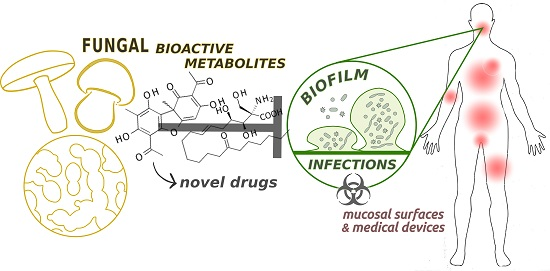
Fungal Metabolites for the Control of Biofilm Infections
Abstract
:1. Biofilm Infections Are a Therapeutic Challenge
2. Fungal Metabolites Reported to Modulate Biofilms of Pathogens
2.1. Biofilm-Modulating Terpenes from Fungi
2.2. Fungal Metabolites of Polyketide Origin for Biofilm-Control
2.3. Amino Acids and Derivatives Controlling Biofilm Formation
2.4. From Papulacandins and Echinocandins to Drugs against Fungal Biofilms
3. Conclusions
4. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MIC | Minimal inhibitory concentration |
| IC50 | Half maximal inhibitory concentration |
References
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Rosen Da, H.T.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.A.; Tran, V.R.; Sugawa, C.; Coticchia, J.M. Identification of Helicobacter pylori biofilms in human gastric mucosa. J. Gastrointest. Surg. 2006, 10, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Dillon, L.R. Catheter outcomes in home infusion. J. Infus. Nurs. 2008, 31, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Camilli, A.; Bassler, B.L. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Schauder, S.; Bassler, B.L. The languages of bacteria. Genes Dev. 2001, 15, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.-R. Controlling Gram-negative pathogenic bacteria by interfering with their biofilm formation. Drug Des. Rev. Online 2005, 2, 13–33. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Novick, R.P. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides 2004, 25, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Mühlschlegel, F.A. Communication in Fungi. Int. J. Microbiol. 2012, 351832. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.A.S.; Oteef, M.D.Y.; Flowers, T.H.; Douglas, L.J. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot. Cell 2006, 5, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.W.; Atkin, A.L.; Hornby, J.M. Quorum sensing in dimorphic fungi: Farnesol and beyond. Appl. Environ. Microbiol. 2006, 72, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Ciccoli, R.; Ivanov, I.; Sczepanski, M.; Deva, R. On mechanism of quorum sensing in Candida albicans by 3(R)-hydroxy-tetradecaenoic acid. Curr. Microbiol. 2011, 62, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Obata, S.; Shimizu, S. Microorganisms for Production of Geranylgeraniol and Analogous Compounds. European Patent EP 1,219,714, 2 July 2002. [Google Scholar]
- Harriott, M.M.; Noverr, M.C. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wargo, M.J.; Hogan, D.A. Fungal—Bacterial interactions: A mixed bag of mingling microbes. Curr. Opin. Microbiol. 2006, 9, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Joly, V.; Pangon, B.; Vallois, J.M.; Abel, L.; Brion, N.; Bure, A.; Chau, N.P.; Contrepois, A.; Carbon, C. Value of antibiotic levels in serum and cardiac vegetations for predicting antibacterial effect of ceftriaxone in experimental Escherichia coli endocarditis. Antimicrob. Agents Chemother. 1987, 31, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.J.; Kristinsson, K.G.; Anderson, R.M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc. Natl. Acad. Sci. USA 1999, 96, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J. Microbiology. Desperately seeking new antibiotics. Science 2008, 321, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Richards, J.J.; Melander, C. Non-microbicidal control of bacterial biofilms with small molecules. Anti-Inf. Agents 2014, 12, 120–138. [Google Scholar] [CrossRef]
- Zhu, J.; Kaufmann, G.F. Quo vadis quorum quenching? Curr. Opin. Pharmacol. 2013, 13, 688–898. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P.; Türck, P.; Abraham, W.-R. Secondary metabolites control the associated bacterial communities of saprophytic Basidiomycotina fungi. Microbes Environ. 2015, 30, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Robles, A.J.; King, J.B.; Powell, D.R.; Miller, A.N.; Mooberry, S.L.; Cichewicz, R.H. Crowdsourcing natural products discovery to access uncharted dimensions of fungal metabolite diversity. Angew. Chem. Int. Ed. Engl. 2014, 53, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.A.; Daglia, M.; Papetti, A.; Stauder, M.; O’Donnell, D.; Ciric, L.; Tymon, A.; Repetto, B.; Signoretto, C.; Houri-Haddad, Y.; et al. Evaluation of plant and fungal extracts for their potential antigingivitis and anticaries activity. J. Biomed. Biotechnol. 2012, 510198. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Papetti, A.; Pruzzo, C.; Zaura, E.; Lingström, P.; Ofek, I.; Pratten, J.; et al. The anti-adhesive mode of action of a purified mushroom (Lentinus edodes) extract with anticaries and antigingivitis properties in two oral bacterial pathogens. BMC Compl. Altern. Med. 2014, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Milli, A.; Tessarolo, F.; Caola, I.; Papetti, A.; Pruzzo, C.; Zaura, E.; et al. Effects of mushroom and chicory extracts on the shape, physiology and proteome of the cariogenic bacterium Streptococcus mutans. BMC Compl. Altern. Med. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2011, 10, 287–307. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Sohr, R.; Schulz, B.; Fleischhacker, M.; Ruhnke, M. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob. Agents Chemother. 2008, 52, 1859–1861. [Google Scholar] [CrossRef]
- Cushion, M.T.; Collins, M.S.; Linke, M.J. Biofilm formation by Pneumocystis spp. Eukaryot. Cell 2009, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.I.A.; Teixeira, P.; Azeredo, J.; Oliveira, R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr. Microbiol. 2009, 59, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Teixeira, P.; Cerca, N.; Azeredo, J.; Oliveira, R. Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Curr. Microbiol. 2011, 63, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-H.; Wei, X.; Ma, M.; Chen, X.-J.; Xu, S.-B. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother. 2012, 56, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Shirtliff, M.; James, C.; Meiller, T. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 2006, 6, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Mekala, S.; Chaffin, W.L. Farnesol-mediated inhibition of Candida albicans yeast growth and rescue by a diacylglycerol analogue. Yeast 2007, 24, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Calfee, M.W.; Farrow, J.M., III; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.M.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-induced apoptosis in Candida albicans. Antimocrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Semighini, C.P.; Hornby, J.M.; Dumitru, R.; Nickerson, K.W.; Harris, S.D. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 2006, 59, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; MacDonald, K.; McMaster, C.R. A chemogenomic screen in Saccharomyces cerevisiae uncovers a primary role for the mitochondria in farnesol toxicity and its regulation by the Pkc1 pathway. J. Biol. Chem. 2007, 282, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Shirtliff, M.E.; Meiller, T.F.; Peters, B.; Jabra-Rizk, M.A. Farnesol a fungal quorum sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia 2008, 10, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Scopel, M.; Abraham, W.-R.; Antunes, A.L.; Barth, A.; Ribeiro, V.B.; Henriques, A.T.; Macedo, A.J. Mevalonolactone: An inhibitor of Staphylococcus epidermidis adherence and biofilm formation. Med. Chem. 2014, 10, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Niikawa, H.; Kobayashi, M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J. Nat. Med. 2013, 67, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Eamvijarn, A.; Gomes, N.M.; Dethoup, T.; Buaruang, J.; Manoch, L.; Silva, A.; Pedro, M.; Marini, I.; Roussis, V.; Kijjoa, A. Bioactive meroditerpenes and indole alkaloids from the soil fungus Neosartorya fischeri (KUFC 6344), and the marine-derived fungi Neosartorya laciniosa (KUFC 7896) and Neosartorya tsunodae (KUFC 9213). Tetrahedron 2013, 69, 8583–8591. [Google Scholar] [CrossRef]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.D.; Tida, S.A.M.S.; Kijjoa, A. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Li, T.X.; Yang, M.H.; Wang, X.B.; Wang, Y.; Kong, L.Y. Synergistic antifungal meroterpenes and dioxolanone derivatives from the endophytic fungus Guignardia sp. J. Nat. Prod. 2015, 78, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.A.; de Melo, M.G.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.; Quintans-Júnior, L.J.; Gelain, D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Pandian, S.K. Usnic acid, a lichen secondary metabolite inhibits group A Streptococcus biofilms. Antonie Leeuwenhoek 2015, 107, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Beema Shafreen, R.M.; Muthamil, S.; Karutha Pandian, S. Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimic. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Riedel, K.; Boustie, J.; Eberl, L.; Berg, G.; Grube, M. Effects of lichen secondary metabolites on bacterial functions and biofilm formation. Planta Med. 2008, 74, PA85. [Google Scholar] [CrossRef]
- Millot, M.; Girardot, M.; Dutreix, L.; Imbert, C.; Mambu, L. Lichen biodiversity: A source of secondary metabolites active against Candida biofilms. Planta Med. 2014, 80, P1N24. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Skindersoe, M.E.; Bjarnsholt, T.; Phipps, R.K.; Christensen, K.B.; Jensen, P.O.; Andersen, J.B.; Koch, B.; Larsen, T.O.; Hentzer, M. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 2005, 151, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P. Use of Comatuslactone to Modulate Microbial Biofilms. Ph.D. Thesis, Technical University Braunschweig, Braunschweig, Germany, 1 April 2014. [Google Scholar]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; van Olphen, A.; Baker, B.J. Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lambu, M.R.; Jamwal, U.; Rani, C.; Chib, R.; Wazir, P.; Mukherjee, D.; Chaubey, A.; Khan, I.A. Escherichia coli N-acetylglucosamine-1-phosphate-uridyltransferase/glucosamine-1-phosphate-acetyltransferase (GlmU) inhibitory activity of terreic acid isolated from Aspergillus terreus. J. Biomol. Screen. 2016, 21, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, Y.; Olesen, S.H.; Becker, A.; Betzi, S.; Schönbrunn, E. The fungal product terreic acid is a covalent inhibitor of the bacterial cell wall biosynthetic enzyme UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA). Biochemistry 2010, 49, 4276–4282. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.F.; Li, P.J.; Li, X.X.; Sun, P.H.; Gao, H.; Liu, X.Z.; Huang, P.; Tang, J.S.; Yao, X.S. New antibacterial isocoumarin glycosides from a wetland soil derived fungal strain Metarhizium anisopliae. Bioorg. Med. Chem. Lett. 2016, 26, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Kluepfel, D.; Bagli, J.; Baker, H.; Charest, M.P.; Kudelski, A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J. Antibiot. 1972, 25, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Kozutsumi, Y.; Nakamura, S.; Fujita, T.; Kawasaki, T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 1995, 211, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Mukherjee, P.K.; Chandra, J.; Roth, M.R.; Welti, R.; Rouabhia, M.; Ghannoum, M.A. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology 2011, 157, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Perdoni, F.; Signorelli, P.; Cirasola, D.; Caretti, A.; Galimberti, V.; Biggiogera, M.; Gasco, P.; Musicanti, C.; Morace, G.; Borghi, E. Antifungal activity of Myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 2015, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Du, L.; King, J.B.; Hall, B.E.; Cichewicz, R.H. Small-molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida isolates. ACS Chem. Biol. 2013, 8, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; You, J.; King, J.B.; Cai, S.; Park, E.; Powell, D.R.; Cichewicz, R.H. Polyketide glycosides from Bionectria ochroleuca inhibit Candida albicans biofilm formation. J. Nat. Prod. 2014, 77, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; You, J.; King, J.B.; Powell, D.R.; Cichewicz, R.H. Waikialoid A suppresses hyphal morphogenesis and inhibits biofilm development in pathogenic Candida albicans. J. Nat. Prod. 2012, 75, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Delbem, A.C.; Fernandes, R.A.; Barbosa, D.B.; Monteiro, D.R. Activity of tyrosol against single and mixed-species oral biofilms. J. Appl. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Studies on the metabolic products of Rosellinia necatrix. I. Isolation and characterization of several physiologically active neutral substances. Bull. Agric. Chem. Soc. Jpn. 1960, 24, 372–381. [Google Scholar]
- Stierle, A.C.; Cardellina, J.H., II; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Dow, J.M. Diffusible signals and interspecies communication in bacteria. Microbiology 2008, 154, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Xu, S.X.; Magarvey, N.A.; McCormick, J.K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. USA 2011, 108, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Scopel, M.; Abraham, W.-R.; Henriques, A.T.; Macedo, A.J. Dipeptide cis-cyclo(leucyl-tyrosyl) produced by sponge associated Penicillium sp. F37 inhibits biofilm formation of the pathogenic Staphylococcus epidermidis. Bioorg. Med. Chem. Lett. 2013, 23, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Diblasi, L.; Arrighi, F.; Silva, J.; Bardón, A.; Cartagena, E. Penicillium commune metabolic profile as a promising source of antipathogenic natural products. Nat. Prod. Res. 2015, 29, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Xu, Y.; Xiong, H.-R.; Qian, P.-Y.; Zhang, S. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World J. Microbiol. Biotechnol. 2009, 25, 399–406. [Google Scholar] [CrossRef]
- Bai, Z.Q.; Lin, X.; Wang, Y.; Wang, J.; Zhou, X.; Yang, B.; Liu, J.; Yang, X.; Wang, Y.; Liu, Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 2014, 95, 194–202. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P.; Abraham, W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and unexpected insights into the modulation of LuxR-type quorum sensing by cyclic dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Estrela, A.B.; Heck, M.G.; Abraham, W.-R. Novel approaches to control biofilm infections. Curr. Med. Chem. 2009, 16, 1512–1530. [Google Scholar] [CrossRef] [PubMed]
- Traxler, P.; Fritz, H.; Fuhrer, H.; Richter, W.J. Papulacandins, a new family of antibiotics with antifungal activity. Structures of papulacandins A, B, C and D. J. Antibiot. 1980, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef] [PubMed]
- VanMiddlesworth, F.; Omstead, M.N.; Schmatz, D.; Bartizal, K.; Fromtling, R.; Bills, G.; Nollstadt, K.; Honeycutt, S.; Zweerink, M.; Garrity, G.; et al. L-687,781, a new member of the papulacandin family of beta-1,3-d-glucan synthesis inhibitors. I. Fermentation, isolation, and biological activity. J. Antibiot. 1991, 44, 45–51. [Google Scholar] [PubMed]
- VanMiddlesworth, F.; Dufresne, C.; Smith, J.; Wilson, K.E. Structure elucidation of L-687,781, a new β-1,3-d-glucan synthesis inhibitor. Tetrahedron 1991, 47, 7563–7568. [Google Scholar] [CrossRef]
- Kaneto, R.; Chiba, H.; Agematu, H.; Shibamoto, N.; Yoshioka, T.; Nishida, H.; Okamoto, R. Mer-WF3010, a new member of the papulacandin family. I. Fermentation, isolation and characterization. J. Antibiot. 1993, 46, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Kaneto, R.; Agematu, H.; Shibamoto, N.; Yoshioka, T.; Nishida, H.; Okamoto, R. Mer-WF3010, a new member of the papulacandin family. II. Structure determination. J. Antibiot. 1993, 46, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Iwadate-Kurihara, Y.; Hosoya, T.; Ishikawa, T.; Miyakoshi, S.; Hamano, K.; Inukai, M. F-10748 A1, A2, B1, B2, C1, C2, D1 and D2, novel papulacandins. J. Antibiot. 2002, 55, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Andoh, T.; Ueki, T.; Masuyoshi, S.; Sugawara, K.; Oki, T. BU-4794F, a new beta-1,3-glucan synthase inhibitor. J. Antibiot. 1993, 46, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Tennant, S.; Frost, D.; O’Beirne, M.J.; Karwowski, J.P.; Humphrey, P.E.; Malmberg, L.-H.; Choi, W.; Brandt, K.D.; West, P.; et al. Discovery of saricandin, a novel papulacandin, from a Fusarium species. J. Antibiot. 1996, 49, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yamashita, M.; Tsurumi, Y.; Kohsaka, M. Chaetiacandin, a novel papulacandin. I. Fermentation, isolation and characterization. J. Antibiot. 1985, 38, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Itoh, Y. Chaetiacandin, a novel papulacandin. II. Structure determination. J. Antibiot. 1985, 38, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, G.; Rasmussen, R.R.; Scherr, M.; Frost, D.; Brandt, K.D.; Choi, W.; Jackson, M.; Karwowski, J.P.; Sunga, G.; Malmberg, L.-H.; et al. Corynecandin: A novel antifungal glycolipid from Coryneum modonium. J. Antibiot. 1997, 50, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Frost, D.J.; Karwowski, J.P.; Humphrey, P.E.; Dahod, S.K.; Choi, W.S.; Brandt, K.; Malmberg, L.-H.; Rasmussen, R.R.; Scherr, M.H.; et al. Fusacandins A and B; novel antifungal antibiotics of the papulacandin class from Fusarium sambucinum. I. Identity of the producing organism, fermentation and biological activity. J. Antibiot. 1995, 48, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Hochlowski, J.E.; Whittern, D.N.; Buko, A.; Alder, L.; McAlpine, J.B. Fusacandins A and B; novel antifungal antibiotics of the papulacandin class from Fusarium sambucinum. II. Isolation and structural elucidation. J. Antibiot. 1995, 48, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Van der Kaaden, M.; Breukink, E.; Pieters, R.J. Synthesis and antifungal properties of papulacandin derivatives. Beilstein J. Org. Chem. 2012, 8, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Römmele, G.; Traxler, P.; Wehrli, W. Papulacandins—The relationship between chemical structure and effect on glucan synthesis in yeast. J. Antibiot. 1983, 36, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of echinocandins in fungal biofilm-related disease: Vascular catheter-related infections, immunomodulation, and mucosal surfaces. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S622–S629. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, M.; Mylonakis, E. Characteristics, clinical relevance, and the role of echinocandins in fungal-bacterial interactions. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S630–S634. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, R.; Keller-Schierlein, W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: Isolation and structural components. Helv. Chim. Acta 1974, 57, 2459–2477. [Google Scholar] [PubMed]
- Keller-Juslén, C.; Kuhn, M.; Loosli, H.R.; Petcher, T.J.; Weber, H.P.; von Wartburg, A. Struktur des Cyclopeptid-Antibiotikums sl 7810 (= Echinocandin B). Helv. Chim. Acta 1976, 17, 4147–4150. [Google Scholar]
- Emri, T.; Majoros, L.; Tóth, V.; Pócsi, I. Echinocandins: Production and applications. Appl. Microbiol. Biotechnol. 2013, 97, 3267–3284. [Google Scholar] [CrossRef] [PubMed]
- Traber, R.; Keller-Juslén, C.; Loosli, H.-R.; Kuhn, M.; Von Wartburg, A. Cyclopeptid-Antibiotika aus Aspergillus-Arten. Struktur der Echinocandine C und D. Helv. Chim. Acta 1979, 62, 1252–1267. [Google Scholar] [CrossRef]
- Mizuno, K.; Yagi, A.; Satoi, S.; Takada, M.; Hayashi, M.; Asano, K.; Matsuda, T. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J. Antibiot. 1977, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yagi, A.; Asano, K.; Mizuno, K.; Watanabe, T. Studies of aculeacin. II. Isolation and characterization of aculeacins B, C, D, E, F, and G. J. Antibiot. 1977, 30, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, R.; Kobayashi, M.; Fujine, K.; Sato, I.; Hashimoto, M.; Takase, S.; Tsurumi, Y.; Fujie, A.; Hino, M.; Hashimoto, S.; et al. FR227673 and FR190293, novel antifungal lipopeptides from Chalara sp. No. 22210 and Tolypocladium parasiticum No. 16616. J. Antibiot. 2006, 59, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, R.; Sakamoto, K.; Hashimoto, M.; Takase, S.; Tsurumi, Y.; Fujie, A.; Hino, M.; Hashimoto, S.; Hori, Y. FR209602 and related compounds, novel antifungal lipopeptides from Coleophoma crateriformis no.738. I. Taxonomy, fermentation, isolation and physico-chemical properties. J. Antibiot. 2006, 59, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.F.; Yue, Q.; Chen, L.; Li, Y.; An, Z.; Frisvad, J.C. Aspergillus mulundensis sp. nov., a new species for the fungus producing the antifungal echinocandin lipopeptides, mulundocandins. J. Antibiot. 2016, 69, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hensens, O.D.; Liesch, J.M.; Zink, D.L.; Smith, J.L.; Wichmann, C.F.; Schwartz, R.E. Pneumocandins from Zalerion arboricola. III. Structure elucidation. J. Antibiot. 1992, 45, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Tscherter, H.; Dreyfuss, M.M. Antibiotics from a Cryptosporiopsis Species and Their Therapeutic Use. Sandoz S.A, 1982. Belgian Patent 889,955, 15 February 1982. [Google Scholar]
- Bills, G.; Li, Y.; Chen, L.; Yue, Q.; Niu, X.M.; An, Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat. Prod. Rep. 2014, 31, 1348–1375. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Chen, L.; Zhang, X.; Li, K.; Sun, J.; Liu, X.; An, Z.; Bills, G.F. Evolution of chemical diversity in echinocandin lipopeptide antifungal metabolites. Eukaryot. Cell 2015, 14, 698–718. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Tomishima, M.; Kayakiri, N.; Araki, T.; Barrett, D.; Akamatsu, S.; Matsumoto, S.; Uchida, S.; Nakai, T.; Takeda, S.; et al. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg. Med. Chem. Lett. 2014, 24, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Sakamoto, K.; Oohata, N.; Tsuboi, M.; Yamashita, M.; Hino, M.; Yamada, M.; Hashimoto, S. Screening and characterization of microorganisms with FR901379 acylase activity. J. Antibiot. 2010, 63, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Balkovec, J.M. Section review: Anti-infectives: Lipopeptide antifungal agents. Expert Opin. Investig. Drugs 1994, 3, 65–82. [Google Scholar] [CrossRef]
- Tomishima, M.; Ohki, H.; Yamada, A.; Maki, K.; Ikeda, F. Novel echinocandin antifungals. Part 1: Novel side-chain analogs of the natural product FR901379. Bioorg. Med. Chem. Lett. 2008, 18, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Tomishima, M.; Ohki, H.; Yamada, A.; Maki, K.; Ikeda, F. Novel echinocandin antifungals. Part 2: Optimization of the side chain of the natural product FR901379. Discovery of micafungin. Bioorg. Med. Chem. Lett. 2008, 18, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Hino, M.; Fujie, A.; Iwamoto, T.; Hori, Y.; Hashimoto, M.; Tsurumi, Y.; Sakamoto, K.; Takase, S.; Hashimoto, S. Chemical diversity in lipopeptide antifungal antibiotics. J. Ind. Microbiol. Biotechnol. 2001, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S. Micafungin: A sulfated echinocandin. J. Antibiot. 2009, 62, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Dietz, A. Antifungal activity of anidulafungin, a product of Aspergillus nidulans, against Aspergillus nidulans. Int. J. Antimicrob. Agents 2009, 33, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D. From natural products to clinically useful antifungals. Biochim. Biophys. Acta 2002, 1587, 224–233. [Google Scholar] [CrossRef]
- Turner, M.S.; Drew, R.H.; Perfect, J.R. Emerging echinocandins for treatment of invasive fungal infections. Expert Opin. Emerg. Drugs 2006, 11, 231–250. [Google Scholar] [CrossRef]
- Balkovec, J.M.; Hughes, D.L.; Masurekar, P.S.; Sable, C.A.; Schwartz, R.E.; Singh, S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Eschenauer, G.; Depestel, D.D.; Carver, P.L. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 2007, 3, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Kim, H.G.; Long, L. Efficacy of aminocandin in the treatment of immunocompetent mice with haematogenously disseminated fluconazole-resistant candidiasis. J. Antimicrob. Chemother. 2007, 59, 556–559. [Google Scholar] [PubMed]
- Warn, P.A.; Sharp, A.; Morrissey, G.; Denning, D.W. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int. J. Antimicrob. Agents 2009, 35, 146–151. [Google Scholar] [PubMed]
- Guembe, M.; Guinea, J.; Marcos-Zambrano, L.J.; Fernández-Cruz, A.; Peláez, T.; Muñoz, P.; Bouza, E. Micafungin at physiological serum concentrations shows antifungal activity against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother. 2014, 58, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; González del Vecchio, M.; Bouza, E.; Guinea, J. Micafungin is more active against Candida albicans biofilms with high metabolic activity. J. Antimicrob. Chemother. 2014, 69, 2984–2987. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Comparison of the antifungal activity of micafungin and amphotericin B against Candida tropicalis biofilms. J. Antimicrob. Chemother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Simitsopoulou, M.; Kyrpitzi, D.; Velegraki, A.; Walsh, T.J.; Roilides, E. Caspofungin at catheter lock concentrations eradicates mature biofilms of Candida lusitaniae and Candida guilliermondii. Antimicrob. Agents Chemother. 2014, 58, 4953–4956. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, E.M.; Oliva, A.; Furustrand Tafin, U.; Perrotet, N.; Borens, O.; Trampuz, A. Antifungal activity against planktonic and biofilm Candida albicans in an experimental model of foreign-body infection. J. Infect. 2016, 72, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Carone, A.; Caggiano, G.; Franchini, C.; Corbo, F.; Montagna, M.T. In vitro interactions between anidulafungin and nonsteroidal anti-inflammatory drugs on biofilms of Candida spp. Bioorg. Med. Chem. 2016, 24, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Bozó, A.; Gesztelyi, R.; Domán, M.; Kardos, G.; Nagy, F.; Tóth, Z.; Majoros, L. Effect of caspofungin and micafungin in combination with farnesol against Candida parapsilosis biofilms. Int. J. Antimicrob. Agents 2016, 47, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Lamoth, F.; Juvvadi, P.R. Potential microbiological effects of higher dosing of echinocandins. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S669–S677. [Google Scholar] [CrossRef] [PubMed]
- Walraven, C.J.; Bernardo, S.M.; Wiederhold, N.P.; Lee, S.A. Paradoxical antifungal activity and structural observations in biofilms formed by echinocandin-resistant Candida albicans clinical isolates. Med. Mycol. 2014, 52, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jimenez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Ohno, H.; Imamura, Y.; Kohno, S.; Miyazaki, Y. The effects of an Hsp90 inhibitor on the paradoxical effect. Jpn. J. Infect. Dis. 2009, 62, 392–393. [Google Scholar] [PubMed]
- Nett, J.E.; Andes, D. Fungal biofilms: In vivo models for discovery of anti-biofilm drugs. Microbiol. Spectr. 2015, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Azie, N.; Andes, D.R. Development of new strategies for echinocandins: Progress in translational research. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S601–S603. [Google Scholar] [CrossRef] [PubMed]
- Fiori, B.; Posteraro, B.; Torelli, R.; Tumbarello, M.; Perlin, D.S.; Fadda, G.; Sanguinetti, M. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob. Agents Chemother. 2011, 55, 3031–3035. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Williams, C.; Lappin, D.F.; Millington, O.; Martins, M.; Ramage, G. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell 2013, 12, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.A.; Teixeira, C.E.C.; Brilhante, R.S.N.; Castelo-Branco, D.S.C.M.; Paiva, M.A.N.; Giffoni Leite, J.J.; Lima, D.T.; Monteiro, A.J.; Sidrim, J.J.C.; Rocha, M.F.G. Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med. Mycol. 2013, 51, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.G.; Carr, J.H.; Starling, C.E.F.; De Resende, M.A.; Donlan, R.M. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob. Agents Chemother. 2009, 53, 4377–4384. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.J.; Piper, K.E.; Nguyen, G.; Steckelberg, J.M.; Patel, R. In vitro activity of anidulafungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 2242–2243. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.J.; Steckelberg, K.E.; Piper, K.E.; Steckelberg, J.M.; Patel, R. In vitro activity of micafungin against planktonic and sessile Candida albicans isolates. Antimicrob. Agents Chemother. 2009, 53, 2638–2639. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Miyagawa, S.; Takeda, O.; Hakariya, M.; Matsumoto, S.; Ohno, H.; Miyazaki, Y. Real-time microscopic observation of Candida biofilm development and effects due to micafungin and fluconazole. Antimicrob. Agents Chemother. 2013, 57, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Susceptibility of Candida albicans biofilms to caspofungin and anidulafungin is not affected by metabolic activity or biomass production. Med. Mycol. 2016, 54, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.S.; Colombo, A.L.; Arthington-Skaggs, B.A. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 2007, 51, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Sherry, L.; Nile, C.J.; Sherriff, A.; Johnson, E.M.; Hanson, M.F.; Williams, C.; Munro, C.A.; Jones, B.J.; Ramage, G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012–2013. Clin. Microbiol. Infect. 2016, 22, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Kontoyiannis, D.P. Micafungin triggers caspase-dependent apoptosis in Candida albicans and Candida parapsilosis biofilms, including caspofungin non-susceptible isolates. Virulence 2015, 6, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Shuford, J.A.; Piper, K.E.; Steckelberg, J.M.; Patel, R. In vitro biofilm characterization and activity of antifungal agents alone and in combination against sessile and planktonic clinical Candida albicans isolates. Diagn. Microbiol. Infect. Dis. 2007, 57, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Simitsopoulou, M.; Peshkova, P.; Tasina, E.; Katragkou, A.; Kyrpitzi, D.; Velegraki, A.; Walsh, T.J.; Roilides, E. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: Characterization of less common bloodstream isolates. Antimicrob. Agents Chemother. 2013, 57, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Spedicato, I.; Picciani, C.; D’Antonio, D.; Piccolomini, R. In vitro pharmacodynamic characteristics of amphotericin B, caspofungin, fluconazole, and voriconazole against bloodstream isolates of infrequent Candida species from patients with hematologic malignancies. Antimicrob. Agents Chemother. 2004, 48, 4453–4456. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Sornakumari, H.; Lency, A.; Kavitha, S.; Natarajaseenivasan, K. Synergistic effect of amphotericin B and tyrosol on biofilm formed by Candida krusei and Candida tropicalis from intrauterine device users. Med. Mycol. 2014, 52, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Valentin, A.; Canton, E.; Peman, J.; Quindos, G. In vitro activity of amphotericin B and anidulafungin against Candida spp. biofilms. Rev. Iberoam. Micol. 2007, 24, 272–277. [Google Scholar] [PubMed]
- Liao, Y.; Yang, S.; Cong, L.; Lu, X.; Ao, J.; Yang, R. In vitro activities of antifungal combinations against biofilms and planktonic forms of clinical Trichosporon asahii isolates. Antimicrob. Agents Chemother. 2014, 58, 7615–7616. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, W.; Sabra, A.; Zahreddine, L.; Khairallah, M.T.; Baroud, M.; Hadi, U.; Matar, G.M. The inhibitory effect of micafungin on biofilm formation by Pseudomonas aeruginosa. Biofouling 2013, 29, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Roilides, E.; Katragkou, A.; Petraitis, V.; Walsh, T.J. The role of echinocandins in Candida biofilm-related vascular catheter infections: In vitro and in vivo model systems. Clin. Infect. Dis. 2015, 61 (Suppl. S6), S618–S621. [Google Scholar] [CrossRef] [PubMed]
- Shuford, J.A.; Rouse, M.S.; Piper, K.E.; Steckelberg, J.M.; Patel, R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J. Infect. Dis. 2006, 194, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Bink, A.; Kucharíková, S.; Neirinck, B.; Vleugels, J.; Van Dijck, P.; Cammue, B.P.A.; Thevissen, K. The nonsteroidal antiinflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J. Infect. Dis. 2012, 206, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Kucharikova, S.; Tournu, H.; Holtappels, M.; Van Dijck, P.; Lagrou, K. In vivo efficacy of anidulafungin against mature Candida albicans biofilms in a novel rat model of catheter-associated candidiasis. Antimicrob. Agents Chemother. 2010, 54, 4474–4475. [Google Scholar] [CrossRef] [PubMed]
- Kucharikova, S.; Sharma, N.; Spriet, I.; Maertens, J.; Van Dijck, P.; Lagrou, K. Activities of systemically administered echinocandins against in vivo mature Candida albicans biofilms developed in a rat subcutaneous model. Antimicrob. Agents Chemother. 2013, 57, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Kucharikova, S.; Neirinck, B.; Sharma, N.; Vleugels, J.; Lagrou, K.; Van Dijck, P. In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J. Antimicrob. Chemother. 2015, 70, 846856. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-G.; Pandit, S.; Xiao, J.; Gregoire, S.; Falsetta, M.L.; Klein, M.I.; Koo, H. Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. Int. J. Oral Sci. 2011, 3, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Ditu, L.M.; Oprea, E.; Litescu, S.; Bucur, M.; Marutescu, L.; Enache, G.; Saviuc, C.; Burlibasa, M.; Traistaru, T.; et al. In vitro study of the inhibitory activity of usnic acid on dental plaque biofilm. Roum. Arch. Microbiol. Immunol. 2009, 68, 215–222. [Google Scholar] [PubMed]
- Cirasola, D.; Sciota, R.; Vizzini, L.; Ricucci, V.; Morace, G.; Borghi, E. Experimental biofilm-related Candida infections. Future Microbiol. 2013, 8, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Cateau, E.; Rodier, M.H.; Imbert, C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J. Antimicrob. Chemother. 2008, 62, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Samples, M.L.; Mercier, R.C.; Spilde, M.N. Activities and ultrastructural effects of antifungal combinations against simulated Candida endocardial vegetations. Antimicrob. Agents Chemother. 2008, 52, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P. Antifungal combinations against simulated Candida albicans endocardial vegetations. Antimicrob. Agents Chemother. 2009, 53, 2629–2631. [Google Scholar] [CrossRef] [PubMed]
- Serefko, A.; Chudzik, B.; Malm, A. In vitro activity of caspofungin against planktonic and sessile Candida sp. cells. Pol. J. Microbiol. 2006, 55, 133–137. [Google Scholar] [PubMed]
- Lazzell, A.L.; Chaturvedi, A.K.; Pierce, C.G.; Prasad, D.; Uppuluri, P.; Lopez-Ribot, J.L. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J. Antimicrob. Chemother. 2009, 64, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Liang, R.; Hicks, J.M.; Barrish, J.; Versalovic, J. Farnesol decreases biofilms of Staphylococcus epidermidis and exhibits synergy with nafcillin and vancomycin. Pediatr. Res. 2011, 70, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Chen, X.; Lim, J.; Kim, S.H.; Kim, S.T.; Cho, Y.H.; Yoon, J.; Park, S. In vivo fluorescence imaging of bacteriogenic cyanide in the lungs of live mice infected with cystic fibrosis pathogens. PLoS ONE 2011, 6, e21387. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Dinakaran, H.; Thomas, D.P.; Chaturvedi, A.K.; Lopez-Ribot, J.L. Characteristics of Candida albicans biofilms grown in a synthetic urine medium. J. Clin. Microbiol. 2009, 47, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, K.; Staes, I.; Delattin, N.; Cammue, B.P.A.; Thevissen, K.; De Brucker, K. Combinatorial drug approaches to tackle Candida albicans biofilms. Expert Rev. Anti-Infect. Ther. 2015, 13, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Griesser, S.S.; Jasieniak, M.; Coad, B.R.; Griesser, H.J. Antifungal coatings by caspofungin immobilization onto biomaterials surfaces via a plasma polymer interlayer. Biointerphases 2015, 10, 04A307. [Google Scholar] [CrossRef] [PubMed]
- Kucharikova, S.; Gerits, E.; De Brucker, K.; Braem, A.; Ceh, K.; Majdic, G.; Spanic, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016, 71, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Rawson, M.; Haggard, W.; Jennings, J.A. Osteocompatibility of biofilm inhibitors. Open Orthop. J. 2014, 8, 442–449. [Google Scholar] [PubMed]
- Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Trusca, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M.; et al. Usnic acid-loaded biocompatible magnetic PLGA-PVA microsphere thin films fabricated by MAPLE with increased resistance to Staphylococcal colonization. Biofabrication 2014, 6, 35002. [Google Scholar] [CrossRef] [PubMed]
- Guzun, A.S.; Stroescu, M.; Jinga, S.I.; Voicu, G.; Grumezescu, A.M.; Holban, A.M. Plackett-Burman experimental design for bacterial cellulose-silica composites synthesis. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 42, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of cefotaxime impregnated chitosan as nano-antibiotics: De novo strategy to combat biofilm forming multi-drug resistant pathogens. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-Activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Bakry, A.; D’Ilario, L.; Francolini, I.; Piozzi, A.; Taresco, V. Release behavior and antibiofilm activity of usnic acid-loaded carboxylated poly(l-lactide) microparticles. Eur. J. Pharm. Biopharm. 2014, 88, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Francolini, I.; Padella, F.; Bellusci, M.; Boni, A.; Innocenti, C.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Design and characterization of antimicrobial usnic acid loaded-core/shell magnetic nanoparticles. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 52, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The fungi: 1, 2, 3 ... 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Wisecaver, J.H.; Slot, J.C.; Rokas, A. The evolution of fungal metabolic pathways. PLoS Genet. 2014, 10, e1004816. [Google Scholar] [CrossRef] [PubMed]
- Borghi, E.; Morace, G.; Borgo, F.; Rajendran, R.; Sherry, L.; Nile, C.; Ramage, G. New strategic insights into managing fungal biofilms. Front. Microbiol. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]









| Experimental Setup for Anti-Biofilm Tests | Target Organisms | Fungal Compounds and Combinations Tested | References |
|---|---|---|---|
| in vitro biofilm formation by clinical isolates | Aspergillus spp. | anid, casp, casp + DNase | [150,151] |
| Candida albicans | anid, casp, mica, farnesol, cas + voriconazole, cas + amb, shearinine, shearinine + amb | [137,150,152,153,154,155,156,157,158,159,160,161,162,163] | |
| Candida spp. (non-albicans) | anid, casp, mica, farnesol, casp + farnesol, mica + farnesol, tyrosol, tyrosol + amb, shearinine, shearinine + amb | [76,138,142,150,152,153,156,157,159,161,163,164,165,166] | |
| Trichosporon asahii | casp, casp + voriconazole | [167] | |
| Pseudomonas aeruginosa | mica | [168] | |
| Group A Streptococcus | usnic acid | [61] | |
| in vivo model of catheter biofilm in rabbit | C. albicans | casp, mica | [169,170] |
| in vivo model of subcutaneous device infection in rat | C. albicans | anid, casp, mica, casp + diclofenac | [171,172,173] |
| C. glabrata | anid, casp, mica | [174] | |
| mixed species oral biofilm, in vitro QS (quorum-sensing) interference in biofilm from dental plaque isolates | C. albicans, C. glabrata, S. mutans | tt-farnesol, tyrosol | [79,175] |
| Gram-positive bacteria | usnic acid | [176] | |
| in vivo model of biofilm infection by clinical isolates in Galleria mellonella larvae | C. albicans | anid | [177] |
| in vitro biofilms in catheters and biomaterials, simulated endocardial vegetation (SEV) | Candida spp. | casp, mica | [178,179,180,181] |
| Staphylococcus aureus, P. aeruginosa | usnic acid | [63] | |
| in vivo murine model of central venous catheter or subcutaneous catheter infection | C. albicans | casp | [182] |
| S. epidermidis | tt-farnesol | [183] | |
| in vivo model of foreign-body infection in guinea pig | C. albicans | casp, anid | [140] |
| in vivo murine model of cystic fibrosis | Burkholderia cepacia, P. aeruginosa | patulin | [184] |
| synthetic urine medium | C. albicans | casp | [185] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrela, A.B.; Abraham, W.-R. Fungal Metabolites for the Control of Biofilm Infections. Agriculture 2016, 6, 37. https://doi.org/10.3390/agriculture6030037
Estrela AB, Abraham W-R. Fungal Metabolites for the Control of Biofilm Infections. Agriculture. 2016; 6(3):37. https://doi.org/10.3390/agriculture6030037
Chicago/Turabian StyleEstrela, Andréia Bergamo, and Wolf-Rainer Abraham. 2016. "Fungal Metabolites for the Control of Biofilm Infections" Agriculture 6, no. 3: 37. https://doi.org/10.3390/agriculture6030037
APA StyleEstrela, A. B., & Abraham, W.-R. (2016). Fungal Metabolites for the Control of Biofilm Infections. Agriculture, 6(3), 37. https://doi.org/10.3390/agriculture6030037