1. Introduction
Botrytis cinerea can infect many agriculturally important food crops with devastating consequences [
1,
2]. It is a significant concern for agribusiness and food supply continuity with a weighty portion of the fungicide industry focusing on its inhibition. This necrotrophic fungus can attack many plant organs and is a serious threat for post-harvest losses. The invasion strategy of
B. cinerea, including the hypersensitive response, has been proposed [
3,
4,
5]. Involvement of the extensively studied secretome has been shown [
6,
7]. These proteomic studies have shown a number of proteins are secreted, several of which contribute to cell death in plant tissues. One of the most abundant is BcSnod1 (also referred to as BcSpl1 [
8]). Closely related though not as ubiquitously expressed is BcSnod2. Both are members of the Ceratoplatanin-family of Snod-like proteins.
Ceratoplatanins, named for the founding member cerato-platanin produced by
Ceratocystis fimbriata [
9], are extracellular disease related proteins [
7,
9]. In the case of
C. fimbriata, cerato-platanin was detected in the fungal cell wall as well [
10]. Ceratoplatanins are moderately hydrophobic with four conserved cysteines that form two intramolecular disulfide bonds. This family induces plant defenses and, in some cases, localized necrosis in the exposed area. Ceratoplatanin knock-outs in different fungi have various effects. In the rice blast fungus,
Magnaporthe grisea, deletion of
msp1 caused a significant reduction in virulence for young rice and barley [
11]. In
Leptosphaeria maculans, the cause of blackleg disease of canola, Ceratoplatanin knock-out mutants were as virulent as the wild-type [
12]. For
B. cinerea, BcSnod1 is required for virulence against tomato and tobacco [
13].
Herein we report recombinant production of both BcSnod1 and BcSnod2. Further we demonstrate that these virulence factors by themselves are able to elicit necrosis in tomato and tobacco. With the ability to make large amounts of functional protein recombinantly, the possibilities for better understanding B. cinerea infection and the roles of Ceratoplatanins are increased. Continued structure function studies are forthcoming, all of which contribute to new possibilities for antifungal intervention to help prevent agricultural losses.
3. Experimental Section
3.1. Molecular Biology
BcSnod1 and BcSnod2 genes were amplified from genomic B. cinerea B05.10 DNA. The primers used for BcSnod1 were 5'-GTGCATATGATCACCGTCTCCTACG and 5'-GTGCTCGAGTTACAATCCACAAGCACTCTTGTCG. For BcSnod2, the primers used were 5'-GTGCATATGATCCAAGTAACCTACG and 5'-GTGCTCGAGCTAAGCCTTACACGGTGATCC. PCR products for both genes were first cloned into pGemTEasy (Promega Corporation, Madison, WI). For subsequent recombinant bacterial expression, BcSnod genes were excised using Nde1 and Xho1 restriction sites and ligated into pET28b (Novagen/EMD/Merck KGaA, Darmstadt, Germany). For yeast expression in Pichia pastoris, excised genes were inserted into pPic9k (Invitrogen/Life Technologies, Grand Island, NY) using EcoR1 and Not1 restriction sites. DNA sequencing confirmed the integrity of all clones.
3.2. Bacterial Protein Production
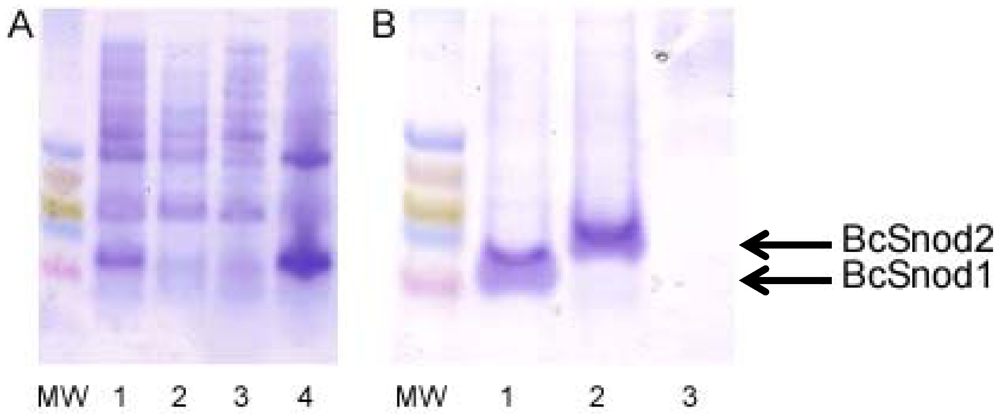
For bacterial expression of both BcSnod1 and BcSnod2, chemically competent Rosetta E. coli were transformed with kanamycin resistant pET28b expression vectors containing the BcSnod protein of interest. Protein expression in Luria broth was induced at an OD600 of 0.8 by adding 1.0 mM isopropyl β-D-thiogalactopyranoside and continued incubation at 37 °C for 4 h. Cells were harvested by centrifugation and stored at −80 °C. After lysis by sonication, insoluble BcSnod protein was collected by centrifugation at 20 kg for 20 min at 0 °C. The pellet, containing BcSnod, was then denatured in 8 M urea with 20 mM Tris pH 7.0, 250 mM NaCl and 3 mM DTT for refolding. Using step-wise dialysis, the protein was refolded by urea removal. Soluble BcSnod was further purified by metal chelation chromatography, eluted with 150 mM imidazole. The final dialysis buffer was 20 mM Tris pH 7.0 and 3 mM DTT.
3.3. Pichia Production
For expression in Pichia pastoris (GS115 His−, ATCC-20864), the BcSnod vector of interest was linearized with BglII and Pichia transformed via electroporation. For electroporation, 80 µL of elctrocompetent cells were mixed with 3 ng of DNA and a 1.5 kV, 25 μF, 200 Ω electroporating pulse was applied. Screening for presence of BcSnod insert was carried out by PCR using the AOX primers. MutS mutants of transformed Pichia were used for recombinant expression. For screening, single colonies were inoculated in minimal glycerol media and grown until the OD600 reached 2.0. The cells were pelleted and resuspended in minimal methanol media for protein expression. Every 12 h, 0.5% methanol was added to the media to maintain expression of protein.
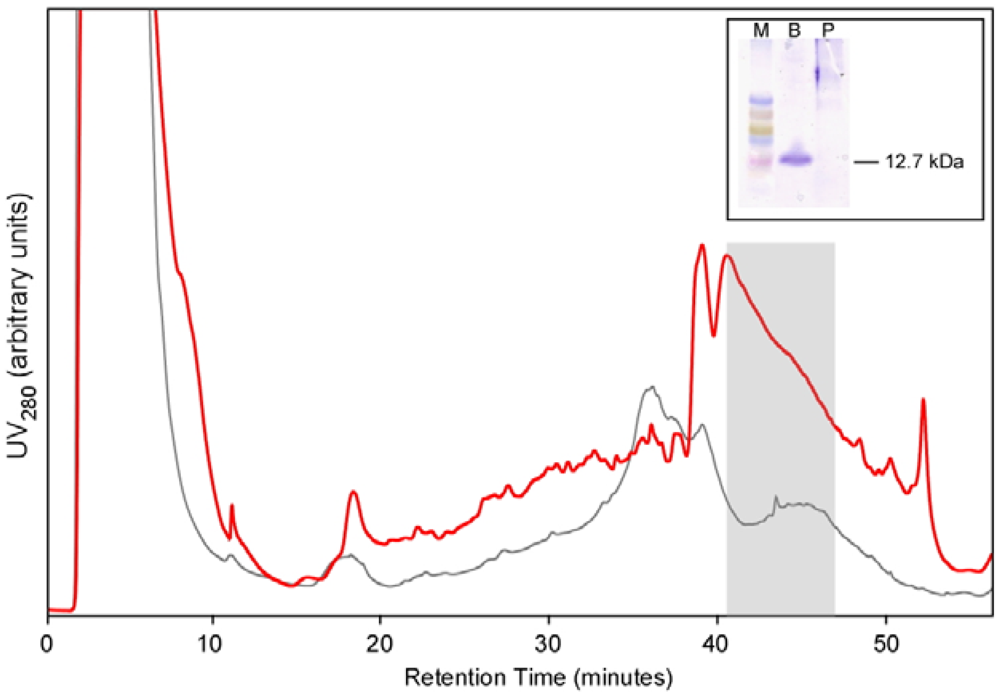
For large scale production, a colony of Pichia expressing BcSnod1 was grown overnight in 1 liter of minimal glycerol media, spun down, and then resuspended in 100 mL of minimal methanol media. Every 12 h, 0.5% methanol was added for protein expression. After 10 days, the supernatant was collected and concentrated to a final volume of 5 mL. The concentrated solution was applied to a C18 HPLC column for purification. A gradient of acetonitrile in 0.05% trifluoroacetic acid was used to purify BcSnod, which eluted at approximately 55% acetonitrile.
3.4. Plant Assays
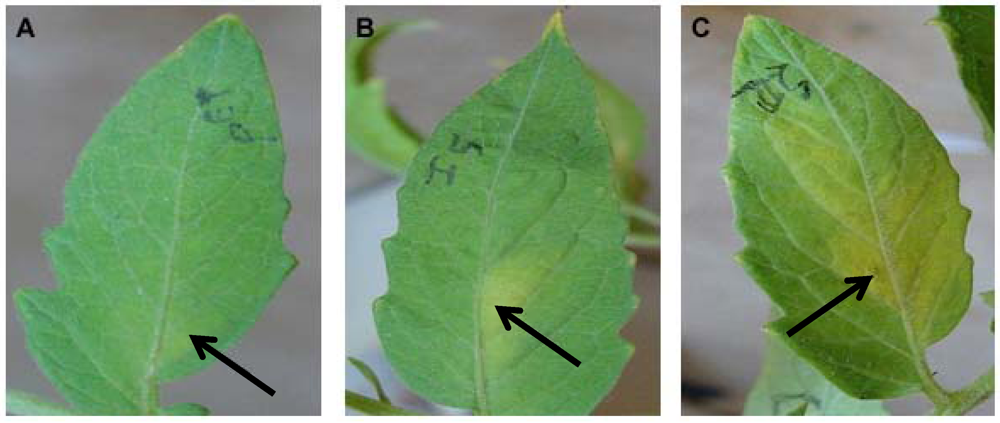
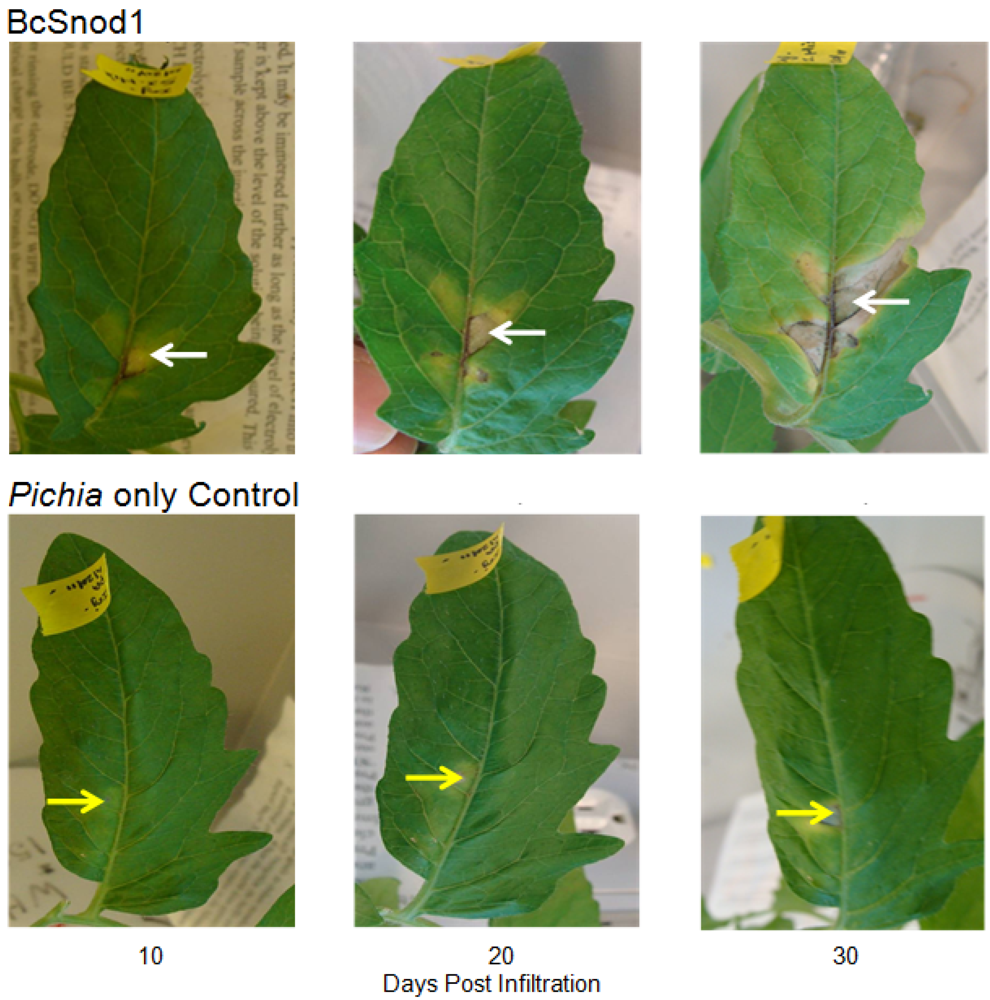
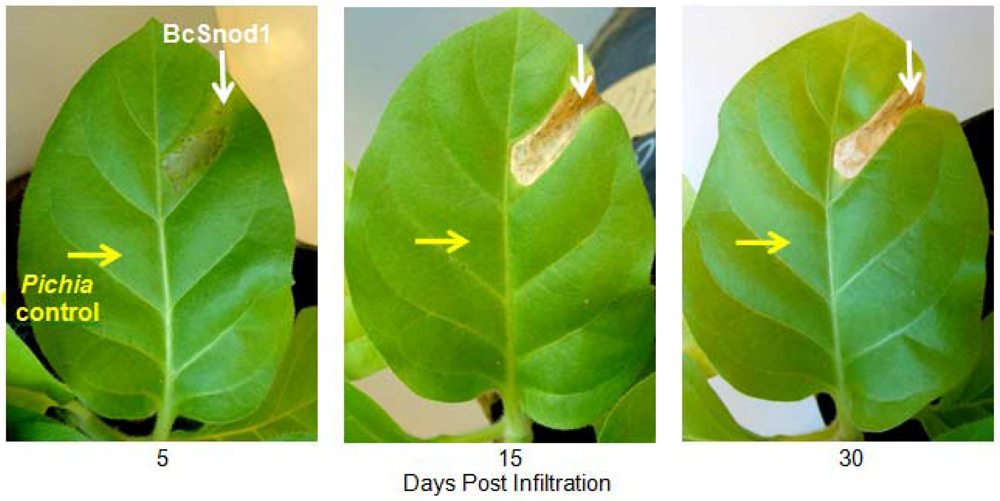
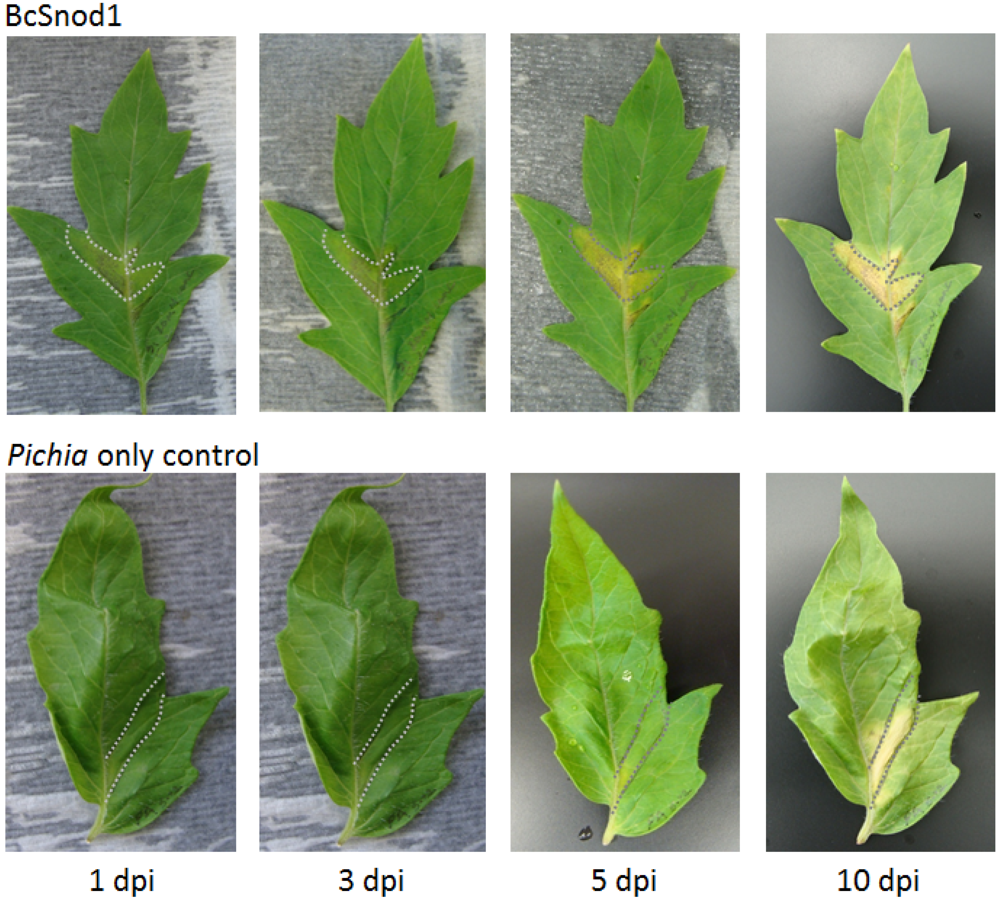
Two month old tomato (Solanum lycopersicum) and four month old tobacco (Nicotiana tabacum var. Xanthi) plants were used for the transient activity assays. All plants were grown in an environmental chamber (24 °C, 14 h, 40W/m2 light intensity, 10 h dark). For detached leaf assays, leaves were cut from the plant and kept in a wet box in the environmental chamber. For the transient assay with bacterially expressed protein, 200 μg of BcSnod1 or BcSnod2 in 100 μL were infiltrated in the midvein of tomato leaves using a syringe and 30.5 gauge needle. For the transient assay with Pichia expressed protein, 15 μg of BcSnod1 protein in 100 μL was infiltrated in the midvein of tomato and tobacco leaves using a syringe and 30.5 gauge needle. In the detached leaf assays, leaves were infiltrated with 100 μL containing 130 μg of protein after plugging out of the plant. In the surface exposed detached leaf assay, 10 µL of 7 μg protein was pipetted on he leaves and kept in wet box in environmental chamber. As a control, extracts from Pichia with no insert were infiltrated or pipetted on the leaves.
4. Conclusions
Understanding plant: pathogen interactions is key in being able to prevent infection and protecting food stuffs. This is especially true for the phytophathogen
B. cinerea, which has multiple means to threaten agriculturally important crops. Like other necrotrophic pathogens,
B. cinerea secretes an array of proteins with functions ranging from cell wall degradation to initiation of host cell death. Early interaction between the host and pathogen leads to secretion of defense molecules and expression of the genes related to the pathogenesis by the host [
14]. The pathogen in return secretes molecules or compounds which disable the plant defense response and help the pathogen to establish an infection. In this melee, BcSnod1 is a potent phytotoxin that causes necrosis simply by surface exposure at sufficient levels. Therefore, understanding BcSnod function and mechanisms of action provides considerable insight into
B. cinerea infectivity, creating possibilities for new antifungal development. Also, insight learned from the BcSnods helps define the functional realm of the Ceratoplatanin family.
Recombinant BcSnod1 obtained from E. coli and Pichia had varied intensities of activity against tomato. The delayed hypersensitive response from bacterially produced BcSnod1 and ill-defined NMR spectra (15N-HSQC data not shown; S.R., H.M., and R.M.) indicate a heterogeneous protein sample with multiple conformations (i.e., a poorly folded protein). In contrast, BcSnod1 expressed in Pichia pastoris was much more effective at inducing necrosis in the area infiltrated, demonstrating BcSnod1 is phytotoxic and that recombinant expression in yeast is more efficient at producing properly folded protein. Some Ceratoplatanins have been shown to have post-translational modifications, others do not. The fact that the bacterially expressed protein could still elicit a response suggests that if there is post-translational modification of BcSnods, it is not absolutely necessary for function though it might facilitate or stabilize protein folding.
BcSnod2 was only obtained from overexpression in
E. coli and had the same activity as
E. coli produced BcSnod1. BcSnod2 caused discoloration in the area of protein infiltration indicating BcSnod2, like BcSnod1, is also phytotoxic. It thus appears that the BcSnods are another example of redundant secreted proteins with overlapping function. However, their differences in yeast expression and preliminary indication of differential temporal expression in the course of infection [
15]suggest greater distinction. Phytotoxicity for BcSnod2 has been demonstrated for the first time, but uncertainty still remains.
Compared to other Ceratoplatanin family members, the activities of BcSnod1 and BcSnod2 from
E. coli were slower at similar concentrations. However the activity of
Pichia produced BcSnod1 displayed a similar time course to what was previously reported. This further supports the conclusion that the bacterially produced protein obtained after renaturation was not homogeneously folded in a fully functional state. The differences in propagation of discoloration or necrosis in tomato versus tobacco may be related to susceptibility of infection. Cell death may help
B. cinerea in spreading infection [
16]. Being a necrotrophic fungus, host cell death leads to utilization of nutrients/macromolecules present in those cells. Secreting Ceratoplatanins might be the mode of action to kill the plants cells and initiate pathogenesis. Further, once pathogenesis is initiated, BcSnods may be involved in expanding
B. cinerea infection causing spread of grey mould. The likening of BcSnods to other Ceratoplatanins also contributes to new antifungal avenues. Transgenic expression of MSP1, the Ceratoplatanin from
M. grisea, conferred increased antibiotic resistance to the host [
11]. Like Ceratoplatanin homolog in
M.grisea, transgenic overexpression of BcSnods could lead to more resistant crops. The differences in BcSnod1 versus BcSnod2 could provide options for expression resulting in even more potent inhibition. Transgenic expression of a single BcSnod may provide different inhibition than when both are expressed simultaneously. Also, determining pathway that lead to necrosis may provide new options to attenuate
B. cinerea infection, possibly extending to other Ceratoplatanin producing fungi.