Development and Evaluation of Poly Herbal Molluscicidal Extracts for Control of Apple Snail (Pomacea maculata)
Abstract
:1. Introduction
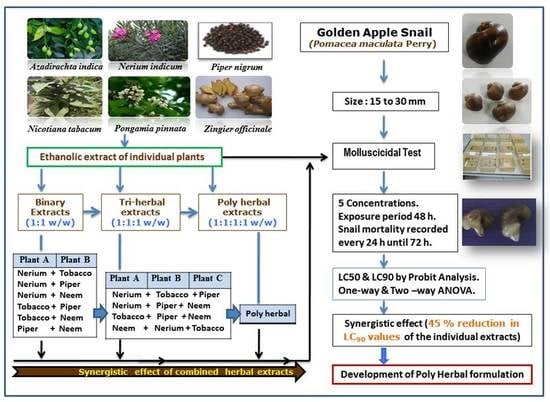
2. Materials and Methods
2.1. Collection of Snails (Pomacea maculata)
2.2. Collection of Plant Materials
2.3. Preparation of Plant Extracts
2.4. Evaluation of the Molluscicidal Potency of Plant Extracts
2.5. Preparation of Binary, Tri, and Poly Herbal Combinations of Plant Extracts
2.6. Preparation of Niclosamide Test Solutions
2.7. Evaluation of Molluscicidal Potency of Plant Extracts
3. Results
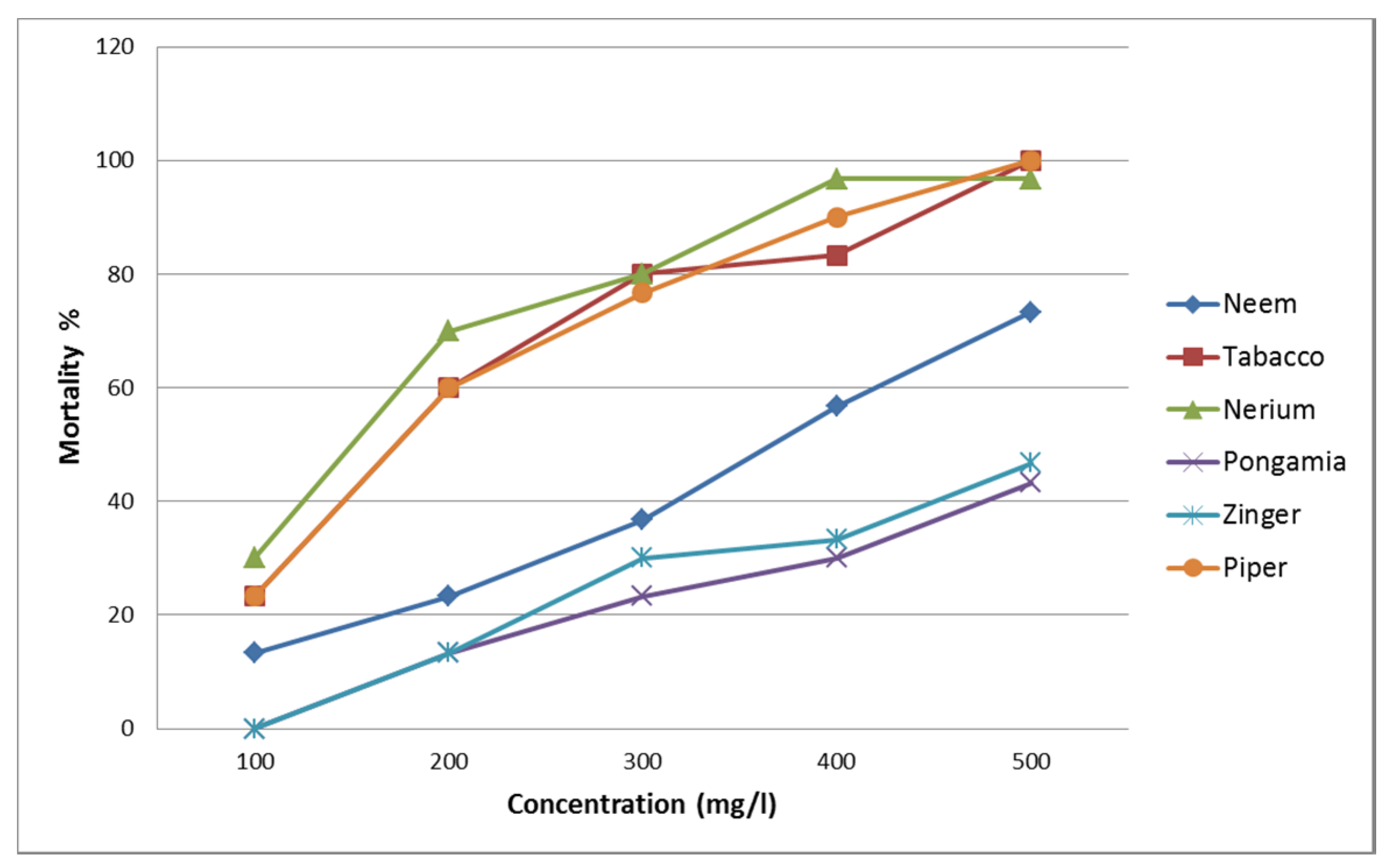
3.1. Molluscicidal Activity of Six Individual Plant Extracts
3.2. Molluscicidal Activity of Binary Combinations of Plant Extracts
3.3. Molluscicidal Activity of Tri and Poly Herbal Extracts
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Rice Research Institute (IRRI), Rice Knowledge Bank. Available online: http://www.knowledgebank.irri.org/step-by-step-production/growth/pests-and-diseases/golden-apple-snails (accessed on 14 November 2016).
- Nghiem, L.T.P.; Soliman, T.; Yeo, D.C.J.; Tan, H.T.W.; Evans, T.A.; Mumford, J.D.; Keller, R.P.; Baker, R.H.A.; Corlett, R.T.; Carrasco, L.R. Economic and Environmental Impacts of Harmful Non-Indigenous Species in Southeast Asia. PLoS ONE 2013, 8, e71255. [Google Scholar] [CrossRef] [PubMed]
- GISD (Global Invasive Species Database); Invasive Species Specialist Group ISSG. The Global Invasive Species Database. Version 2015.1. Available online: http://www.issg.org/database (accessed on 1 June 2016).
- Salleh, N.H.M.; Arbain, D.; Daud, M.Z.M.; Pilus, N.; Nawi, R. Distribution and Management of Pomecae canaliculata in the Northern Region of Malaysia: Mini Review. APCBE Procedia 2012, 2, 129–134. [Google Scholar] [CrossRef]
- Arfan, A.G.; Muhamad, R.; Omar, D.; Nor Azwady, A.A.; Manjeri, G. Distribution of two Pomacea spp. in rice fields of Peninsular Malaysia. Annu. Res. Rev. Biol. 2014, 4, 4123–4136. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Chen, X.; Luo, S. Insect Damage Reduction while Maintaining Rice Yield in Duck-Rice Farming Compared with Mono Rice Farming. J. Sustain. Agric. 2009, 33, 801–809. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, J.; Song, C.; Luo, M.; Zhao, B.; Quan, G.; An, M. Integrated Management to Control Golden Apple Snails (Pomacea canaliculata) in Direct Seeding Rice Fields: An Approach Combining Water Management and Rice-Duck Farming. Agroecol. Sustain. Food Syst. 2014, 38, 264–282. [Google Scholar] [CrossRef]
- Plant Health Australia. Contingency Plan: Golden Apple Snail; Plant Health Australia: Deakin, Australia, 2009; p. 15. [Google Scholar]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural Toxins for Use in Pest Management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, A. Metabolic Changes in Freshwater Harmful Snail Lymnaea acuminata Due to Aqueous Extract of Bark and Leaf of Euphorbia pulcherima Plant. Am.-Eurasian J. Toxicol. Sci. 2010, 2, 13–19. [Google Scholar]
- Li, X.; Deng, F.; Shan, X.; Pan, J.; Yu, P.; Mao, Z. Effects of the molluscicidal agent GA-C13:0, a natural occurring ginkgolic acid, on snail mitochondria. Pestic. Biochem. Physiol. 2012, 103, 115–120. [Google Scholar] [CrossRef]
- Prakash, A.; Rao, J.; Nandagopal, V. Managing resistance to insecticides is a key for the future of crop protection. J. Biopestic. 2008, 1, 154–169. [Google Scholar]
- El-Wakeil, N.E. Botanical pesticides and their mode of action. Gesunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Teixeira, T.; Rosa, J.S.; Rainha, N.; Baptista, J.; Rodrigues, A. Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra (Müller, 1774). Chemosphere 2012, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruamthum, W.; Visetson, S.; Milne, J.R.; Bullangpoti, V. Toxicity of botanical insecticides on golden apple snail (Pomacea canaliculata). Commun. Agric. Appl. Biol. Sci. 2010, 75, 191–197. [Google Scholar] [PubMed]
- Joshi, R.C.; Meepagala, K.M.; Sturtz, G.; Cagauan, A.G.; Mendoza, C.O.; Dayan, F.E.; Duke, S.O. Molluscicidal activity of vulgarone B from Artemisia douglasiana (Besser) against the invasive, alien, mollusc pest, Pomacea canaliculata (Lamarck). Int. J. Pest Manag. 2005, 51, 175–180. [Google Scholar] [CrossRef]
- Shukla, S.; Singh, V.K.; Singh, D.K. The effect of single, binary, and tertiary combination of few plant derived molluscicides alone or in combination with synergist on different enzymes in the nervous tissues of the freshwater snail Lymnaea (Radix) acuminata (Lamark). Pestic. Biochem. Physiol. 2006, 85, 167–173. [Google Scholar] [CrossRef]
- Plan, M.R.R.; Saska, I.; Cagauan, A.G.; Craik, D.J. Backbone Cyclised Peptides from Plants Show Molluscicidal Activity against the Rice Pest Pomacea canaliculata (Golden Apple Snail). J. Agric. Food Chem. 2008, 56, 5237–5241. [Google Scholar] [CrossRef] [PubMed]
- Keni, M.F.; Latip, S.N.H.M. Azadirachta indica seed as potential biopesticides for controlling golden apple snail, Pomacea canaliculata in rice cultivation. In Proceedings of the 2013 IEEE Business Engineering and Industrial Applications Colloquium (BEIAC), Langkawi, Malaysia, 7–9 April 2013; pp. 251–256.
- Massaguni, R.; Latip, S.N.H.M. Assesssment the Molluscicidal Properties of Azadirachtin against Golden Apple Snail, Pomacea Canaliculata. Malays. J. Anal. Sci. 2015, 19, 781–789. [Google Scholar]
- Benchawattananon, R.; Boonkong, U. The toxicity of leave crude extract from neem tree (Azadirachta indica Juss.) and Garlic (Allium sativom L.) on mortality rate of golden apple snail (Pomacea sp.). In Proceedings of the 32nd Congress on Science and Technology of Thailand, Bangkok, Thailand, 10–12 October 2006.
- Musman, M.; Kamaruzzaman, S.; Karina, S.; Rizqi, R.; Arisca, F. A preliminary study on the anti hatching of freshwater golden apple snail Pomacea canaliculata (Gastropoda: Ampullariidae) eggs from Barringtonia racemosa (Magnoliopsida: Lecythidaceae) seeds extract. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2013, 6, 394–398. [Google Scholar]
- Demetillo, M.T.; Baguio, M.L.; Limitares, D.E.; Madjos, G.G.; Abrenica-Adamat, L.R. Effect of Cymbopogon citratus (lemon grass) crude leaf extracts on the developmental stages of Pomacea canaliculata (golden apple snail). Adv. Environ. Sci. 2015, 7, 460–467. [Google Scholar]
- Zhang, H.; Xu, H.H.; Song, Z.J.; Chen, L.Y.; Wen, H.J. Molluscicidal activity of Aglaia duperreana and the constituents of its twigs and leaves. Fitoterapia 2012, 83, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.S.; Ndjoko, K.; Hostettmann, K. Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponins. Crop Prot. 2008, 27, 310–319. [Google Scholar] [CrossRef]
- Kijprayoona, S.; Toliengb, V.; Petsoma, A.; Chaicharoenpongb, C. Molluscicidal activity of Camellia oleifera seed meal. Sci. Asia 2014, 40, 393–399. [Google Scholar] [CrossRef]
- Quijano, M.; Riera-Ruiz, C.; Barragan, A.; Miranda, M.; Orellana, T.; Manzano, P. Molluscicidal activity of the aqueous extracts from Solanum mammosum L., Sapindus saponaria L. and Jatropha curcas L. against Pomacea canaliculata. Emir. J. Food Agric. 2014, 26, 871–877. [Google Scholar] [CrossRef]
- Tangkoonboribun, R.S.S. Molluscicide from Tobacco Waste. J. Agric. Sci. 2009, 1, 76. [Google Scholar] [CrossRef]
- Cowie, R.H.; Hayes, K.A. Invasive ampullariid snails: Taxonomic confusion and some preliminary resolution based on DNA sequences. In Proceedings of the APEC Symposium on the Management of the Golden Apple Snail, Pingtung, Taiwan, 6–11 September 2005.
- Hayes, K.A.; Cowie, R.H.; Thiengo, S.C.; Strong, E.E. Comparing apples with apples: Clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zool. J. Linn. Soc. 2012, 166, 723–753. [Google Scholar] [CrossRef]
- Burela, S.; Martín, P.R. Evolutionary and functional significance of lengthy copulations in a promiscuous apple snail, Pomacea canaliculata (Caenogastropoda: Ampullariidae). J. Molluscan Stud. 2011, 77, 54–64. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martín, P.R. Juvenile growth and survival of the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) reared at different constant temperatures. SpringerPlus 2013, 2, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Snail Control in Prevention of Bihariasis Monograph; World Health Organization: Geneva, Switzerland, 1965; Volume 50, pp. 124–138. [Google Scholar]
- Chauhan, S.; Singh, A. Impact of Taraxerol in combination with extract of Euphorbia tirucalli plant on biological parameters of Lymnaea acuminata. Rev. Inst. Med. Trop. São Paulo 2011, 53, 265–270. [Google Scholar] [CrossRef] [PubMed]
- El-Din, A.S.; El-Sayed, K.; Mahmoud, M. Effect of ethanolic extract of Dalbergia sissoo plant parts on Biomphalaria alexandrina snail, the intermediate host of Schistosoma mansoni. Medical Malacology Laboratory. J. Evolut. Biol. Res. 2011, 3, 95–100. [Google Scholar]
- Taguiling, N.K. Effect of Combined Plant Extracts on Golden Apple Snail (Pomacea canaliculata (Lam.)) and Giant Earthworm (Pheretima sp). Int. J. Agric. Crop Sci. 2015, 8, 55–60. [Google Scholar]
- Dai, L.; Wang, W.; Dong, X.; Hu, R.; Nan, X. Molluscicidal activity of cardiac glycosides from Nerium indicum against Pomacea canaliculata and its implications for the mechanisms of toxicity. Environ. Toxicol. Pharmacol. 2011, 32, 226–232. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines for evaluation of plant molluscicides. In Phytolacca Dodecandra (Endod) Dublin; Lemma, A., Heyneman, D., Silangwa, S., Eds.; Tycooly International Publishing Limited: Dublin, Ireland, 1983; pp. 121–124. [Google Scholar]
- Yadav, R.; Singh, A. Combinations of binary and tertiary toxic effects of extracts of Euphorbia pulcherima latex powder with other plant derived molluscicides against freshwater vector snails. Internet J. Toxicol. 2008, 7, 2. [Google Scholar]
- Olofintoye, L.K. Comparative evaluation of Molluscicidal effects of Securidaca longepedunculata (Fres.) and Tephrosia bracteolata (Guilland Perr) on Bulinus globosus. J. Parasitol. Vector Biol. 2010, 2, 44–47. [Google Scholar]
- Sunita, K.; Kumar, P.; Singh, V.K.; Singh, D.K. In vitro phytotherapy of vector snails by binary combinations of larvicidal active components in effective control of fascioliasis. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.G.; Singh, D.K. Combinations of Azadirachta indica and Cedrus deodara oil with piperonyl butoxide, MGK-264 and Embelia ribes against Lymnaea acuminata. Chemosphere 2001, 44, 1691–1695. [Google Scholar] [CrossRef]
- Dai, L.; Qian, X.; Nan, X.; Zhang, Y. Effect of cardiac glycosides from Nerium indicum on feeding rate, digestive enzymes activity and ultrastructural alterations of hepatopancreas in Pomacea canaliculata. Environ. Toxicol. Pharmacol. 2014, 37, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Okwute, S.K. Plants as Potential Sources of Pesticidal Agents: A Review. In Pesticides—Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]

| No. | Scientific Name | Family | Parts Used |
|---|---|---|---|
| 1 | Azadirachta indica A. Juss. | Meliaceae | Neem kernel oil (cold processed) |
| 2 | Nicotiana tabacum L. | Solanaceae | Leaves |
| 3 | Nerium indicum Mill. | Apocynaceae | Leaves |
| 4 | Pongamia pinnata L. | Fabaceae | Seed oil |
| 5 | Zingiber officinale L. | Zingiberaceae | Rhizome |
| 6 | Piper nigrum L. | Piperaceae | Seeds |
| No | Plant A | Plant B |
|---|---|---|
| 1 | Nerium (Nerium indicum) | Tobacco (Nicotiana tabacum) |
| 2 | Nerium (Nerium indicum) | Piper (Piper nigrum) |
| 3 | Nerium (Nerium indicum) | Neem (Azadirachta indica) |
| 4 | Tobacco (Nicotiana tabacum) | Piper (Piper nigrum) |
| 5 | Tobacco (Nicotiana tabacum) | Neem (Azadirachta indica) |
| 6 | Piper (Piper nigrum) | Neem (Azadirachta indica) |
| No. | Plant A | Plant B | Plant C |
|---|---|---|---|
| 1 | Nerium | Tobacco | Piper |
| 2 | Nerium | Piper | Neem |
| 3 | Tobacco | Piper | Neem |
| 4 | Nerium | Neem | Tobacco |
| Plant Name | Hetero-Geneity | Chi Square | p | LC50 | Fiducial Limits (mg/L) | LC90 | Fiducial Limits (mg/L) | ||
|---|---|---|---|---|---|---|---|---|---|
| LL | UL | LL | UL | ||||||
| Azadirachta indica | 0.999 | 3.863 | 0.920 | 365 | 321 | 420 | 624 | 541 | 768 |
| Nicotiana tabacum | 0.765 | 11.68 | 0.232 | 205 | 172 | 237 | 375 | 334 | 436 |
| Nerium indicum | 0.052 | 26.114 | 0.001 | 179 | 130 | 222 | 341 | 289 | 430 |
| Pongamia pinnata | 0.996 | 4.904 | 0.842 | 512 | 445 | 641 | 804 | 666 | 1111 |
| Zingiber officinale | 0.973 | 7.024 | 0.634 | 485 | 425 | 592 | 767 | 643 | 1028 |
| Piper nigrum | 0.704 | 12.575 | 0.183 | 202 | 170 | 232 | 359 | 320 | 417 |
| Concentration (mg/L) | Percentage Mortality of Pomacea maculata | |||||
|---|---|---|---|---|---|---|
| Binary Combination of Plant Extracts | ||||||
| Nerium and Tobacco | Nerium and Piper | Nerium and Neem | Tobacco and Piper | Tobacco and Neem | Piper and Neem | |
| 100 | 63.33 ± 5.7 | 63.33 ± 5.7 | 63.33 ± 5.7 | 36.60 ± 5.7 | 36.60 ± 5.7 | 36.60 ± 5.7 |
| 200 | 90.00 ± 10 | 76.60 ± 15.2 | 70.00 ± 10 | 66.60 ± 5.7 | 56.60 ± 5.7 | 56.60 ± 5.7 |
| 300 | 100 ± 0.00 | 93.30 ± 5.7 | 80.00 ± 10 | 80.00 ± 10 | 73.30 ± 5.7 | 76.60 ± 5.7 |
| 400 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 93.30 ± 5.7 | 83.30 ± 5.7 | 93.30 ± 5.7 |
| 500 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 96.60 ± 5.7 | 93.30 ± 5.7 | 100 ± 0.00 |
| Concentration (mg/L) | Percentage Mortality of Pomacea maculata | ||||
|---|---|---|---|---|---|
| Tri and Poly Herbal Combination of Plant Extracts | |||||
| Nerium, Tobacco, and Piper | Nerium, Piper, and Neem | Tobacco, Piper, and Neem | Nerium, Neem, and Tobacco | Nerium, Tobacco, Piper and Neem | |
| 50 | 86.60 ± 5.7 | 46.66 ± 5.7 | 36.70 ± 5.7 | 93.30 ± 5.7 | 66.60 ± 15.2 |
| 100 | 96.60 ± 5.7 | 73.30 ± 5.7 | 70.00 ± 10 | 96.60 ± 5.7 | 83.30 ± 5.7 |
| 150 | 100 ± 0.00 | 86.60 ± 5.7 | 80.00 ± 10 | 100 ± 0.00 | 90.00 ± 10 |
| 200 | 96.60 ± 5.7 | 96.60 ± 5.7 | 86.70 ± 5.7 | 100 ± 0.00 | 100 ± 0.00 |
| 250 | 100 ± 0.00 | 96.60 ± 5.7 | 93.30 ± 5.7 | 96.60 ± 5.7 | 96.60 ± 5.7 |
| LC (mg/L) 48 h | Nerium | Tobacco | Piper | Neem | Nerium and Tobacco | Nerium, Tobacco, and Piper | Nerium, Tobacco, and Neem |
|---|---|---|---|---|---|---|---|
| LC50 | 179.36 | 205.71 | 202.02 | 365.10 | 100.18 | 73.91 | 62.60 |
| LC90 | 341.57 | 375.84 | 359.90 | 624.67 | 177.71 | 180.35 | 191.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhakaran, G.; Bhore, S.J.; Ravichandran, M. Development and Evaluation of Poly Herbal Molluscicidal Extracts for Control of Apple Snail (Pomacea maculata). Agriculture 2017, 7, 22. https://doi.org/10.3390/agriculture7030022
Prabhakaran G, Bhore SJ, Ravichandran M. Development and Evaluation of Poly Herbal Molluscicidal Extracts for Control of Apple Snail (Pomacea maculata). Agriculture. 2017; 7(3):22. https://doi.org/10.3390/agriculture7030022
Chicago/Turabian StylePrabhakaran, Guruswamy, Subhash Janardhan Bhore, and Manikam Ravichandran. 2017. "Development and Evaluation of Poly Herbal Molluscicidal Extracts for Control of Apple Snail (Pomacea maculata)" Agriculture 7, no. 3: 22. https://doi.org/10.3390/agriculture7030022