Mitigating Global Warming Potential and Greenhouse Gas Intensities by Applying Composted Manure in Cornfield: A 3-Year Field Study in an Andosol Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Set Up
2.2.1. Experiment 1: Effect of Manure and Inorganic Fertilizer
2.2.2. Experiment 2: Effect of Additional Spring Manure Application
2.3. Measurement of Soil and Climatic Variables
Total Soil N and Soil C Analysis
2.4. Greenhouse Gas Flux Measurement
2.5. Estimation of NECB, GWP and GHGI
2.6. Data Analysis
3. Results
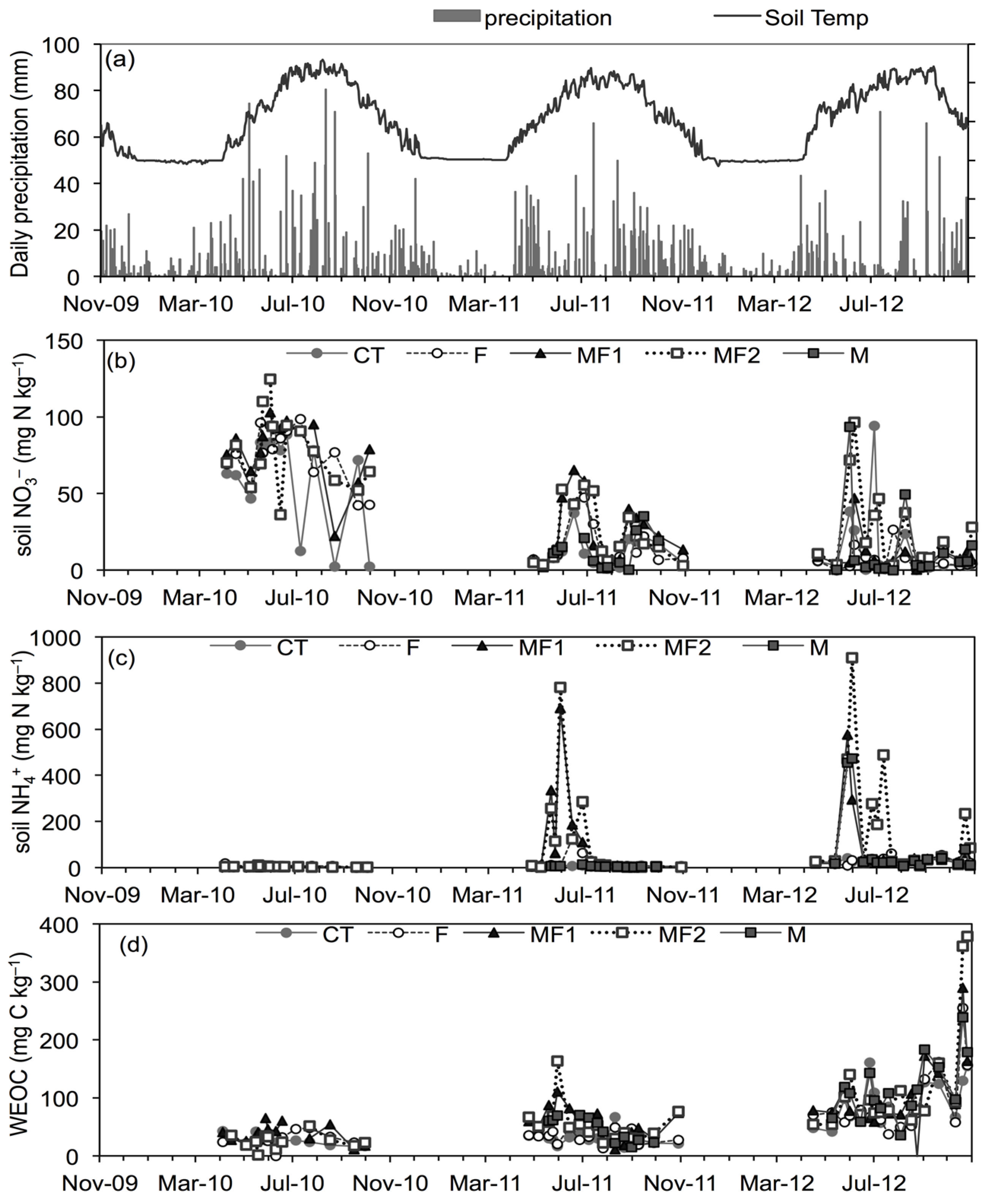
3.1. Soil and Environmental Variables
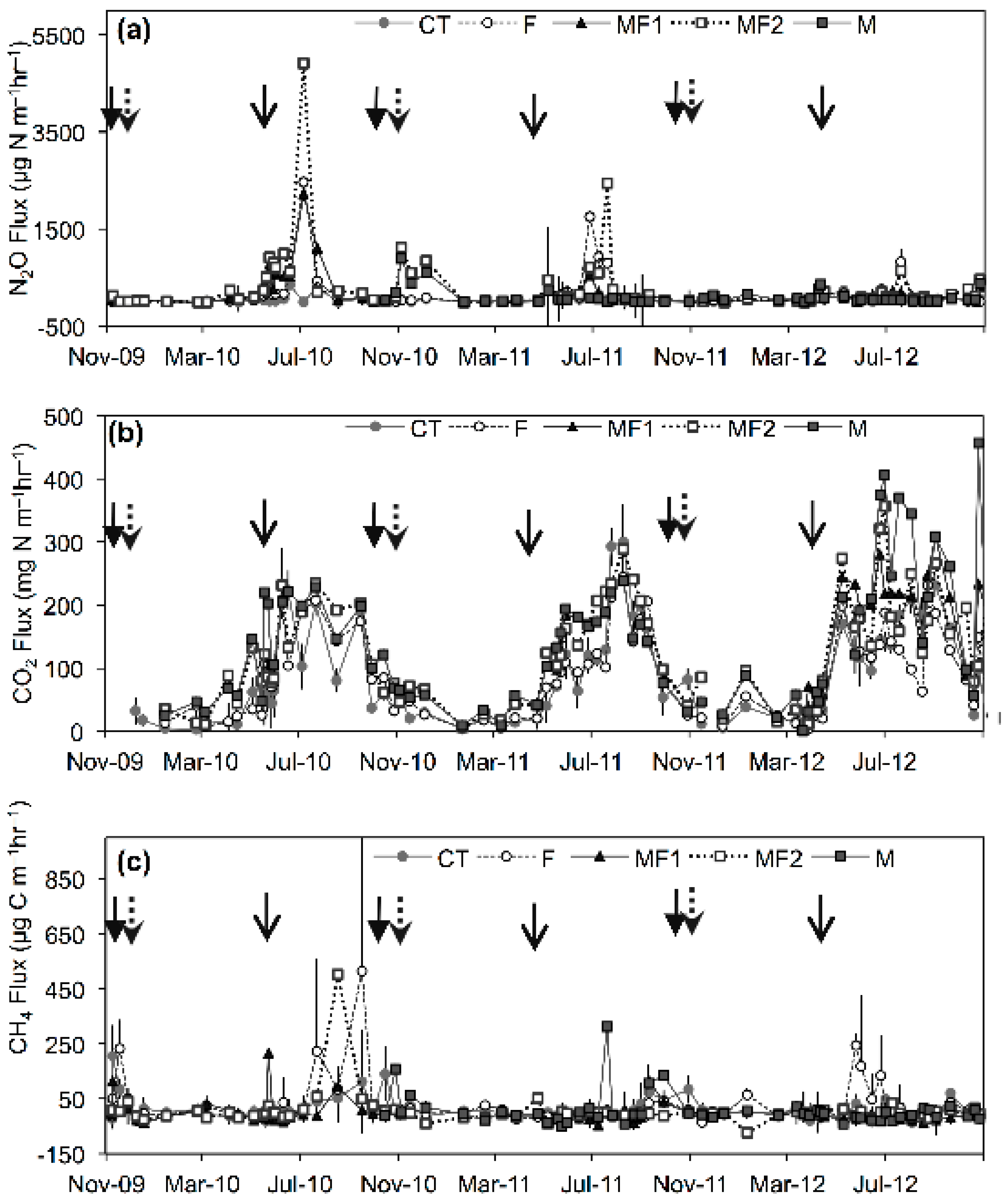
3.2. Greenhouse Gas Emissions-Experiment 1
3.2.1. Nitrous Oxide Emissions
3.2.2. Carbon Dioxide Emissions
3.2.3. Methane Emissions
3.3. Effect of Additional Spring Manure Application on Greenhouse Gas Emissions-Experiment 2
3.4. Factors Controlling GHG Emissions
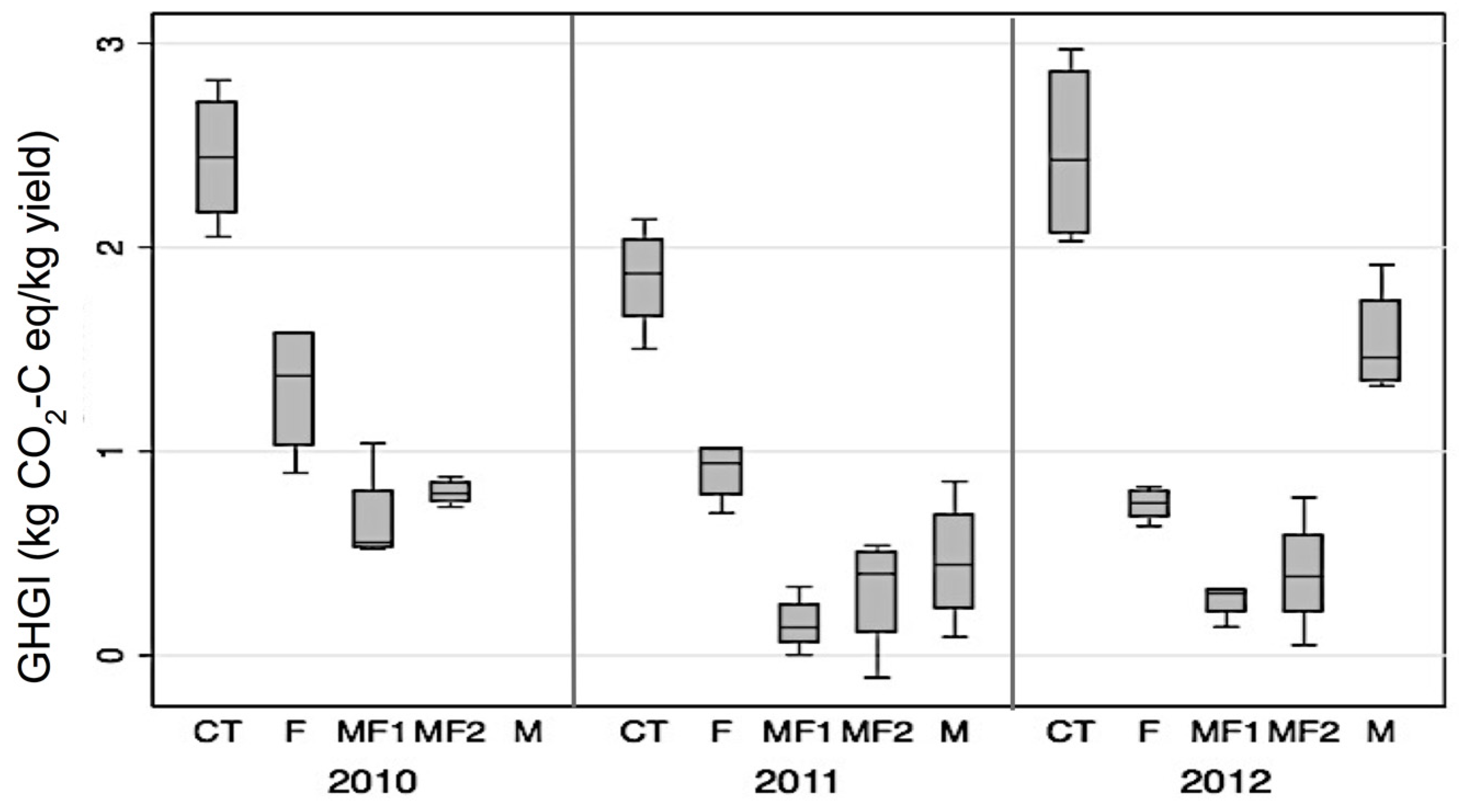
3.5. Effect of Fertilizer and Manure on NPP, NECB, GWP and GHGI
4. Discussion
4.1. Influence of Inorganic Fertilizer and Manure Application on GHG Emissions
4.2. Soil and Environmental Factors Controlling GHG Emissions
4.3. Effect of Fertilizer and Manure on NECB and GWP
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Innternational Panel Climate Change. Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; Mccarl, B.; Ogle, S.; Mara, F.O.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 811–922. [Google Scholar]
- Alluvione, F.; Bertora, C.; Zavattaro, L.; Grignani, C. Nitrous Oxide and Carbon Dioxide Emissions Following Green Manure and Compost Fertilization in Corn. Soil Sci. Soc. Am. J. 2010, 74, 384. [Google Scholar] [CrossRef]
- Tate, K.R. Soil methane oxidation and land-use change—From process to mitigation. Soil Biol. Biochem. 2015, 80, 260–272. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Vieira, F.C.B.; Zanatta, J.A.; De Cássia Piccolo, M.; Dieckow, J. Methane emission from soil under long-term no-till cropping systems. Soil Tillage Res. 2012, 124, 1–7. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Skiba, U.; Sheppard, L.J.; Ball, B.; Roberts, J.D.; Smith, K.A.; Fowler, D. The effect of nitrogen deposition and seasonal variability on methane oxidation and nitrous oxide emission rates in an upland spruce plantation and moorland. Atmos. Environ. 1997, 31, 3693–3706. [Google Scholar] [CrossRef]
- Saggar, S. Estimation of nitrous oxide emission from ecosystems and its mitigation technologies. Agric. Ecosyst. Environ. 2010, 136, 189–191. [Google Scholar] [CrossRef]
- Dobbie, K.; Smith, K.; Management, R.; Building, D.; Road, M. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Mazzetto, A.M.; Barneze, A.S.; Feigl, B.J.; van Groenigen, J.W.; Oenema, O.; Cerri, C.C. Temperature and moisture affect methane and nitrous oxide emission from bovine manure patches in tropical conditions. Soil Biol. Biochem. 2014, 76, 242–248. [Google Scholar] [CrossRef]
- Allen, D.E.; Kingston, G.; Rennenberg, H.; Dalal, R.C.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Li, L.; Han, X.; You, M.; Horwath, W.R. Nitrous oxide emissions from Mollisols as affected by long-term applications of organic amendments and chemical fertilizers. Sci. Total Environ. 2013, 452–453, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Nepstad, D.C.; Ishida, F.Y.; Brando, P.M. Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2008, 14, 2582–2590. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Volume 9. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO); International Fertilizer Industry Association (IFA). IFA Global Estimates of Gaseous Emissions of NH3, NO and N2O from Agricultural Land; FAO: Rome, Italy, 2001. [Google Scholar]
- Zhang, W.; Yu, Y.; Li, T.; Sun, W.; Huang, Y. Net greenhouse gas balance in China’s croplands over the last three decades and its mitigation potential. Environ. Sci. Technol. 2014, 48, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Kim, P.J.; Inubushi, K. Mitigating yield-scaled greenhouse gas emissions through combined application of soil amendments: A comparative study between temperate and subtropical rice paddy soils. Sci. Total Environ. 2015, 529, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pathak, H.; Byjesh, K.; Chakrabarti, B.; Aggarwal, P.K. Potential and cost of carbon sequestration in Indian agriculture: Estimates from long-term field experiments. Filed Crops Res. 2011, 120, 102–111. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Accordi, J.; Costa, F.; Vieira, B.; Dieckow, J. Mitigating greenhouse gas emissions from a subtropical Ultisol by using long-term no-tillage in combination with legume cover crops. Soil Tillage Res. 2016, 161, 86–94. [Google Scholar] [CrossRef]
- Barton, L.; Hoyle, F.C.; Stefanova, K.T.; Murphy, D.V. Incorporating organic matter alters soil greenhouse gas emissions and increases grain yield in a semi-arid climate. Agric. Ecosyst. Environ. 2016, 231, 320–330. [Google Scholar] [CrossRef]
- Sommer, R.; Mukalama, J.; Kihara, J.; Koala, S.; Winowiecki, L.; Bossio, D. Nitrogen dynamics and nitrous oxide emissions in a long-term trial on integrated soil fertility management in Western Kenya. Nutr. Cycl. Agroecosyst. 2016, 105, 229–248. [Google Scholar] [CrossRef]
- Huth, N.I.; Thorburn, P.J.; Radford, B.J.; Thornton, C.M. Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: A simulation study. Agric. Ecosyst. Environ. 2010, 136, 351–357. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Bertrand, N.; Côté, D. Carbon Dioxide and Nitrous Oxide Emissions following Fall and Spring Applications of Pig Slurry to an Agricultural Soil. Soil Sci. Soc. Am. J. 2004, 68, 1410. [Google Scholar] [CrossRef]
- Smith, K.A.; McTaggart, I.P.; Tsuruta, H. Emissions of N2O and NO associated with nitrogen fertilization in intensive agriculture, and the potential for mitigation. Soil Use Manag. 1997, 13, 296–304. [Google Scholar] [CrossRef]
- Cui, F.; Yan, G.; Zhou, Z.; Zheng, X.; Deng, J. Annual emissions of nitrous oxide and nitric oxide from a wheat–maize cropping system on a silt loam calcareous soil in the North China Plain. Soil Biol. Biochem. 2012, 48, 10–19. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Lal, R.; James, R. Crop management effects on soil carbon sequestration on selected farmers’ fields in northeastern Ohio. Soil Tillage Res. 2005, 81, 265–276. [Google Scholar] [CrossRef]
- Ryals, R.; Kaiser, M.; Torn, M.S.; Berhe, A.A.; Silver, W.L. Impacts of organic matter amendments on carbon and nitrogen dynamics in grassland soils. Soil Biol. Biochem. 2014, 68, 52–61. [Google Scholar] [CrossRef]
- Lentz, R.D.; Lehrsch, G.A. Manure and Fertilizer Effects on Carbon Balance and Organic and Inorganic Carbon Losses for an Irrigated Corn Field. Soil Sci. Soc. Am. J. 2014, 78, 987. [Google Scholar] [CrossRef]
- Rees, R.M.; Bingham, I.J.; Baddeley, J.A.; Watson, C.A. The role of plants and land management in sequestering soil carbon in temperate arable and grassland ecosystems. Geoderma 2005, 128, 130–154. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Christie, P.; Ju, X. Net global warming potential and greenhouse gas intensity in a double-cropping cereal rotation as affected by nitrogen and straw management. Biogeosciences 2013, 10, 7897–7911. [Google Scholar] [CrossRef]
- Limin, A.; Shimizu, M.; Mano, M.; Ono, K.; Miyata, A.; Wada, H.; Nozaki, H.; Hatano, R. Manure application has an effect on the carbon budget of a managed grassland in southern Hokkaido, Japan. Soil Sci. Plant Nutr. 2015, 61, 857–872. [Google Scholar] [CrossRef]
- Clayton, H.; McTaggart, I.P.; Parker, J.; Swan, L.; Smith, K.A. Nitrous oxide emissions from fertilised grassland: A 2-year study of the effects of N fertiliser form and environmental conditions. Biol. Fertil. Soils 1997, 25, 252–260. [Google Scholar] [CrossRef]
- Shimizu, M.; Hatano, R.; Arita, T. The effect of fertilizer and manure application on CH4 and N2O emissions from managed grasslands in Japan. Soil Sci. Plant Nutr. 2013, 59, 69–86. [Google Scholar] [CrossRef]
- Acton, S.D.; Baggs, E.M. Interactions between N application rate, CH4 oxidation and N2O production in soil. Biogeochemistry 2011, 103, 15–26. [Google Scholar] [CrossRef]
- Robertson, P.G.; Grace, P.R. Greenhouse gas gluxes in tropical and temperate agriculture: The need for a full-cost accounting of global warming potentials. Environ. Dev. Sustain. 2004, 6, 51–63. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, B.; Liu, C.; Zhou, Z.; Yao, Z.; Wang, Y.; Wang, Y.; Yang, L.; Zhu, J.; Huang, Y.; et al. Quantifying net ecosystem carbon dioxide exchange of a short-plant cropland with intermittent chamber measurements. Glob. Biogeochem. Cycles 2008, 22, 1–13. [Google Scholar] [CrossRef]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Rastetter, E.B.; Lovett, G.M.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Wang, J.; Xu, X.; Huang, Q.; Shen, Q. Mitigating net global warming potential and greenhouse gas intensities by substituting chemical nitrogen fertilizers with organic fertilization strategies in rice–wheat annual rotation systems in China: A 3-year fi eld experiment. Ecol. Eng. 2015, 81, 289–297. [Google Scholar] [CrossRef]
- Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Liu, X.J. Net Global Warming Potential and Greenhouse Gas Intensity in Irrigated Cropping Systems in Northeastern Colorado. J. Environ. Qual. 2006, 35, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Ding, W.; Zaman, M.; Cai, Z.; Wang, Y.; Zhang, X.; Zhou, B. Nitrous oxide emissions from a rainfed-cultivated black soil in Northeast China: Effect of fertilization and maize crop. Biol. Fertil. Soils 2012, 48, 973–979. [Google Scholar] [CrossRef]
- Roth, B.; Jones, M.; Burke, J.; Williams, M. The Effects of Land-Use Change from Grassland to Miscanthus x giganteus on Soil N2O Emissions. Land 2013, 2, 437–451. [Google Scholar] [CrossRef]
- Shimizu, M.; Marutani, S.; Desyatkin, A.R.; Jin, T.; Hata, H.; Hatano, R. The effect of manure application on carbon dynamics and budgets in a managed grassland of Southern Hokkaido, Japan. Agric. Ecosyst. Environ. 2009, 130, 31–40. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Adhya, T.K.; Rao, K.S.; Manna, M.C. Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Tillage Res. 2012, 124, 119–130. [Google Scholar] [CrossRef]
- Toma, Y.; Hatano, R. Effect of crop residue C:N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan: Original article. Soil Sci. Plant Nutr. 2007, 53, 198–205. [Google Scholar] [CrossRef]
- Shimizu, M.; Limin, A.; Desyatkin, A.R.; Jin, T.; Mano, M.; Ono, K.; Miyata, A.; Hata, H.; Hatano, R. Effect of manure application on seasonal carbon fluxes in a temperate managed grassland in Southern Hokkaido, Japan. Catena 2015, 133, 474–485. [Google Scholar] [CrossRef]
- Katayanagi, N.; Ryusuke, H. N2O emissions during the freezing and thawing periods from six fields in a livestock farm, southern Hokkaido, Japan. Soil Sci. Plant Nutr. 2012, 58, 261–271. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Lal, R.; Jarecki, M.K. Nitrous oxide and methane emissions from long-term tillage under a continuous corn cropping system in Ohio. Soil Tillage Res. 2009, 104, 247–255. [Google Scholar] [CrossRef]
- Cui, F.; Zheng, X.; Liu, C.; Wang, K.; Zhou, Z.; Deng, J. Assessing biogeochemical effects and best management practice for a wheat–maize cropping system using the DNDC model. Biogeosciences 2014, 11, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Collins, H.P.; Alva, A.K.; Streubel, J.D.; Fransen, S.F.; Frear, C.; Chen, S.; Kruger, C.; Granatstein, D. Greenhouse Gas Emissions from an Irrigated Silt Loam Soil Amended with Anaerobically Digested Dairy Manure. Soil Sci. Soc. Am. J. 2011, 75, 2206. [Google Scholar] [CrossRef]
- Sistani, K.R.; Jn-Baptiste, M.; Lovanh, N.; Cook, K.L. Atmospheric emissions of nitrous oxide, methane, and carbon dioxide from different nitrogen fertilizers. J. Environ. Qual. 2011, 40, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O: N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shimizu, M.; Hatano, R. Evaluation of N2O and CO2 hot moments in managed grassland and cornfield, southern Hokkaido, Japan. Catena 2015, 133, 1–13. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain. Sci. Total Environ. 2007, 373, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, S.; Bertolini, T.; Vitale, L.; Tommasi, P.; Ottaiano, L.; Oliva, M.; Amato, U.; Fierro, A.; Magliulo, V. The influence of management and environmental variables on soil N2O emissions in a crop system in Southern Italy. Plant Soil 2010, 343, 83–96. [Google Scholar] [CrossRef]
- Cai, Y.; Ding, W.; Luo, J. Nitrous oxide emissions from Chinese maize–wheat rotation systems: A 3-year field measurement. Atmos. Environ. 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; McGeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563–564, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flessa, H. Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J. Geophys. Res. 1995, 100, 115–124. [Google Scholar] [CrossRef]
- MacKenzie, A.F.; Fan, M.X.; Cadrin, F. Nitrous oxide emission as affected by tillage, corn–soybean–alfalfa rotations and nitrogen fertilization. Can. J. Soil Sci. 1997, 77, 145–152. [Google Scholar] [CrossRef]
- Collins, H.P.; Streubel, J.D.; Frear, C.; Chen, S.; Granatstein, D.; Kruger, C.; Alva, A.K.; Fransen, S.F. Application of AD Dairy Manure Effluent to Fields and Associated Impacts; Center for Sustaining Agriculture and Natural Resources: Leavenworth, WA, USA, 2010; pp. 1–25. [Google Scholar]
- Akiyama, H.; McTaggart, I.P.; Ball, B.C.; Scott, A. N2O, NO, and NH3 emissions from soil after the application of organic fertilizers, urea and water. Water Air Soil Pollut. 2004, 156, 113–129. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle Manure Amendments Can Increase the pH of Acid Soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Nagano, H.; Kato, S.; Ohkubo, S.; Inubushi, K. Emmisions of carbon dioxide, methane, and nitrous oxide from short and long-term organic farming Andosols in central Japan. Jpn. Soc. Soil Sci. Plant Nutr. J. 2012, 58, 793–801. [Google Scholar] [CrossRef]
- Owen, J.J.; Parton, W.J.; Silver, W.L. Long-term impacts of manure amendments on carbon and greenhouse gas dynamics of rangelands. Glob. Chang. Biol. 2015, 21, 4533–4547. [Google Scholar] [CrossRef] [PubMed]
- Ussiri, D.A.N.; Lal, R. Long-term tillage effects on soil carbon storage and carbon dioxide emissions in continuous corn cropping system from an alfisol in Ohio. Soil Tillage Res. 2009, 104, 39–47. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Li, S.; Xie, H. Impact of carbon source on nitrous oxide emission from anoxic/oxic biological nitrogen removal process and identification of its emission sources. Environ. Sci. Pollut. Res. 2013, 20, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.L.; Chiang, S.C.; Merka, W.C.; Pancorbo, O.C.; Thompson, S.A. Nitrous oxide and carbon dioxide emissions from pelletized and nonpelletized poultry litter incorporated into soil. Plant Soil 1994, 163, 189–195. [Google Scholar] [CrossRef]
- Ryals, R.; Silver, W.L. Effects of organic matter amendments on net primary productivity and greenhouse gas emissions in annual grasslands. Ecol. Appl. 2012, 23, 46–59. [Google Scholar] [CrossRef]
- Choudhary, M.A.; Akramkhanov, A.; Saggar, S. Nitrous oxide emissions in soils cropped with maize under long-term tillage and under permanent pasture in New Zealand. Soil Tillage Res. 2001, 62, 61–71. [Google Scholar] [CrossRef]
- Mosier, A.; Schime, D.; Valentine, D.; Bronson, K.; Parton, W. Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature 1991, 350, 56–58. [Google Scholar] [CrossRef]
- Sehy, U.; Ruser, R.; Munch, J.C. Nitrous oxide fluxes from maize fields: Relationship to yield, site-specific fertilization, and soil conditions. Agric. Ecosyst. Environ. 2003, 99, 97–111. [Google Scholar] [CrossRef]
- Clough, T.J.; Kelliher, F.M.; Sherlock, R.R.; Ford, C.D. Lime and Soil Moisture Effects on Nitrous Oxide Emissions from a Urine Patch. Soil Sci. Soc. Am. J. 2004, 68, 1600–1609. [Google Scholar] [CrossRef]
- Pan, Y.; Ye, L.; Yuan, Z. Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Environ. Sci. Technol. 2013, 47, 8408–8415. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hu, C.S.; Ming, H.; Zhang, Y.M.; Li, X.X.; Dong, W.X.; Oenema, O. Concentration profiles of CH4, CO2 and N2O in soils of a wheat-maize rotation ecosystem in North China Plain, measured weekly over a whole year. Agric. Ecosyst. Environ. 2013, 164, 260–272. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, C.; Ming, H.; Oenema, O.; Schaefer, D.A.; Dong, W.; Zhang, Y.; Li, X. Methane, Carbon Dioxide and Nitrous Oxide Fluxes in Soil Profile under a Winter Wheat-Summer Maize Rotation in the North China Plain. PLoS ONE 2014, 9, e98445. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, C. Greenhouse gas emissions and mitigation measures in Chinese agroecosystems. Agric. For. Meteorol. 2007, 142, 270–277. [Google Scholar] [CrossRef]
- Phillips, R.L.; Wick, A.F.; Liebig, M.A.; West, M.S.; Daniels, W.L. Biogenic emissions of CO2 and N2O at multiple depths increase exponentially during a simulated soil thaw for a northern prairie Mollisol. Soil Biol. Biochem. 2012, 45, 14–22. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Wu, L.-H.; Sun, N.; Ding, X.-S.; Li, J.-W.; Wang, B.-R.; Li, D.-C. Soil CO2 and N2O Emissions in Maize Growing Season Under Different Fertilizer Regimes in an Upland Red Soil Region of South China. J. Integr. Agric. 2014, 13, 604–614. [Google Scholar] [CrossRef]
- Bond-lamberty, B.; Smith, A.P.; Bailey, V. Temperature and moisture effects on greenhouse gas emissions from deep active-layer boreal soils. Biogeosciences 2016, 13, 6669–6681. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Soil Respiration under Maize Crops: Effects of Water, Temperature, and Nitrogen Fertilization. Soil Sci. Soc. Am. J. 2007, 71, 944. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Zhang, B.; Lu, M.; Luo, Y.; Liu, L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, H.Q. Does a general temperature-dependent Q10 model of soil respiration exist at biome and global scale? J. Integr. Plant Biol. 2005, 47, 1288–1302. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Wang, T.; Sun, J.; Shen, Z. Temperature sensitivity of soil respiration in different ecosystems in China. Soil Biol. Biochem. 2009, 41, 1008–1014. [Google Scholar] [CrossRef]
- Liu, H.S.; Li, L.H.; Han, X.G.; Huang, J.H.; Sun, J.X.; Wang, H.Y. Respiratory substrate availability plays a crucial role in the response of soil respiration to environmental factors. Appl. Soil Ecol. 2006, 29, 284–293. [Google Scholar] [CrossRef]
- Zhai, L.-M.; Liu, H.-B.; Zhang, J.-Z.; Huang, J.; Wang, B.-R. Long-Term Application of Organic Manure and Mineral Fertilizer on N2O and CO2 Emissions in a Red Soil from Cultivated Maize–Wheat Rotation in China. Agric. Sci. China 2011, 10, 1748–1757. [Google Scholar] [CrossRef]
- Anthoni, P.M.; Freibauer, A.; Kolle, O.; Schulze, E.D. Winter wheat carbon exchange in Thuringia, Germany. Agric. For. Meteorol. 2004, 121, 55–67. [Google Scholar] [CrossRef]
- West, T.O.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Yan, G.; Zheng, X.; Cui, F.; Yao, Z.; Zhou, Z. Two-year simultaneous records of N2O and NO fluxes from a farmed cropland in the northern China plain with a reduced nitrogen addition rate by one-third. Agric. Ecosyst. Environ. 2013, 178, 39–50. [Google Scholar] [CrossRef]
| Year | F | MF1 | MF2 |
|---|---|---|---|
| 2010 | 84.3 ± 10.3 a | 110.9 ± 15.9 a,b | 96.6 ± 15.9 a |
| 2012 | 107.3 ± 16.6 a | 127.6 ± 16.7 c | 119.8 ± 15.7 b,c |
| Year | Plot | Manure | NPP | CO2 | NECB | CH4 kg C | N2O kg N | GWP Mg CO2-C eq |
|---|---|---|---|---|---|---|---|---|
| Mg C | ||||||||
| 2010 | CT | 0 | 2.9 ± 1.1 c | 6.8 ± 0.8 a,b,c | −6.5 ± 0.8 c | 1.5 ± 1.7 a,b | 3.9 ± 1.2 a | 7.1 ± 1.0 c,d |
| F | 0 | 6.5 ± 2.2 a,d | 6.9 ± 1.1 a,b,c | −6.2 ± 1.1 b,c | 5.1 ± 4.6 b | 17.4 ± 16.1 a,b,c,d | 8.5 ± 2.2 d | |
| MF1 | 8.9 ± 0.4 | 7.0 ± 3.7 a,b,d | 10.2 ± 0.7 c | −1.7 ± 0.7 a,d | 0.5 ± 1.4 a,b | 22.9 ± 11.3 b,c,d | 4.7 ± 1.7 a,b,c,d | |
| MF2 | 8.9 ± 0.4 | 6.1 ± 2.3 a,d,e | 8.9 ± 0.5 b,c | −0.5 ± 0.5 a | 3.7 ± 3.9 a,b | 33.5 ± 2.3 d | 4.9 ± 0.4 a,b,c,d | |
| 2011 | CT | 0 | 3.5 ± 1.3 c,e | 6.5 ± 0.9 a,b,c | −5.7 ± 0.9 b,c | 0.3 ± 1.0 a,b | 5.8 ± 2.3 a,b | 6.4 ± 0.9 b,c,d |
| F | 0 | 7.6 ± 1.3 a,b | 6.1 ± 1.1 a,d | −5.1 ± 1.1 b,c,e | −0.2 ± 0.6 a | 13.6 ± 8.7 a,b,c | 6.8 ± 1.1 b,c,d | |
| MF1 | 6.7 ± 0.5 | 9.9 ± 1.2 b | 7.8 ± 1.1 a,b,c | 0.3 ± 1.1 a | 0.4 ± 0.2 a,b | 14.3 ± 2.2 a,b,c | 1.5 ± 1.3 a | |
| MF2 | 6.7 ± 0.5 | 9.9 ± 1.4 b | 8.3 ± 1.6 a,b,c | 0.2 ± 1.6 a | −0.6 ± 0.7 a | 25.2 ± 11.9 c,d | 3.0 ± 2.8 a,b | |
| M | 6.7 ± 0.5 | 4.3 ± 1.6 c,d,e | 7.8 ± 1.1 a,b,c | −0.5 ± 1.1 a | 1.3 ± 2.1 a,b | 11.7 ± 2.3 a,b,c | 2.0 ± 1.4 a | |
| 2012 | CT | 0 | 2.7 ± 0.4 c | 6.8 ± 1.2 a,b,d | −6.2 ± 1.2 b,c | −0.2 ± 0.1 a | 3.6 ± 0.7 a | 6.6 ± 1.2 b,c,d |
| F | 0 | 6.3 ± 0.8 a,d,e | 4.9 ± 0.3 d | −3.7 ± 0.3 b,d,e | 1.9 ± 2.8 a,b | 7.1 ± 3.3 a,b | 4.6 ± 0.5 a,b,c,d | |
| MF1 | 6.7 ± 0.4 | 8.4 ± 1.3 a,b | 8.8 ± 0.6 a,b,c | −1.3 ± 0.6 a,d | −0.7 ± 0.2 a | 7.7 ± 1.2 a,b | 2.2 ± 0.7 a | |
| MF2 | 6.7 ± 0.4 | 8.7 ± 1.1 a,b | 9.8 ± 2.2 c | −2.4 ± 2.1 a,d,e | 0.0 ± 0.6 a,b | 8.8 ± 4.0 a,b,c | 3.5 ± 2.5 a,b,c | |
| M | 6.7 ± 0.4 | 2.9 ± 0.9 c | 10.3 ± 0.5 c | −3.7 ± 0.5 b,d,e | −0.6 ± 0.3 a | 5.6 ± 1.7 a,b | 4.4 ± 0.8 a,b,c | |
| Plot | Manure | NPP | CO2 | NECB | N2O kg N | CH4 kg C | GWP Mg CO2-C eq |
|---|---|---|---|---|---|---|---|
| Mg C | |||||||
| M1 | 7.3 ± 0.3 a | 3.94 ± 0.46 b | 8.28 ± 1.70 | 0.59 ± 1.7 b | 7.19 ± 1.77 | −0.04 ± 0.37 | 0.33 ± 1.75 a |
| MF | 7.3 ± 0.3 a | 6.99 ± 2.52 a | 8.71 ± 1.64 | 0.20 ± 1.6 b | 10.00 ± 1.83 | −0.29 ± 0.24 | 1.08 ± 1.74 a |
| MM | 12.3 ± 0.4 b | 7.12 ± 1.00 a | 8.30 ± 1.72 | 5.22 ± 1.7 a | 7.41 ± 1.39 | 0.18 ± 0.23 | −4.27 ± 1.81 b |
| Variable | N2O Flux (μg N m−2·h−1) | CH4 Flux (μg C m−2·h−1) | CO2 Flux (mg C m−2·h−1) |
|---|---|---|---|
| Soil temperature | 0.174 ** | 0.163 ** | 0.776 ** |
| WFPS | 0.084 | 0.205 ** | –0.341 ** |
| Soil pH | –0.281 ** | –0.060 | 0.162 * |
| NO3− | 0.242 ** | 0.031 | –0.265 ** |
| NH4+ | –0.049 | –0.067 | 0.069 |
| WEOC | 0.031 | –0.113 | 0.105 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukumbuta, I.; Shimizu, M.; Hatano, R. Mitigating Global Warming Potential and Greenhouse Gas Intensities by Applying Composted Manure in Cornfield: A 3-Year Field Study in an Andosol Soil. Agriculture 2017, 7, 13. https://doi.org/10.3390/agriculture7020013
Mukumbuta I, Shimizu M, Hatano R. Mitigating Global Warming Potential and Greenhouse Gas Intensities by Applying Composted Manure in Cornfield: A 3-Year Field Study in an Andosol Soil. Agriculture. 2017; 7(2):13. https://doi.org/10.3390/agriculture7020013
Chicago/Turabian StyleMukumbuta, Ikabongo, Mariko Shimizu, and Ryusuke Hatano. 2017. "Mitigating Global Warming Potential and Greenhouse Gas Intensities by Applying Composted Manure in Cornfield: A 3-Year Field Study in an Andosol Soil" Agriculture 7, no. 2: 13. https://doi.org/10.3390/agriculture7020013







