxicities in Crop Plants; Bennett, W.F., Ed.; APS Press—The American Phytopathological Society: St. Paul, MN, USA, 1993; pp. 99–103. [Google Scholar]
- Ohyama, T.; Takayama, K.; Akagi, A.; Saito, A.; Higuchi, K.; Sato, T. Development of an N-free culture solution for cultivation of nodulated soybean with less pH fluctuation by the addition of potassium bicarbonate. Agriculture 2023, 13, 739. [Google Scholar] [CrossRef]
- Foote, B.D.; Howell, R.W. Phosphorus Tolerance and Sensitivity of Soybeans as Related to Uptake and Translocation. Plant Physiol. 1964, 39, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Gremaud, M.F.; Harper, J.E. Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol. 1989, 89, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Isaka, M.; Saito, A.; Higuchi, K. Effects of nodulation on metabolite concentrations in xylem sap and in the organs of soybean plants supplied with different N forms. Metabolites 2023, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yashima, H.; Ohtake, N.; Sueyoshi, K.; Akao, S.; Ohyama, T. Possible involvement of photosynthetic supply in changes of nodule characteristics of hypernodulating soybeans. Soil Sci. Plant Nutr. 1999, 45, 187–196. [Google Scholar] [CrossRef]
- Yasuda, K.; Kojima, T. Comparison of three methods for the determination of total phosphorus in industrial effluents and effect of iron on those methods. J. Environ. Lab. Assoc. 1984, 9, 71–75. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 18th ed.; Section 4500-P E. 4-115-116; American Public Health Association: Washington, DC, USA, 1992.
- MEdical and PHarmaceutical Statistics (MEPHAS). Available online: www.gen-info.osaka-u.ac.jp/MEPHAS/mokuji1-e.html (accessed on 24 May 2002).
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2020, 72, 199–223. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Huang, T.K.; Yang, S.Y.; Hong, Y.T.; Huang, S.M.; Wang, F.N.; Chiang, S.F.; Tsai, S.Y.; Lu, W.C.; Chiou, T.J. Identification of plant vacuolar transporters mediating phosphate storage. Nat. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Boisson, A.M.; Gout, E.; Bligny, R.; Douce, R.; Aubert, S. Phosphate (Pi) starvation effect on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: An in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol. 2009, 151, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kobae, Y.; Mizuno, T.; Hata, S. Identification and expression analysis of arbuscular mycorrhiza-inducible phosphate transporter genes of soybean. Biosci. Biotechnol. Biochem. 2012, 76, 309–313. [Google Scholar] [CrossRef]
- Esteves, E.; Locatelli, G.; Bou, N.A.; Ferrarezi, R.S. Sap analysis: A powerful tool for monitoring plant nutrition. Horticulturae 2021, 7, 426. [Google Scholar] [CrossRef]
- Kan, M.; Yamazaki, K.; Fujiwara, T.; Kamiya, T. A simple and high-throughput method for xylem sap collection. BioTechniques 2019, 67, 242–245. [Google Scholar] [CrossRef]
- Noodén, L.D.; Singh, S.; Letham, D.S. Correlation of xylem sap cytokinin levels with monocarpic senescence in soybean. Plant Physiol. 1990, 93, 33–39. [Google Scholar] [CrossRef]
- Subramanian, S.; Cho, U.-H.; Keyes, C.; Yu, O. Distinct changes in soybean xylem sap proteome in response to pathogenic and symbiotic microbe interactions. BMC Plant Biol. 2009, 9, 119. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamura, Y.; Higuchi, K.; Saito, A.; Ohyama, T. Absorption and Transport of Phosphorus in Nodulated Soybean Plants and Diagnosis of Phosphorus Status Using Xylem Sap Analysis. Agriculture 2024, 14, 403. https://doi.org/10.3390/agriculture14030403
Yamamura Y, Higuchi K, Saito A, Ohyama T. Absorption and Transport of Phosphorus in Nodulated Soybean Plants and Diagnosis of Phosphorus Status Using Xylem Sap Analysis. Agriculture. 2024; 14(3):403. https://doi.org/10.3390/agriculture14030403
Chicago/Turabian StyleYamamura, Yoshiaki, Kyoko Higuchi, Akihiro Saito, and Takuji Ohyama. 2024. "Absorption and Transport of Phosphorus in Nodulated Soybean Plants and Diagnosis of Phosphorus Status Using Xylem Sap Analysis" Agriculture 14, no. 3: 403. https://doi.org/10.3390/agriculture14030403





