Seasonal Dynamics of Epigeic Arthropods under the Conditions of Ecological Management of the Triticum aestivum Crop
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levy, A.A.; Feldman, M. Evolution and origin of bread wheat. Plant Cell 2022, 34, 2549–2567. [Google Scholar] [CrossRef]
- Farooq, M.U.; Bashir, M.F.; Khan, M.U.S.; Iqbal, B.; Ali, Q. Role of crispr to improve abiotic stress tolerance in crop plants. Biol. Clin. Sci. Res. J. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Batáry, P.; Fischer, J.; Báldi, A.; Crist, T.O.; Tscharntke, T. Does habitat heterogeneityincrease farmland biodiversity? Front. Ecol. Environ. 2011, 33, 152–153. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Mass-floweringcrops enhance wild bee abundance. Oecologia 2013, 172, 477–484. [Google Scholar] [CrossRef]
- Bouvier, J.C.; Ricci, B.; Agerberg, J.; Lavigne, C. Apple orchard pest control strategies affect bird communities in southeastern France. Environ. Toxicol. Chem. 2011, 30, 212–219. [Google Scholar] [CrossRef]
- Crowder, D.W.; Northfield, T.D.; Strand, M.R.; Snyder, W.E. Organic agriculture promotes evenness and natural pest control. Nature 2010, 466, 109–112. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Legrand, A.; Gaucherel, C.; Baudry, J. Long-term effects of organic, conventional, and integrated crop systems on Carabids. Agron. Sust. Dev. 2011, 31, 515–524. [Google Scholar] [CrossRef]
- Langraf, V.; Petrovičová, K.; Schlarmannová, J.; Chovancová, Z. Changes in the community structure of epigeic arthropods in the conditions of ecological farming of pea (Pisum sativum L.). Chil. J. Agric. Res. 2022, 82, 527–536. [Google Scholar]
- Dyman, T.; Yashchenko, S.; Mazur, T.; Dyman, N.; Zagoruy, L. Comparative analysis of the diversity of bees in agroecosystem habitats. Tehnol. Virobnictva Pererobki Produktìv Tvarinnictva 2022, 2, 70–77. [Google Scholar] [CrossRef]
- Barnes, A.D.; Allen, K.; Kreft, H.; Corre, M.D.; Jochum, M.; Veldkamp, E. Direct and cascading impacts of tropical land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 2017, 1, 1511–1519. [Google Scholar] [CrossRef]
- Vignozzi, N.; Agnelli, A.E.; Brandi, G.; Gagnarli, E.; Goggioli, D.; Lagomarsino, A.; Pellegrini, S.; Simoncini, S.; Simoni, S.; Valboa, G.; et al. Soil ecosystem functions in a high-density olive orchard managed by different soil conservation practices. Appl. Soil Ecol. 2019, 134, 64–76. [Google Scholar] [CrossRef]
- Ernst, D.; Kolenčík, M.; Šebesta, M.; Ubaďurišová, L.; Ďúranová, H.; Kšiňan, S. Agronomic Investigation of Spray Dispersion of Metal-Based Nanoparticles on Sunflowers in Real-World Environments. Plants 2023, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Brygadyrenko, V.V. Community structure of litter invertebrates of forest belt ecosystems in the Ukrainian steppe zone. Int. J. Environ. Res. 2015, 9, 1183–1192. [Google Scholar]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Faly, L.I.; Kolombar, T.M.; Prokopenko, E.V.; Pakhomov, O.Y.; Brygadyrenko, V.V. Structure of litter macrofauna communities in poplar plantations in an urban ecosystem in Ukraine. Biosyst. Divers. 2017, 25, 29–38. [Google Scholar] [CrossRef]
- Berg, M.P.; Bengtsson, J. Temporal and spatial variability in soil food web structure. Oikos 2007, 116, 1789–1804. [Google Scholar] [CrossRef]
- Kolesnikova, A.; Lapteva, E.; Degteva, S.; Taskaeva, A.; Kudrin, A.; Vinogradova, Y. Biodiversity of foodplain soils in the European north-east of Russia. In River Basin Management; IntechOpen: London, UK, 2016; pp. 271–294. [Google Scholar]
- Boháč, J.; Jahnov, Z. Land Use Changes and Landscape Degradation in Central and Eastern Europe in the Last Decades: Epigeic Invertebrates as Bioindicators of Landscape Changes. In Environmental Indicators; Springer: Berlin/Heidelberg, Germany, 2015; pp. 395–420. [Google Scholar]
- Boetzl, A.F.; Sponsler, D.; Albrecht, M.; Batáry, P.; Birkhofer, K.; Knapp, M.; Krauss, J.; Maas, B.; Martin, A.E.; Sirami, C.; et al. Distance functions of carabids in crop fields depend on functional traits, crop type and adjacent habitat: A synthesis. Proc. R. Soc. B 2024, 291, 1–10. [Google Scholar] [CrossRef]
- Fazekašová, D.; Bobuľovská, L. Soil organisms as an Indicator of Quality and Environmental Stress in the Soil Ecosystem. Zivotn. Prostr. 2012, 46, 103–106. [Google Scholar]
- Coleman, D.C.; Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology, Ecology, and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: New York, NY, USA, 2015; Chapter 5; pp. 111–149. [Google Scholar]
- Menta, C.; Remelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Murphy, B.W. Soil Organic Matter and Soil Function—Review of the Literature and Underlying Data; Department of the Environment: Canberra, Australia, 2014. [Google Scholar]
- Ondrasek, G.; Bakić, H.; Zovko, M.; Filipović, L.; Meriño-Gergichevich, C.; Savić, R.; Rengel, Z. Biogeochemistry of soil organic matter in agroecosystems & environmental implications. Sci. Total Environ. 2019, 658, 1559–1573. [Google Scholar]
- Schierwater, B.; DeSalle, R. Invertebrate Zoology: A Tree of Life Approach; CRC Press: London, UK, 2021; 644p. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- R Version 4.1.3; The R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Balqees, N.; Ali, Q.; Malik, A. Genetic evaluation for seedling traits of maize and wheat under biogas wastewater, sewage water and drought stress conditions. Biol. Clin. Sci. Res. J. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Javed, A.; Muhammad, S.; Ali, Q.; Manzoor, T. An Overview of Leaf Rust Resistance Genes in Triticum aestivum. Bull. Biol. Allied Sci. Res. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.; Pasher, J.; Smith, A.; Javorek, S.; King, D.; Lindsay, K.F.; Mitchell, S.; Tischendorf, L. Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Burel, F.; Butet, A.; Delettre, Y.R.; de la Pena, N.M. Differential response of selected taxa tolandscape context and agricultural intensification. Landsc. Urban Plan. 2004, 67, 195–204. [Google Scholar] [CrossRef]
- Magdoff, F. Ecological agriculture: Principles, practices, and constraints. Renew. Agric. Food Syst. 2007, 22, 109–117. [Google Scholar] [CrossRef]
- Kubicová, Ľ.; Dobák, D.; Kádeková, Z. Trends in consumption of milk and dairy products in Slovakia after EUaccession. Zesz. Nauk. SGGW Polityki Eur. Finans. I Mark. 2014, 12, 90–97. [Google Scholar]
- Porhajašová, J.; Babošová, M. Impact of arable farming management on the biodiversity of Carabidae (Coleoptera). Saudi J. Biol. Sci. 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Whalen, J.K.; Hamel, C. Effects of key soil organisms on nutrient dynamics in temperate agroecosystems. J. Crop Improv. 2004, 11, 175–207. [Google Scholar] [CrossRef]
- Schuster, N.R.; Peterson, J.A.; Gilley, J.E.; Schott, L.R.; Schmidt, A.M. Soil arthropod abundance and diversityfollowing land application of swine slurry. Agric. Sci. 2019, 10, 150–163. [Google Scholar]
- Menta, C.; Conti, F.D.; Fondón, L.C.; Staffilani, F.; Remelli, S. Soil Arthropod Responses in Agroecosystem: Implications of Different Management and Cropping Systems. Agronomy 2020, 10, 982. [Google Scholar] [CrossRef]
- Magura, T.; Ferrante, M.; Lövei, L.G. Only habitat specialists become smaller with advancing urbanization. Glob. Ecol. Biogeogr. 2020, 29, 1978–1987. [Google Scholar] [CrossRef]
- Diehl, E.; Wolters, V.; Birkhofer, K. Arable weeds in organically managed wheat fields foster carabid beetles byresource and structure-mediated effects. Arthropod-Plant Interact. 2012, 6, 75–82. [Google Scholar] [CrossRef]
- Diehl, E.; Mader, V.L.; Wolters, V.; Birkhofer, K. Management intensity and vegetation complexity affect web-building spiders and their prey. Oecologia 2013, 173, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bote, J.L.; Romero, A.J. Epigeic soil arthropod abundance under different agricultural land uses. Span. J. Agric. Res. 2012, 10, 55–61. [Google Scholar] [CrossRef]
- Liu, R.T.; Zhu, F.; Steinberger, Y. Effect of shrub microhabitats on aboveground and belowground arthropod distributionin a desertified steppe ecosystem. Pol. J. Ecol. 2015, 63, 534–548. [Google Scholar]
- Sticht, C.; Schrader, S.; Giesemann, A.; Weigel, H.J. Atmospheric CO2 enrichment induces life strategy- and species-specific responses of collembolans in the rhizosphere of sugar beet and winter wheat. Soil Biol. Biochem. 2008, 40, 1432–1445. [Google Scholar] [CrossRef]
- Magro, S.; Gutiérrez-López, M.; Casado, M.A.; Jiménez, M.D.; Trigo, D.; Mola, I. Soil functionality at the roadside: Zooming in on a microarthropod community in an anthropogenic soil. Ecol. Eng. 2013, 60, 81–87. [Google Scholar] [CrossRef]
- Holmstrup, M.; Maraldo, K.; Krogh, P.H. Combined effect of copper and prolonged summer drought on soil Microarthropods in the field. Environ. Pollut. 2007, 146, 525–533. [Google Scholar] [CrossRef]
- Bianchni, F.; Booij, C.; Tscherntke, T. Sustainable pest regulation in agricultural landscape: A review on landscape composition. Biodiversity and natural pest control. Proc. R. Soc. 2006, 273, 1715–1727. [Google Scholar]
- Bagyaraj, D.J.; Nethravathi, C.J.; Nitin, K.S. Soil Biodiversity and Arthropods: Role in Soil Fertility. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems; Springer: Singapore, 2016; pp. 17–51. [Google Scholar]
- Ghiglieno, I.; Simonetto, A.; Donna, P.; Tonni, M.; Valenti, L.; Bedussi, F. Soil Biological Quality Assessment to Improve Decision Support in the Wine Sector. Agronomy 2019, 9, 593. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Simonetto, A.; Orlando, F.; Donna, P.; Tonni, M.; Valenti, L. Response of the Arthropod Community to Soil Characteristics and Management in the Franciacorta Viticultural Area (Lombardy, Italy). Agronomy 2020, 10, 740. [Google Scholar] [CrossRef]
- Jasinski, M.; Twardowski, J.; Tendziagolska, E. The occurrence of soil mesofauna in organic crops. J. Res. Appl. Agric. Eng. 2016, 61, 193–199. [Google Scholar]
- Fazekašová, D.; Bobuľovská, L.; Angelovičová, L. Biological activity of soil as an indicator of soil fertility. Nővénytérmeles 2013, 62, 205–208. [Google Scholar]
- Langellotto, G.A.; Denno, R.F. Responses of invertebrate natural enemies to complex-structured habitats: A meta-analytical synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef]
- Lenoir, L.; Lennartsson, T. Effects of timing of grazing on arthropod communities in semi-natural grasslands. J. Insect Sci. 2010, 10, 5–24. [Google Scholar] [CrossRef]
- Moço, S.K.M.; Gama-Rodrigues, F.E.; Gama-Rodrigues, C.A.; Machado, R.C.R.; Baligar, C.V. Relationships between invertebrate communities, litter quality and soil attributes under different cacao agroforestry systems in the south of Bahia. Appl. Soil Ecol. 2010, 46, 347–354. [Google Scholar] [CrossRef]


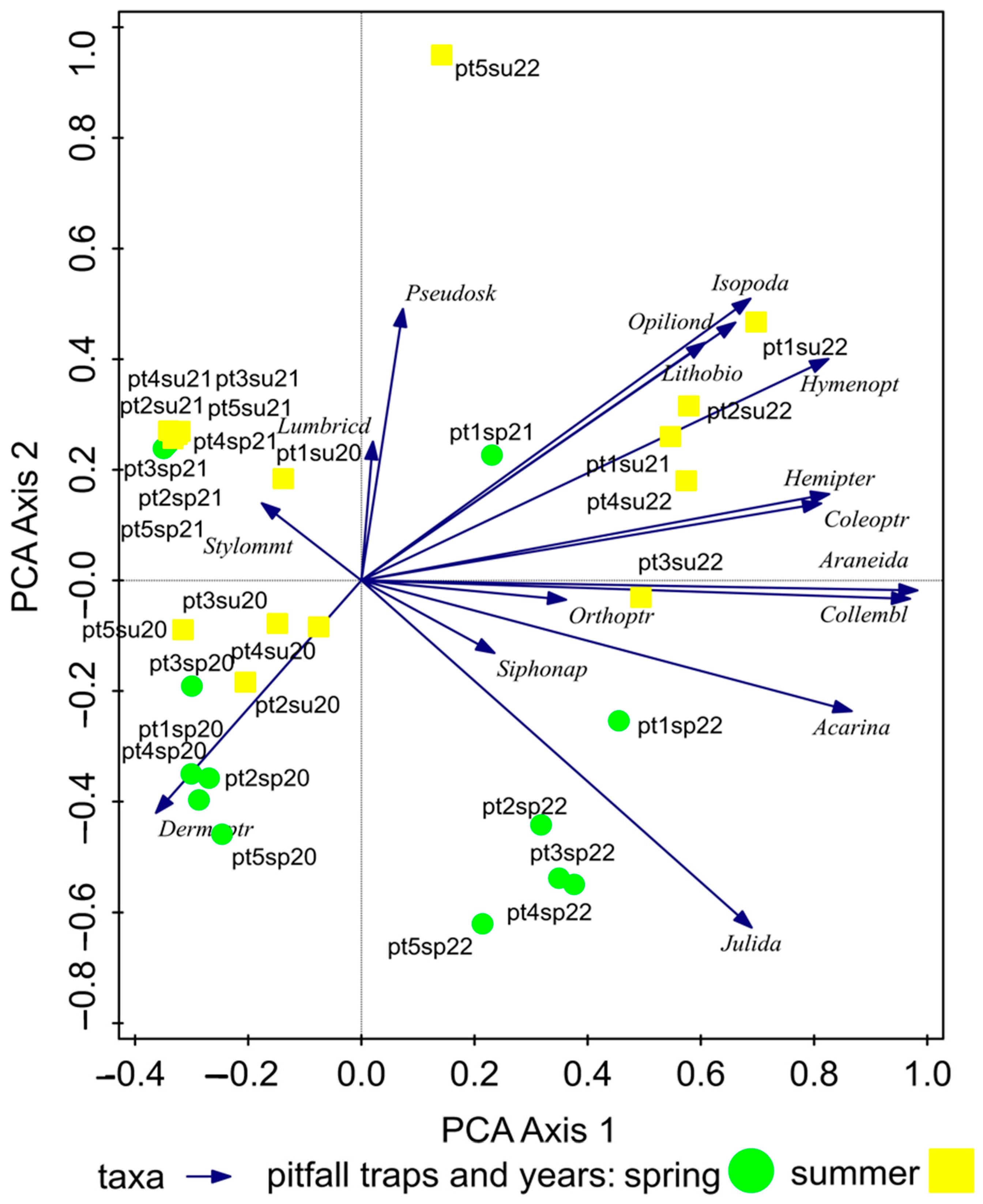
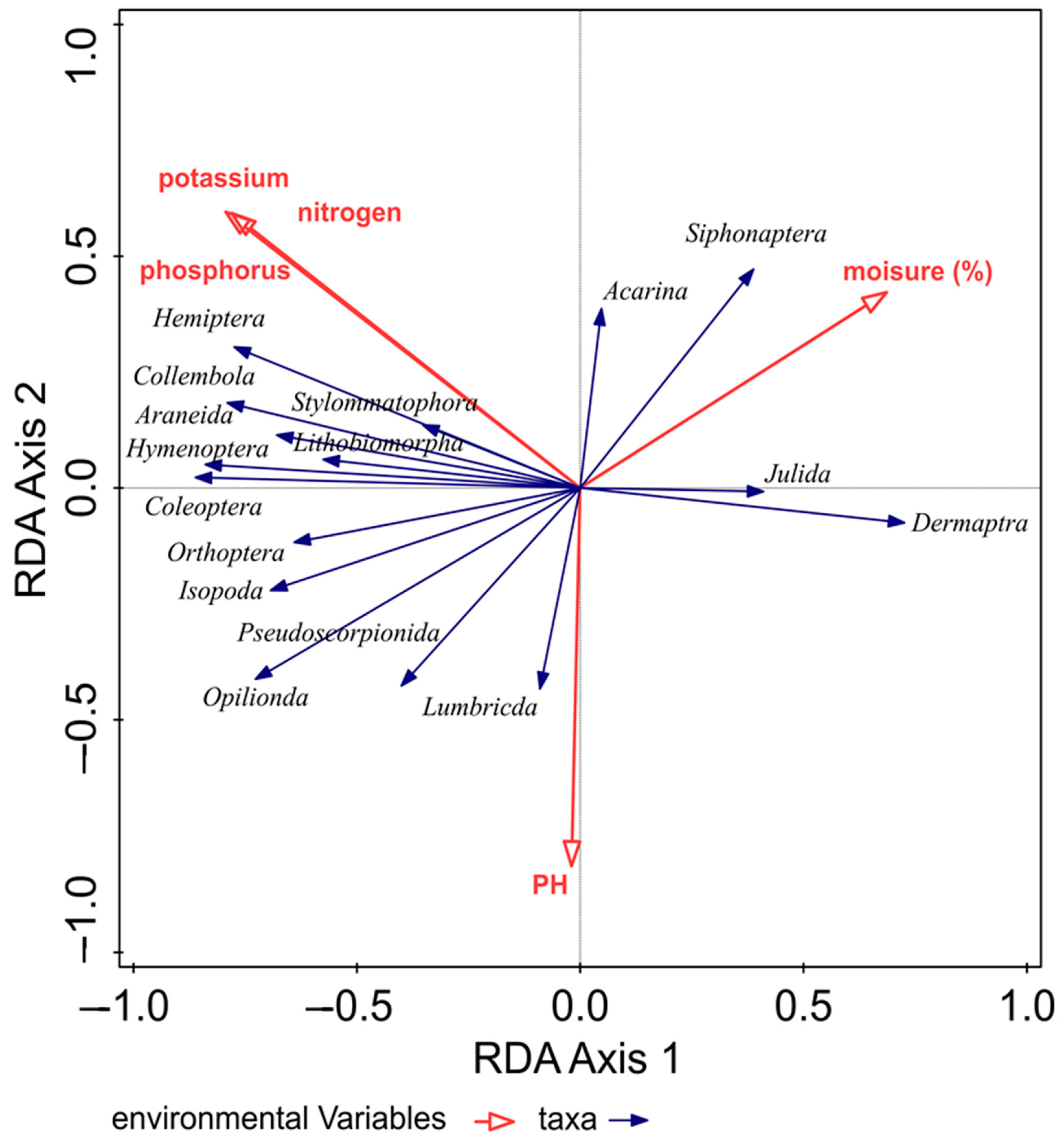

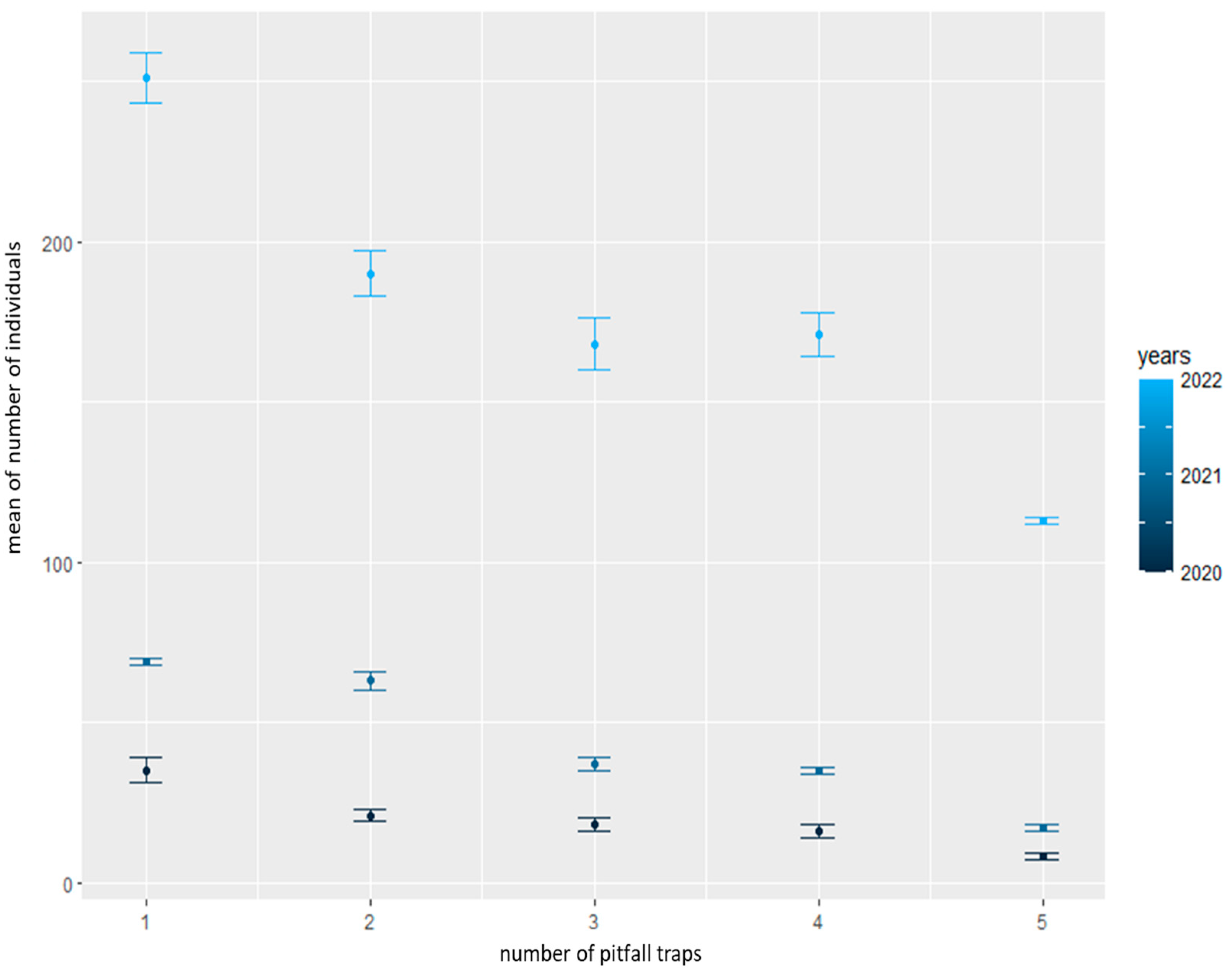
| Period/Taxa | Year 2020 | Year 2021 | Year 2022 | ∑ N | ∑% | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| spring | 345 | 7.86% | 547 | 12.46% | 3499 | 79.69% | 4391 | 100.00% |
| Acarina | 0 | 0.00% | 6 | 0.14% | 163 | 3.71% | 169 | 3.85% |
| Araneida | 0 | 0.00% | 35 | 0.80% | 200 | 4.55% | 235 | 5.35% |
| Coleoptera | 125 | 2.85% | 300 | 6.83% | 1854 | 42.22% | 2279 | 51.90% |
| Collembola | 0 | 0.00% | 4 | 0.09% | 384 | 8.75% | 388 | 8.84% |
| Dermaptera | 35 | 0.80% | 0 | 0.00% | 0 | 0.00% | 35 | 0.80% |
| Hemiptera | 0 | 0.00% | 0 | 0.00% | 10 | 0.23% | 10 | 0.23% |
| Hymenoptera | 0 | 0.00% | 97 | 2.21% | 25 | 0.57% | 122 | 2.78% |
| Isopoda | 0 | 0.00% | 22 | 0.50% | 5 | 0.11% | 27 | 0.61% |
| julida | 161 | 3.67% | 74 | 1.69% | 847 | 19.29% | 1082 | 24.64% |
| Lithobiomorpha | 0 | 0.00% | 0 | 0.00% | 1 | 0.02% | 1 | 0.02% |
| Lumbricida | 12 | 0.27% | 1 | 0.02% | 4 | 0.09% | 17 | 0.39% |
| Opilionida | 0 | 0.00% | 5 | 0.11% | 0 | 0.00% | 5 | 0.11% |
| Orthoptera | 12 | 0.27% | 3 | 0.07% | 5 | 0.11% | 20 | 0.46% |
| Siphonaptera | 0 | 0.00% | 0 | 0.00% | 1 | 0.02% | 1 | 0.02% |
| summer | 725 | 6.84% | 886 | 8.36% | 8986 | 84.80% | 10,597 | 100.00% |
| Acarina | 0 | 0.00% | 2 | 0.02% | 62 | 0.59% | 64 | 0.60% |
| Araneida | 16 | 0.15% | 10 | 0.09% | 534 | 5.04% | 560 | 5.28% |
| Coleoptera | 537 | 5.07% | 680 | 6.42% | 5084 | 47.98% | 6301 | 59.46% |
| Collembola | 0 | 0.00% | 8 | 0.08% | 2371 | 22.37% | 2379 | 22.45% |
| Dermaptera | 4 | 0.04% | 0 | 0.00% | 0 | 0.00% | 4 | 0.04% |
| Hemiptera | 0 | 0.00% | 0 | 0.00% | 56 | 0.53% | 56 | 0.53% |
| Hymenoptera | 25 | 0.24% | 44 | 0.42% | 474 | 4.47% | 543 | 5.12% |
| Isopoda | 2 | 0.02% | 59 | 0.56% | 66 | 0.62% | 127 | 1.20% |
| julida | 77 | 0.73% | 2 | 0.02% | 238 | 2.25% | 317 | 2.99% |
| Lithobiomorpha | 0 | 0.00% | 1 | 0.01% | 5 | 0.05% | 6 | 0.06% |
| Lumbricida | 0 | 0.00% | 49 | 0.46% | 0 | 0.00% | 49 | 0.46% |
| Opilionida | 0 | 0.00% | 4 | 0.04% | 56 | 0.53% | 60 | 0.57% |
| Orthoptera | 64 | 0.60% | 3 | 0.03% | 40 | 0.38% | 107 | 1.01% |
| Pseudoskorpionida | 0 | 0.00% | 14 | 0.13% | 0 | 0.00% | 14 | 0.13% |
| Stylommatophora | 0 | 0.00% | 10 | 0.09% | 0 | 0.00% | 10 | 0.09% |
| ∑ | 1070 | 7.14% | 1433 | 9.56% | 12,485 | 83.30% | 14,988 | 100.00% |
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.985 | 0.046 | 0.998 | 0.895 | 0.009 | 0.999 | 0.915 | 0.031 | 0.895 | 0.885 | 0.008 | 0.969 | 1.000 | 0.009 | 1 |
| 0.028 | 0.900 | 0.958 | 0.041 | 0.978 | 0.865 | 0.031 | 0.889 | 0.876 | 0.009 | 0.901 | 0.911 | 0.050 | 2 | |
| 0.049 | 0.046 | 0.008 | 0.041 | 0.031 | 0.992 | 0.042 | 0.034 | 0.010 | 0.014 | 0.042 | 0.010 | 3 | ||
| 1.000 | 0.009 | 0.895 | 0.895 | 0.036 | 0.785 | 0.875 | 0.009 | 0.954 | 0.812 | 0.023 | 4 | |||
| 0.046 | 0.659 | 0.575 | 0.042 | 0.986 | 0.951 | 0.006 | 0.701 | 0.784 | 0.043 | 5 | ||||
| 0.008 | 0.034 | 0.045 | 0.009 | 0.030 | 0.911 | 0.018 | 0.013 | 0.895 | 6 | |||||
| 0.975 | 0.029 | 0.989 | 0.966 | 0.031 | 0.975 | 0.994 | 0.033 | 7 | ||||||
| 0.047 | 0.900 | 0.995 | 0.043 | 0.879 | 0.895 | 0.021 | 8 | |||||||
| 0.030 | 0.042 | 1.000 | 0.018 | 0.021 | 1.000 | 9 | ||||||||
| 0.957 | 0.008 | 0.898 | 0.912 | 0.050 | 10 | |||||||||
| 0.007 | 0.895 | 0.942 | 0.019 | 11 | ||||||||||
| 0.015 | 0.042 | 0.800 | 12 | |||||||||||
| 0.932 | 0.037 | 13 | ||||||||||||
| 0.008 | 14 |
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.775 | 0.045 | 0.788 | 0.685 | 0.034 | 0.789 | 0.705 | 0.014 | 0.685 | 0.675 | 0.012 | 0.759 | 0.790 | 0.027 | 1 |
| 0.042 | 0.745 | 0.859 | 0.013 | 0.879 | 0.766 | 0.010 | 0.789 | 0.776 | 0.050 | 0.801 | 0.811 | 0.050 | 2 | |
| 0.018 | 0.006 | 0.014 | 0.046 | 0.027 | 0.548 | 0.021 | 0.014 | 0.013 | 0.050 | 0.019 | 0.014 | 3 | ||
| 0.776 | 0.034 | 0.665 | 0.671 | 0.033 | 0.561 | 0.651 | 0.010 | 0.730 | 0.588 | 0.049 | 4 | |||
| 0.042 | 0.560 | 0.476 | 0.020 | 0.887 | 0.853 | 0.040 | 0.602 | 0.686 | 0.038 | 5 | ||||
| 0.041 | 0.050 | 0.018 | 0.005 | 0.010 | 0.896 | 0.021 | 0.046 | 0.758 | 6 | |||||
| 0.627 | 0.033 | 0.640 | 0.617 | 0.019 | 0.627 | 0.645 | 0.026 | 7 | ||||||
| 0.467 | 0.900 | 0.995 | 0.043 | 0.879 | 0.895 | 0.021 | 8 | |||||||
| 0.018 | 0.039 | 0.758 | 0.018 | 0.009 | 0.875 | 9 | ||||||||
| 0.712 | 0.005 | 0.746 | 0.865 | 0.010 | 10 | |||||||||
| 0.011 | 0.678 | 0.875 | 0.039 | 11 | ||||||||||
| 0.018 | 0.037 | 0.986 | 12 | |||||||||||
| 0.811 | 0.010 | 13 | ||||||||||||
| 0.050 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langraf, V.; Petrovičová, K. Seasonal Dynamics of Epigeic Arthropods under the Conditions of Ecological Management of the Triticum aestivum Crop. Agriculture 2024, 14, 482. https://doi.org/10.3390/agriculture14030482
Langraf V, Petrovičová K. Seasonal Dynamics of Epigeic Arthropods under the Conditions of Ecological Management of the Triticum aestivum Crop. Agriculture. 2024; 14(3):482. https://doi.org/10.3390/agriculture14030482
Chicago/Turabian StyleLangraf, Vladimír, and Kornélia Petrovičová. 2024. "Seasonal Dynamics of Epigeic Arthropods under the Conditions of Ecological Management of the Triticum aestivum Crop" Agriculture 14, no. 3: 482. https://doi.org/10.3390/agriculture14030482




