Assessing the Potential for Ion Selective Electrodes and Dual Wavelength UV Spectroscopy as a Rapid on-Farm Measurement of Soil Nitrate Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Type and Sampling
2.2. Background Soil Analysis
| Soil | pH | EC (µS cm−1) | Moisture content (g g−1) | Total C (%) | Total N (%) |
|---|---|---|---|---|---|
| Eutric Cambisol | 6.53 | 59.4 | 0.25 | 3.5 | 0.29 |
| Dystric Gleysol | 6.53 | 59.4 | 0.28 | 1.1 | 0.10 |
| Haplic Podzol | 5.34 | 12.9 | 0.70 | 6.1 | 0.57 |
2.3. Ion Selective Electrode (ISE)
2.4. NO3− Determination Using Ion Selective Electrode Rapid Test Method
2.5. Nitrate Extraction and Determination by the Standard Lab Method
2.6. NO3− Determination by Dual Wavelength UV Spectroscopy
2.7. Evaluation of the Methods across a Broad Range of Soils
| Sample | Soil | pH | EC (µS cm−1) | Moisture content (g g−1) | Organic matter (%) |
|---|---|---|---|---|---|
| 1 | DG | 6.04 | 19.3 | 0.33 | 5 |
| 2 | EC | 6.04 | 13.9 | 0.38 | 7 |
| 3 | HP | 5.85 | 6.4 | 0.51 | 10 |
| 4 | HP | 6.07 | 35.1 | 0.32 | 5 |
| 5 | HP | 4.85 | 43.2 | 0.52 | 18 |
| 6 | HP | 5.65 | 16.5 | 0.81 | 17 |
| 7 | HP | 5.89 | 84.6 | 0.36 | 9 |
| 8 | EC | 6.38 | 20.3 | 0.19 | 7 |
| 9 | EC | 6.19 | 13.8 | 0.17 | 5 |
| 10 | EC | 6.65 | 55.0 | 0.17 | 8 |
| 11 | EC | 7.14 | 34.3 | 0.18 | 6 |
| 12 | EC | 6.48 | 43.5 | 0.29 | 7 |
| 13 | EC | 6.51 | 47.8 | 0.39 | 12 |
| 14 | EC | 6.61 | 18.1 | 0.25 | 4 |
| 15 | EC | 6.29 | 49.8 | 0.52 | 10 |
| 16 | EC | 6.23 | 45.7 | 0.30 | 8 |
| 17 | DG | 5.37 | 69.7 | 0.63 | 8 |
| 18 | HP | 4.53 | 32.6 | 0.37 | 5 |
| 19 | EC | 5.18 | 6.8 | 0.30 | 11 |
| 20 | EC | 6.28 | 45.7 | 0.24 | 6 |
| 21 | EC | 6.84 | 50.0 | 0.20 | 6 |
| 22 | EC | 5.39 | 38.6 | 0.39 | 5 |
| 23 | EC | 5.52 | 90.2 | 0.46 | 10 |
2.8. Statistical Analysis
3. Results and Discussion
3.1. Efficiency of the Rapid Extraction Method
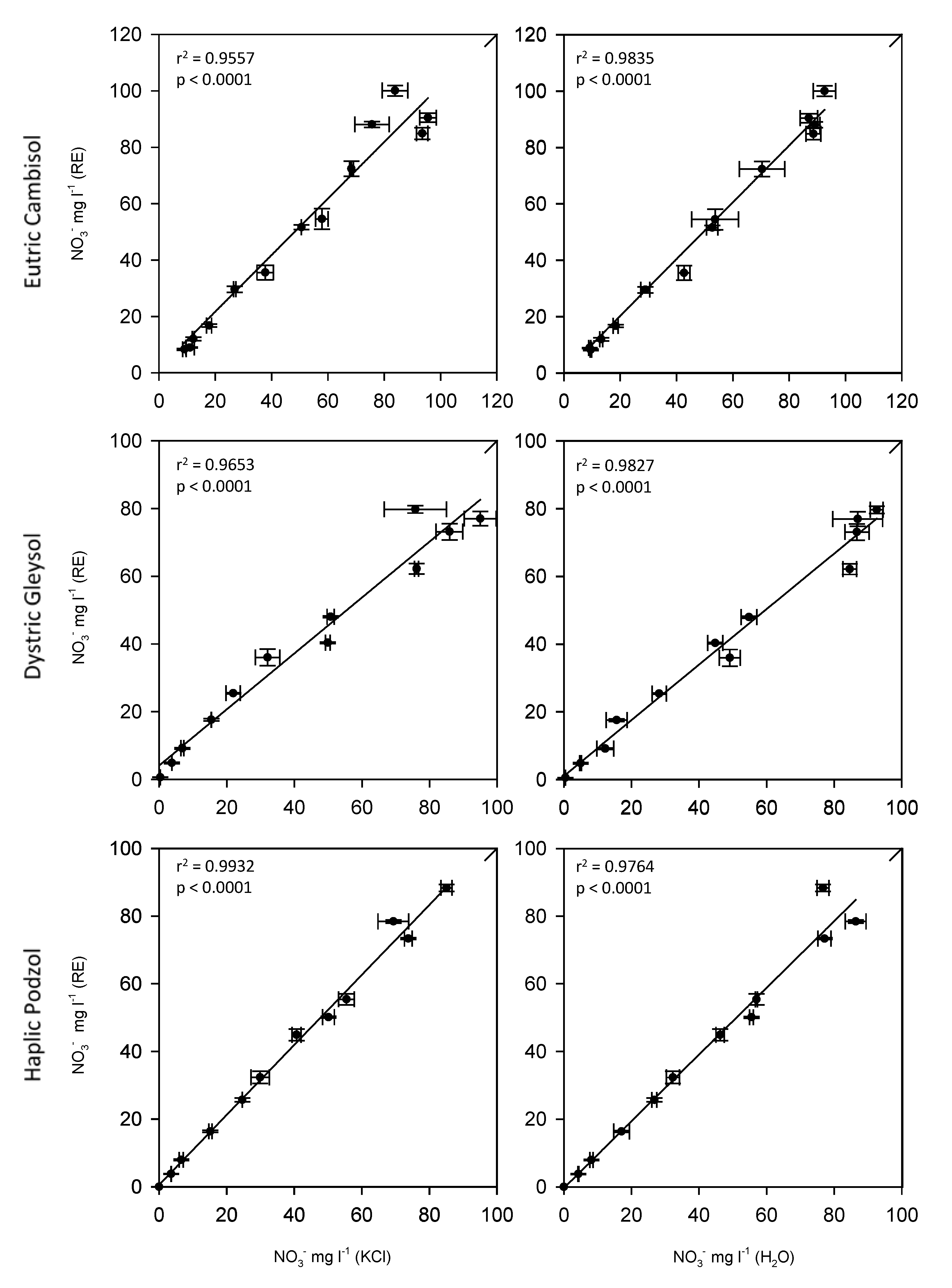
3.2. Comparison of the ISE Rapid Test with the Standard Lab Method

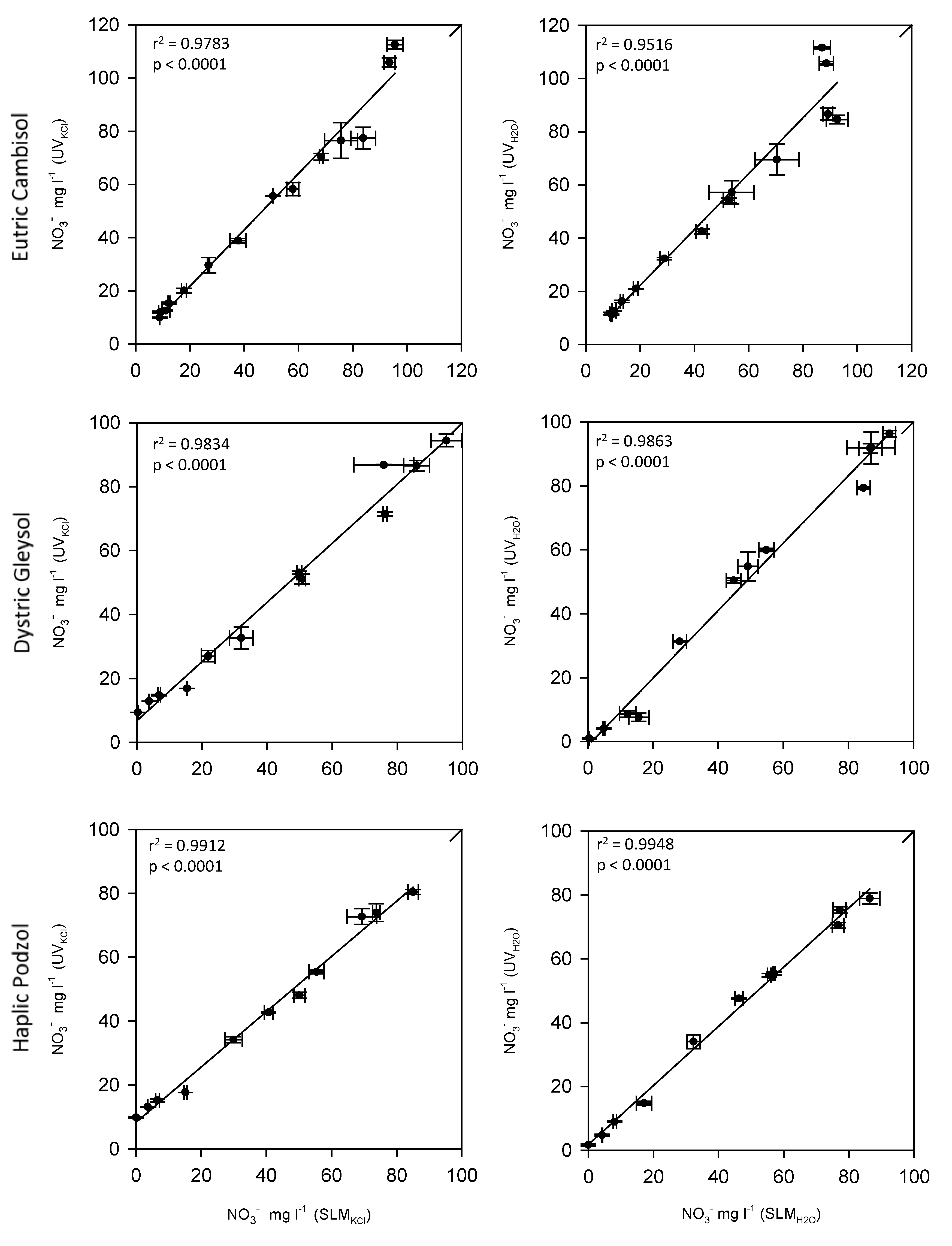
3.3. Comparison of UV Spectroscopy with the Standard Lab Method
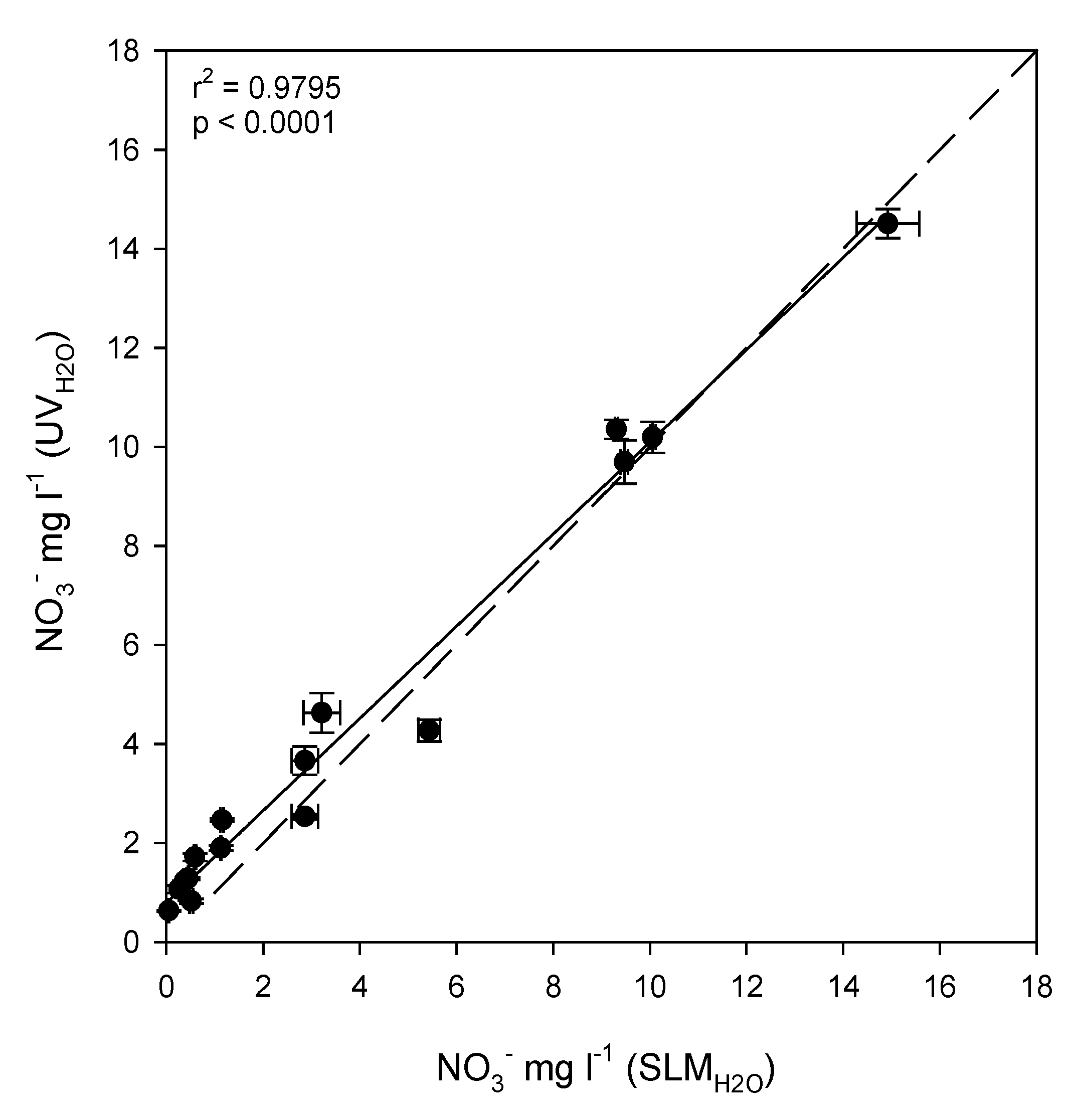
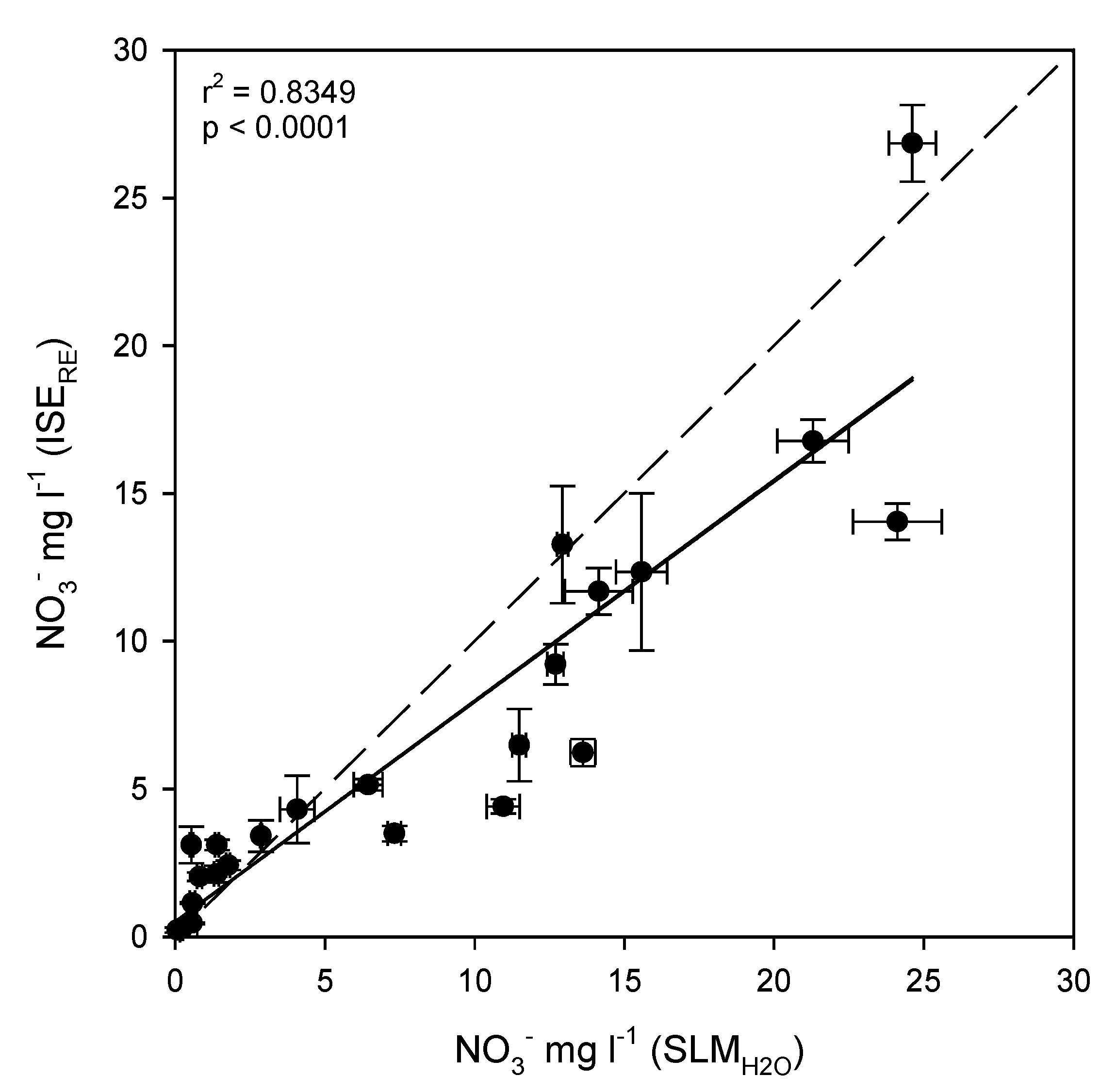
3.4. Evaluation of ISEs for Soil Nitrate Determination
3.5. Evaluation of UV Spectroscopy for Soil Nitrate Determination
3.6. Implications for Fertiliser Application Guidelines
4. Conclusions
Acknowledgments
References
- Cassman, K.; Dobermann, A.; Walters, D.; Yang, H. Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 2003, 28, 315–358. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA. 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Iversen, T.; Grant, R.; Nielsen, K. Nitrogen enrichment of European inland and marine waters with special attention to Danish policy measures. Environ. Pollut. 1998, 102, 771–780. [Google Scholar] [CrossRef]
- Anonymous. EEA Signals, A European Environment Agency Update on Selected Issues; European Environment Agency: Luxembourg, 2004. [Google Scholar]
- Olfs, H.W.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil- and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Grizetti, B. European Nitrogen Assessment; Sutton, M.A., Howard, C.M., Erisman, J.M., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK, 2011; Chapter 17; pp. 379–404. [Google Scholar]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO 2002, 31, 132–140. [Google Scholar]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Env. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Anonymous. Fertiliser Manual (RB209), 8th ed.; Department of Environment, Farming and Rural Affairs (Defra), HMSO: London, UK, 2010. [Google Scholar]
- Jemison, J.M.; Fox, R.H. A quick-test procedure for soil and plant-tissue nitrates using test strips and a hand-held reflectometer. Commun. Soil Sci. Plant Anal. 1988, 19, 1569–1582. [Google Scholar] [CrossRef]
- Roth, G.W.; Beegle, D.B.; Fox, R.H.; Toth, J.D.; Piekielek, W.P. Development of a quick test kit method to measure soil nitrate. Commun. Soil Sci. Plant Anal. 1991, 22, 191–200. [Google Scholar] [CrossRef]
- Hartz, T.K. A quick test procedure for soil nitrate-nitrogen. Commun. Soil Sci. Plant Anal. 1994, 25, 511–515. [Google Scholar] [CrossRef]
- Wetselaar, R.; Smith, G.D.; Angus, J.F. Field measurement of soil nitrate concentrations. Commun. Soil Sci. Plant Anal. 1998, 29, 729–739. [Google Scholar] [CrossRef]
- Hartz, T.K.; Smith, R.F.; Lestrange, M.; Schulbach, K.F. On-farm monitoring of soil and crop nitrogen status by nitrate-selective electrode. Commun. Soil Sci. Plant Anal. 1993, 24, 2607–2615. [Google Scholar] [CrossRef]
- Schmidhalter, U. Development of a quick on-farm test to determine nitrate levels in soil. J. Plant Nutr. Soil Sc. 2005, 168, 432–438. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis Part 3—Chemical Methods; SSSA Book series No. 5; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Adamchuk, V.I.; Lund, E.D.; Sethuramasamyraja, B.; Morgan, M.T.; Dobermann, A.; Marx, D.B. Direct measurement of soil chemical properties on-the-go using ion-selective electrodes. Comput. Electron. Agric. 2005, 48, 272–294. [Google Scholar] [CrossRef]
- Edwards, A.C.; Hooda, P.S.; Cook, Y. Determination of nitrate in water containing dissolved organic carbon by ultraviolet spectroscopy. Int. J. Environ. Anal. Chem. 2001, 80, 49–59. [Google Scholar] [CrossRef]
- Miller, A.J.; Zhen, R.G. Measurement of intracellular nitrate concentrations in chara using nitrate-selective microelectrodes. Planta. 1991, 184, 47–52. [Google Scholar]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y.; Ji, F. A novel pH sensing membrane based on an ionic liquid-polymer composite. Microchimica Acta. 2012, 176, 229–234. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shaw, R.; Williams, A.P.; Miller, A.; Jones, D.L. Assessing the Potential for Ion Selective Electrodes and Dual Wavelength UV Spectroscopy as a Rapid on-Farm Measurement of Soil Nitrate Concentration. Agriculture 2013, 3, 327-341. https://doi.org/10.3390/agriculture3030327
Shaw R, Williams AP, Miller A, Jones DL. Assessing the Potential for Ion Selective Electrodes and Dual Wavelength UV Spectroscopy as a Rapid on-Farm Measurement of Soil Nitrate Concentration. Agriculture. 2013; 3(3):327-341. https://doi.org/10.3390/agriculture3030327
Chicago/Turabian StyleShaw, Rory, A. Prysor Williams, Anthony Miller, and Davey L. Jones. 2013. "Assessing the Potential for Ion Selective Electrodes and Dual Wavelength UV Spectroscopy as a Rapid on-Farm Measurement of Soil Nitrate Concentration" Agriculture 3, no. 3: 327-341. https://doi.org/10.3390/agriculture3030327
APA StyleShaw, R., Williams, A. P., Miller, A., & Jones, D. L. (2013). Assessing the Potential for Ion Selective Electrodes and Dual Wavelength UV Spectroscopy as a Rapid on-Farm Measurement of Soil Nitrate Concentration. Agriculture, 3(3), 327-341. https://doi.org/10.3390/agriculture3030327




