Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Entry Paths of Eroded Particles into the Streams and Rivers
2.1.1. Agriculturally Caused Erosion, Including Drainage and Earth Road Construction
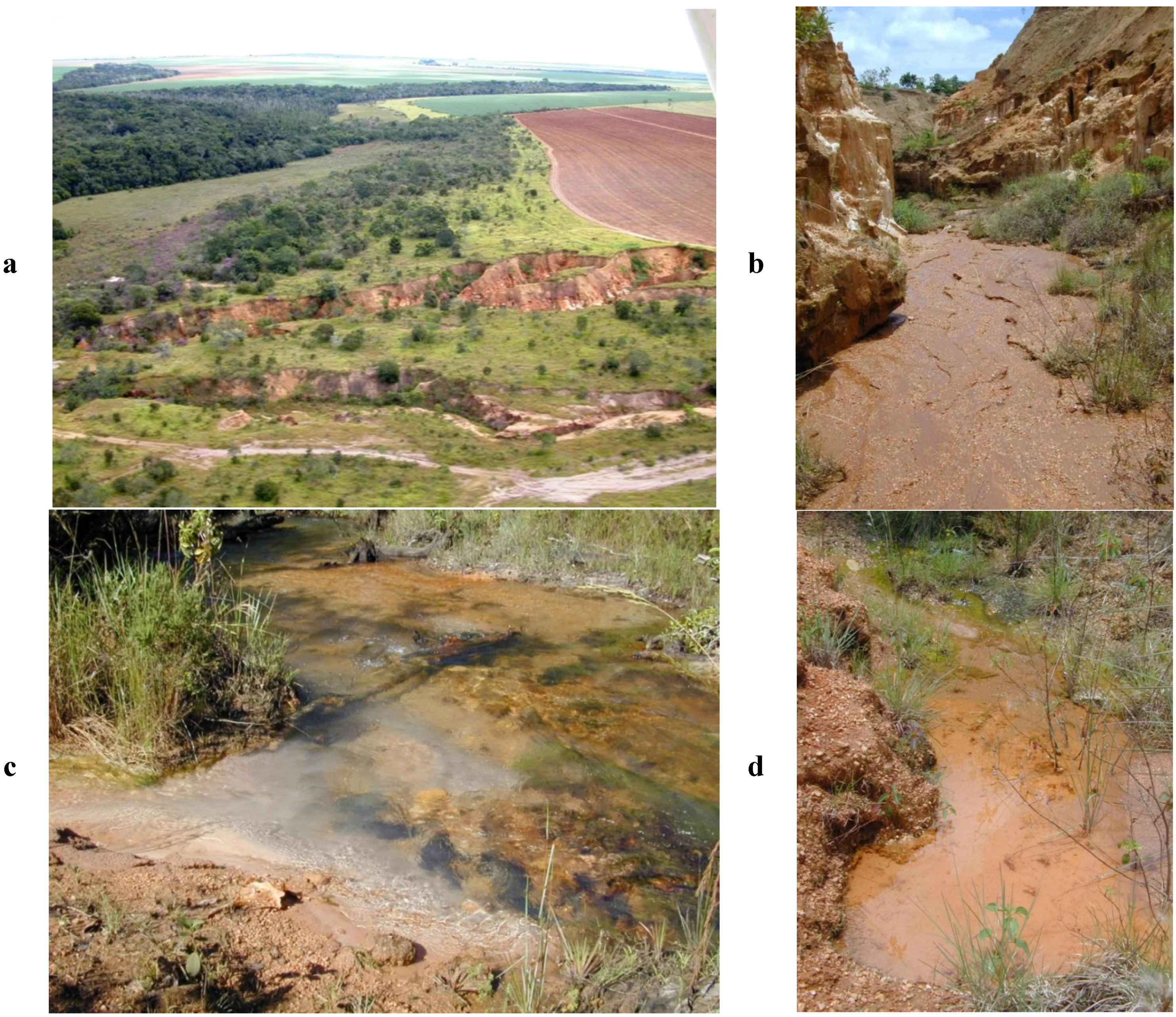
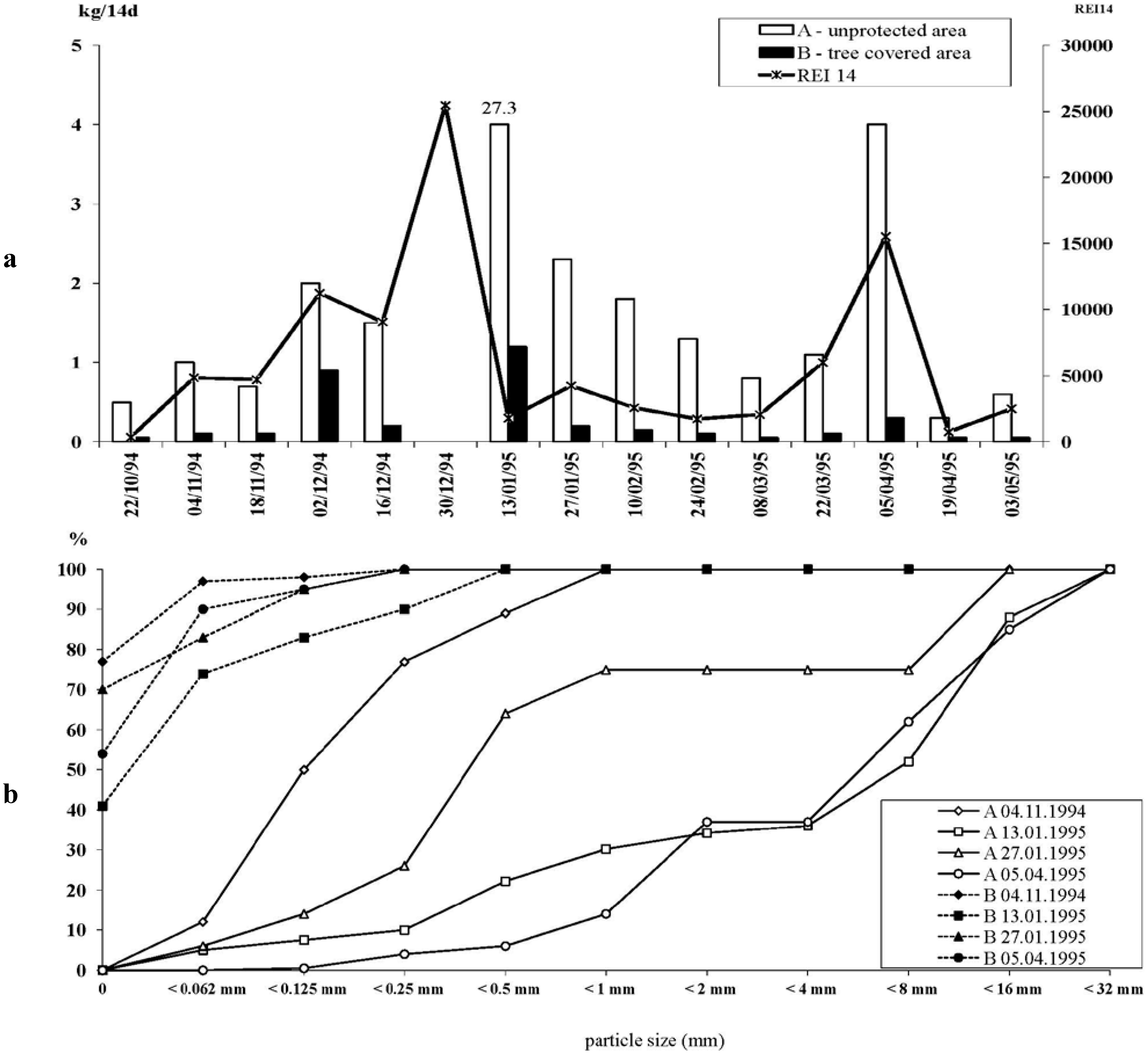
2.1.2. Erosion from Artisanal, Small-Scale Gold Mining Activities

2.2. Effects of Increased Sediment Load on Habitats and Biota in and Near Streams
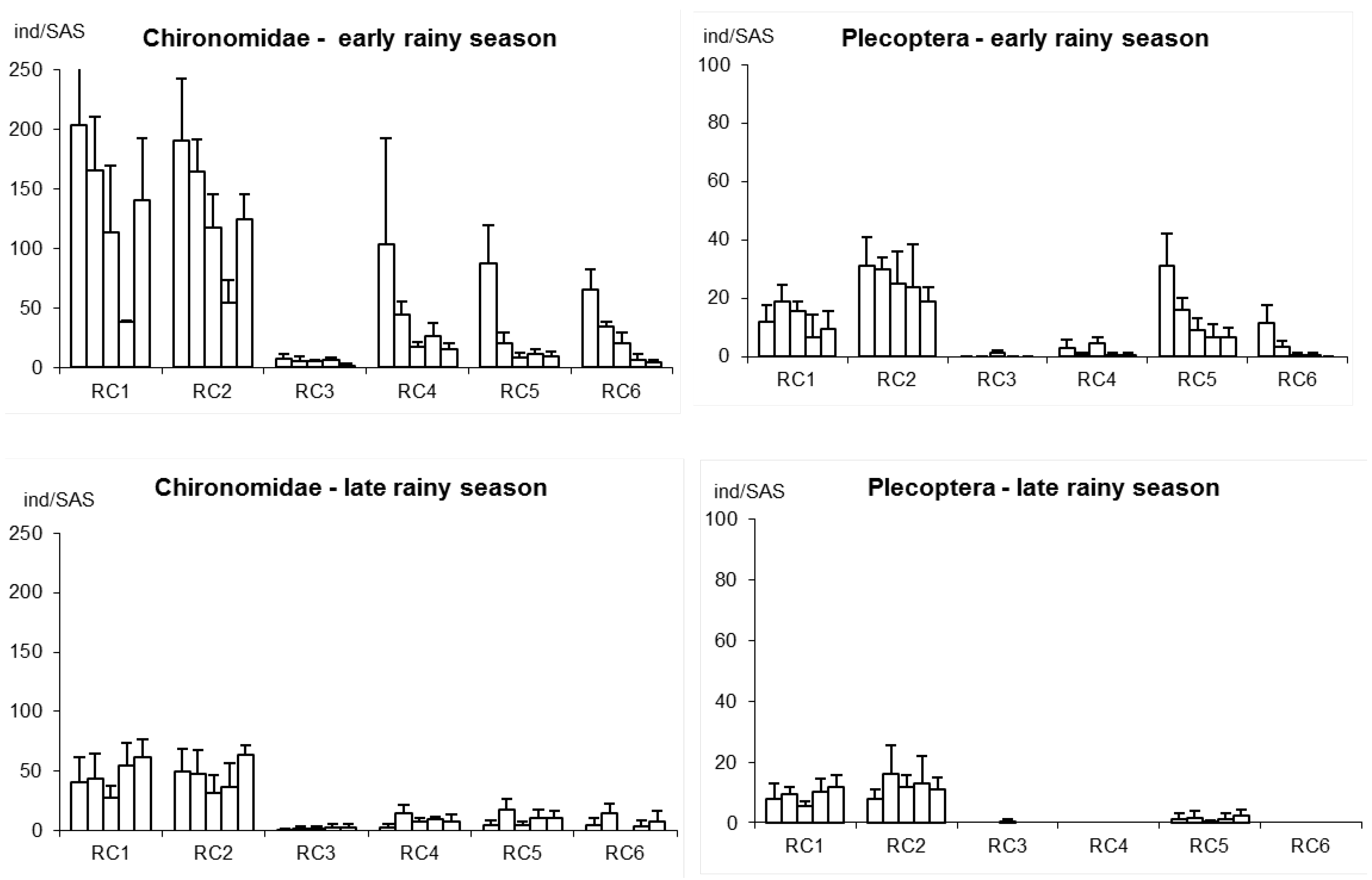
2.2.1. Effects on Stream Habitat Structures
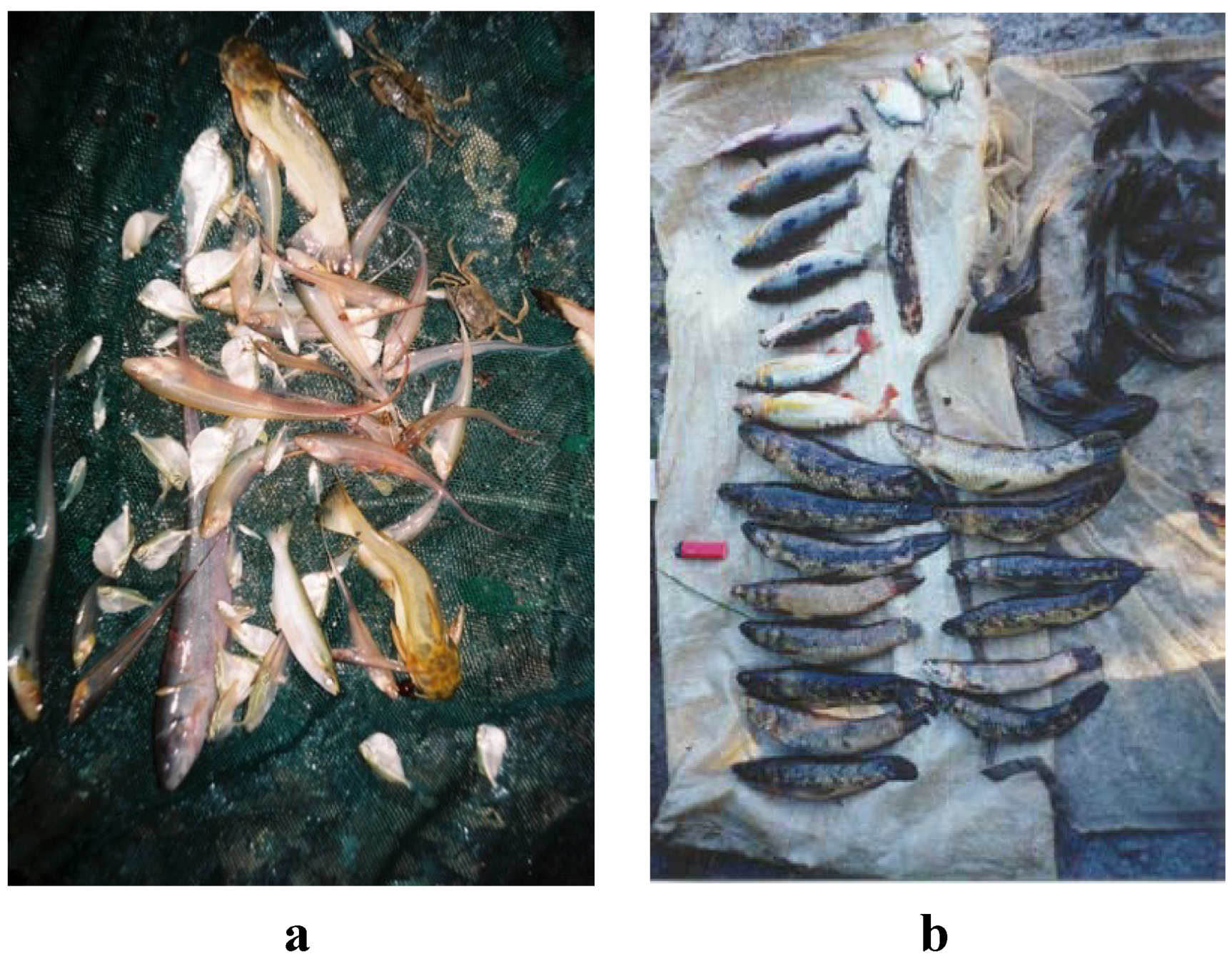
2.2.2. Effects on Stream Plants and Animals
| Undisturbed Stream Maykabuka Creek | Mining-Impacted Stream Mamanari Creek | |
|---|---|---|
| Monthly flow (m3 s−1) | 0.54–1.65 | 0.92–3.92 |
| Riparian rain forest | Undisturbed | Undisturbed |
| Total suspended solids (mg L−1) | 19.0–28.9 | 318–2469 |
| Turbidity (NTU) | 28.2–31.1 | 424–2874 |
| Secchi disc visibility (cm) | >50 | <10 |
| Sediment yield (tonnes year km2) | 13 | 310 (of which 95.6% produced by the gold mine) |
| Thickness of layer of fine sediment on the streambed (cm) | 0 | 12.8 (runs)–33.2 (pools) (maximum 57 cm) |
| Substrate diversity (Shannon-Wiener index) | 1.57 | 0.70 |
| Number of fish species | 68 | 56 |
| Fish diversity (Shannon-Wiener index) | 3.19–3.39 | 2.60–2.70 |
| Erythrinidae (% of total number of fishes caught) | 3.48 | 0.41 |
| Gasteropelecidae (%) | 4.81 | 18.61 |
| Gymnotiformes (%) | 3.54 | 12.62 |
| Auchenipteridae (%) | 1.20 | 3.33 |
| Callichthyidae (%) | 14.65 | 0.62 |
| Cichlidae (%) | 5.98 | 0.58 |
| Juvenile fishes (%) | 57.3 | 14.1 |
| Food fishes (% of total fish biomass) | 33.2 | 13.4 |
2.3. Effects on Riparian Habitat Structures


2.4. Effects on Riparian Animals and Plants
3. Possible Actions
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soils and food sufficiency. A review. Agron. Sustain. Dev. 2009, 29, 113–133. [Google Scholar] [CrossRef]
- Quinton, J.N.; Govers, G.; Van Oost, K.; Bardgett, R.D. The impact of agricultural soil erosion on biogeochemical cycling. Nature Geosci. 2010, 3, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil erosion and the global carbon budget. Environ. Inter. 2003, 29, 437–450. [Google Scholar] [CrossRef]
- Pimentel, D.; Harvey, C.; Resosudarmo, P.; Sinclair, K.; Kurz, D.; McNair, M.; Christ, S.; Shrpirtz, L.; Fitton, L.; Saffouri, R.; et al. Environmental and economic costs of soil erosion and conservation benefits. Science 1995, 267, 1117–1123. [Google Scholar] [CrossRef]
- Sioli, H. Das Wasser im Amazonasgebiet. Forsch. Fortschr. 1950, 26, 274–280. [Google Scholar]
- Ryan, P.A. Environmental effects of sediments on New Zealand streams: A review. New Zealand J. Mar. Freshwater Res. 1991, 25, 207–221. [Google Scholar] [CrossRef]
- Malmqvist, B.; Rundle, S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002, 29, 134–153. [Google Scholar]
- Castello, L.; McGrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebre, P.A.; Petry, P.; Macedo, M.N.; Reno, V.F.; Arantes, C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 2013, 6, 217–229. [Google Scholar] [CrossRef]
- Boulton, A.J.; Boyero, L.; Covich, A.P.; Dobson, M.; Lake, P.S.; Pearson, R.G. Are Tropical Streams Ecologically Different from Temperate Streams? In Tropical Stream Ecology; Dudgeon, D., Ed.; Elsevier: Amsterdam, the Netherlands, 2008; pp. 257–284. [Google Scholar]
- Wantzen, K.M.; Junk, W.J. The Importance of Stream-Wetland-Systems for Biodiversity: A Tropical Perspective. In Biodiversity in Wetlands: Assessment, Function and Conservation; Gopal, B., Junk, W.J., Davies, J.A., Eds.; Backhuys: Leiden, the Netherlands, 2000; pp. 11–34. [Google Scholar]
- Wantzen, K.M.; Mathooko, J.; Yule, C.; Pringle, C.M. Organic Matter Processing in Tropical Streams. In Tropical Stream Ecology; Dudgeon, D., Ed.; Elsevier: Amsterdam, the Netherlands, 2008; pp. 43–64. [Google Scholar]
- Jacobsen, D.; Encalada, A. The macroinvertebrate fauna of Ecuadorian highland streams in the wet and dry season. Arch. Hydrobiol. 1998, 142, 53–70. [Google Scholar]
- Lewis, W.M., Jr.; Hamilton, S.K.; Saunders, J.F., III. Rivers of Northern South America. In River and Stream Ecosystems; Cushing, C.E., Cummins, K.W., Minshall, G.W., Eds.; Elsevier: New York, NY, USA, 1995; pp. 219–256. [Google Scholar]
- Wantzen, K.M. Physical pollution: Effects of gully erosion in a tropical clear-water stream. Aquat. Conserv. 2006, 16, 733–749. [Google Scholar] [CrossRef]
- Wantzen, K.M. Cerrado Streams—Characteristics of a threatened freshwater ecosystem type on the tertiary shields of South America. Amazoniana 2003, 17, 485–502. [Google Scholar]
- Johnson, D.L.; Lewis, L.A. Land Degradation: Creation and Destruction; Blackwell: London, UK, 1995; pp. 1–335. [Google Scholar]
- Nepstad, D.; McGrath, D.; Alencar, A.; Barros, A.C.; Carvalho, G.; Santilli, M.; Vera Diaz, M.C. Frontier governance in Amazonia. Science 2009, 295, 629–631. [Google Scholar]
- Veiga, M.M.; Hinton, J.J. Abandoned artisanal gold mines in the Brazilian Amazon: A legacy of mercury pollution. Nat. Resour. For. 2002, 26, 13–24. [Google Scholar]
- Heemskerk, M. Livelihood decision making and environmental degradation: Small-scale gold mining in the Suriname Amazon. Soc. Nat. Resour. 2002, 15, 327–344. [Google Scholar] [CrossRef]
- Swenson, J.J.; Carter, C.E.; Domec, J.C.; Delgado, C.I. Gold mining in the Peruvian Amazon: Global prices, deforestation, and mercury imports. PLoS One 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Ouboter, P.E.; Landburg, G.; Quik, J.; Mol, J.; van der Lugt, F. Mercury levels in pristine and gold mining impacted aquatic ecosystems of Suriname, South America. Ambio 2012, 41, 873–882. [Google Scholar] [CrossRef]
- Balogh, S.J.; Meyer, M.L.; Johnson, D.K. Transport of mercury in three contrasting river basins. Environ. Sci. Technol. 1998, 32, 456–462. [Google Scholar] [CrossRef]
- Secretaria de Assuntos Estratégicos (SAE) Impacto da revisão do Código Florestal: como viabilizar o grande desafio adiante? Internet report. Available online: http://www.sae.gov.br/site/?p=15735 (accessed on 24 September 2013).
- Silva, J.A.A.; Nobre, A.D.; Manzatto, C.V.; Joly, C.A.; Rodrigues, R.R.; Skorupa, L.A.; Nobre, C.A.; Ahrens, S.; May, P.H.; Sá, T.D.A.; et al. O Código Florestal e a Ciência: Contribuição para o Diálogo; Sociedade Brasileira para o Progresso da Ciência & Academia Brasileira de Ciências: São Paulo, Brazil, 2011; pp. 1–124. [Google Scholar]
- Wantzen, K.M.; Siqueira, A.; Nunes da Cunha, C.; Sa, M.F.P. Stream-valley systems of the Brazilian Cerrado: Impact assessment and conservation scheme. Aquat. Conserv. 2006, 16, 713–732. [Google Scholar] [CrossRef]
- Harding, J.S.; Benfield, E.F.; Bolstad, P.V.; Helfman, G.S.; Jones, E.B.D., III. Stream biodiversity: The ghost of land use past. Proc. Natl. Acad. Sci. USA 1998, 95, 14843–14847. [Google Scholar] [CrossRef]
- Batlle-Bayer, L. Changes in organic carbon stocks upon land use conversion in the Brazilian Cerrado: A review. Agric. Ecosyst. Environ. 2010, 137, 47–58. [Google Scholar] [CrossRef]
- Couto, E.G. O uso da terra e o garimpo na bacia do rio Sao Lourenco, Mato Grosso: Reflexos No Ambiente; FEMA/UFMT-CCA: Cuiabá, Mato Grosso, Brazil, 1990; p. 206. [Google Scholar]
- Wantzen, K.M. Influence of Man-Made Siltation on Habitat Structure and Biotic Communities of Cerrado Streams of Mato Grosso. Ph.D. Thesis, Herbert Utz Verlag, Munich, 1997; p. 186. [Google Scholar]
- Wantzen, K.M. Effects of siltation on benthic communities in clear water streams in Mato Grosso, Brazil. Verh. Int. Ver. Limnol. 1998, 26, 1155–1159. [Google Scholar]
- DellaSala, D.A.; Karr, J.R.; Olson, D.M. Roadless areas and clean water. J. Soil Water Conserv. 2011, 66, 78–84. [Google Scholar] [CrossRef]
- Peterson, G.D.; Heemskerk, M. Deforestation and forest regeneration following small-scale gold mining in the Amazon: The case of Suriname. Environ. Conserv. 2001, 28, 117–126. [Google Scholar]
- Centrum voor Landbouwkundig Onderzoek in Suriname (CELOS), Assessments of the extend of land categories, land uses and changes for the Second National Communication—AFOLU GHC Inventory 2011. CELOS, Natural Resources and Environmental Assessment: Paramarib, Suriname, 2011; unpublished work.
- Mol, J.H.; Ouboter, P.E. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conserv. Biol. 2004, 18, 201–214. [Google Scholar] [CrossRef]
- Power, M.E. The importance of sediment in the grazing ecology and size class interactions of the armored catfish, Ancistrus spinosus. Environ. Biol. Fish. 1984, 10, 173–181. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Smith, D.G. Turbidity, suspended sediment, and water clarity: A review. J. Amer. Water Resources Ass. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Nejstgaard, J.; Sœdberg, E.; Sørnes, T. Optical control of fish and zooplankton populations. Limnol. Oceanogr. 2004, 49, 233–238. [Google Scholar] [CrossRef]
- Walker, I. Amazonian Streams and Small Rivers. In Limnology in Brazil; Tundisi, J.G., Bicudo, C.E.M., Matsamura-Tundisi, T., Eds.; Brazilian Academy of Sciences: Rio de Janeiro, Brazil, 1995; pp. 167–194. [Google Scholar]
- Lau, D.C.P.; Leung, K.M.Y.; Dudgeon, D. What does stable isotope analysis reveal about trophic relationships and the relative importance of allochthonous and autochthonous resources in tropical streams? A synthetic study from Hong Kong. Freshwater Biol. 2009, 54, 127–141. [Google Scholar] [CrossRef]
- Brito, E.F.; Moulton, T.P.; de Souza, M.L.; Bunn, S.E. Stable isotope analysis indicates microalgae as the predominant food source of fauna in a coastal forest stream, south-east Brazil. Austral Ecol. 2006, 31, 623–633. [Google Scholar] [CrossRef]
- Newcombe, C.P.; MacDonald, D.D. Effects of suspended sediments on aquatic ecosystems. North Amer. J. Fish. Manage. 1991, 11, 72–82. [Google Scholar] [CrossRef]
- Waters, T.F. Sediments in Streams—Sources, Biological Effects, and Control; American Fisheries Society: Bethesda, MD, USA, 1995; p. 250. [Google Scholar]
- Kleeberg, A.; Köhler, J.A.N.; Sukhodolova, T.; Sukhodolov, A. Effects of aquatic macrophytes on organic matter deposition, resuspension and phosphorus entrainment in a lowland river. Freshwater Biol. 2010, 55, 326–345. [Google Scholar] [CrossRef]
- Brookes, A. Response of aquatic vegetation to sedimentation downstream from river channelisation works in England and Wales. Biol. Conserv. 1986, 38, 351–367. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Junk, W.J. Aquatic-terrestrial linkages from streams to rivers: Biotic hot spots and hot moments. Arch. Hydrobiol. Suppl. 2006, 158, 595–611. [Google Scholar]
- Horeau, V.; Cerdan, P.; Champeau, A.; Richard, S. Importance of aquatic invertebrates in the diet of rapids-dwelling fish in the Sinnamary River, French Guiana. J. Trop. Ecol. 1998, 14, 851–864. [Google Scholar] [CrossRef]
- Odinetz Collart, O.; Jégu, M.; Thatcher, V.; Tavares, A.S. Les prairies aquatiques de l’Amazonie bresilienne. RSTOM Actualités 1996, 49, 8–14. [Google Scholar]
- Cope, W.G.; Bringolf, R.B.; Buchwalter, D.B.; Newton, T.J.; Ingersoll, C.G.; Wang, N.; Augspurger, T.; Dwyer, F.J.; Barnhart, M.C.; Neves, R.J.; et al. Differential exposure, duration, and sensitivity of unionoidean bivalve life stages to environmental contaminants. J. North Amer. Benthological Soc. 2008, 27, 451–462. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Pinto-Silva, V. Uso de substratos artificiais para macroinvertebrados bentônicos para a avaliação do impacto de assoreamento em nascentes dos tributrios do Pantanal do Mato Grosso, Brasil. Revista Brasileira de Recursos Hídricos 2006, 11, 99–107. [Google Scholar]
- Bash, J. Effects of Turbidity and Suspended Solids on Salmonids; Center for Streamside Studies, University of Washington: Seattle, WA, USA, 2001; p. 74. [Google Scholar]
- Lloyd, D.S. Turbidity as a water quality standard for salmonid habitats in Alska. North Amer. J. Fish. Manage. 1987, 7, 34–45. [Google Scholar] [CrossRef]
- Bruton, M.N. The effects of suspensoids on fish. Hydrobiologia 1985, 125, 221–242. [Google Scholar] [CrossRef]
- Gende, S.M.; Edwards, R.T.; Willson, M.F.; Wipfli, M.S. Pacific salmon in aquatic and terrestrial ecosystems. BioScience 2002, 52, 917–928. [Google Scholar] [CrossRef]
- Carolsfeld, J.; Harvey, C.; Ross, C.; Baer, A. Migratory Fishes of South America: Biology, Fisheries and Conservation; International Development Centre & the World Bank: Ottawa, Ontario, Canada, 2003; p. 372. [Google Scholar]
- Cederholm, C.J.; Salo, E.O. The Effects of Logging Road Landslide Siltation on the Salmon and Trout Spawning Gravels of Stequaleho Creek and Clearwater River Basin, Jefferson County, Washington, 1972–1978; Fisheries Research Institute, University of Washington: Seattle, WA, USA, 1979; p. 90. [Google Scholar]
- Van der Sluijs, I.; Gray, S.M.; Amorim, M.C.P.; Candolin, U.; Hendry, A.P.; Krahe, R.; Maan, M.F.; Utne-Palm, A.C.; Wagner, H.J.; Wong, N.B.M. Communication in troubled waters: Responses of fish communication systems to changing environments. Evol. Ecol. 2011, 25, 623–640. [Google Scholar] [CrossRef]
- Keenleyside, M.H.A. Some aspects of schooling in fish. Behaviour 1955, 8, 183–249. [Google Scholar] [CrossRef]
- Keenleyside, M.H.A.; Bietz, B.F. The reproductive behavior of Aequidens vittatus (Pisces, Cichlidae) in Surinam, South America. Environ. Biol. Fish. 1981, 6, 87–94. [Google Scholar] [CrossRef]
- Seehausen, O.; Van Alphen, J.J.M.; Witte, F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 1997, 277, 1808–1811. [Google Scholar] [CrossRef]
- Dias, A.M.; Tejerina-Garro, F.L. Changes in the structure of fish assemblages in streams along an undisturbed-impacted gradient, upper Parana River basin, Central Brazil. Neotrop. Ichthyol. 2010, 8, 587–598. [Google Scholar] [CrossRef]
- Mol, J.H.; You, K.W.T.; Vrede, I.; Flynn, A.; Ouboter, P.; van der Lugt, F. Fishes of Lely and Nassau Mountains, Suriname. In a Rapid Biological Assessment of the Lely and Nassau Plateaus, Suriname (with Additional Information on the Brownsberg Plateau); Alonso, L.E., Mol, J.H., Eds.; Conservation International: Arlington, TX, USA, 2007; pp. 107–118. [Google Scholar]
- Lujan, N.K.; Roach, K.A.; Jacobsen, D.; Winemiller, K.O.; Vargas, V.M.; Ching, V.R.; Maestre, J.A. Aquatic community structure across an Andes-to-Amazon fluvial gradient. J. Biogeogr. 2013. [Google Scholar]
- Moyle, P.B.; Leidy, R.A. Loss of Biodiversity in Aquatic Ecosystems: Evidence from Fish Faunas. In Conservation Biology; Fiedler, P.L., Jain, S.K., Eds.; Chapman & Hall: New York, NY, USA, 1992; pp. 127–169. [Google Scholar]
- Burcher, C.L.; McTammany, M.E.; Benfield, E.F.; Helfman, G.S. Fish assemblage responses to forest cover. Environ. Manage. 2008, 41, 336–346. [Google Scholar] [CrossRef]
- Casatti, L.; Ferreira, C.P.; Carvalho, F.R. Grass-Dominated stream sides exhibit low fish diversity and dominance by guppies: An assessment of two tropical pasture river basins. Hydrobiologia 2009, 632, 273–283. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Nunes da Cunha, C.; Siqueira, A.J.B. Cerrado Stream Valleys and their Vegetation: Structure, Impacts by Erosion and Recuperation Strategies. In the Pantanal: Ecology, Biodiversity and Sustainable Management of a Large Neotropical Seasonal Wetland; Junk, W.J., da Silva, C.J., Nunes da Cunha, C., Wantzen, K.M., Eds.; Pensoft: Moscow and Sofia, Russia, 2011; pp. 143–165. [Google Scholar]
- Valentin, C.; Poesem, J.; Li, Y. Gully erosion: Impacts, factors and control. Catena 2005, 63, 132–153. [Google Scholar] [CrossRef]
- Hammond, D.S.; Rosales, J.; Ouboter, P.E. Managing the Freshwater Impacts of Surface Mining in Latin America; IDB Technical Note 519; Inter-American Development Bank: Washington, DC, USA, 2013; p. 36. [Google Scholar]
- Wang, J.J.; Lu, X.X.; Liew, S.C.; Zhou, Y. Retrieval of suspended sediment concentrations in large turbid rivers using Landsat ETM+: An example from the Yangtze River, China. Earth Surf. Process. Landf. 2009, 34, 1082–1092. [Google Scholar] [CrossRef]
- CARBIOCIAL (Carbon Sequestration, Biodiversity and Social Structures in Southern Amazonia: Models and Implementation of Carbon-Optimized Land Management Strategies) Project. Available online: http://www.carbiocial.de/ (accessed on 24 September 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wantzen, K.M.; Mol, J.H. Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems. Agriculture 2013, 3, 660-683. https://doi.org/10.3390/agriculture3040660
Wantzen KM, Mol JH. Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems. Agriculture. 2013; 3(4):660-683. https://doi.org/10.3390/agriculture3040660
Chicago/Turabian StyleWantzen, Karl M., and Jan H. Mol. 2013. "Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems" Agriculture 3, no. 4: 660-683. https://doi.org/10.3390/agriculture3040660




