Fluorescence and Reflectance Sensor Comparison in Winter Wheat
Abstract
:1. Introduction
- Which sensors have been the most useful in regards to practical field handling?
- Which indices are statistically significant for assessments of the N treatments?
- Which fluorescence and spectroscopy indices can be used to estimate the wheat yield at an early development stage; i.e., what kind of index combination can derive a more exact yield prediction?
2. Materials and Methods
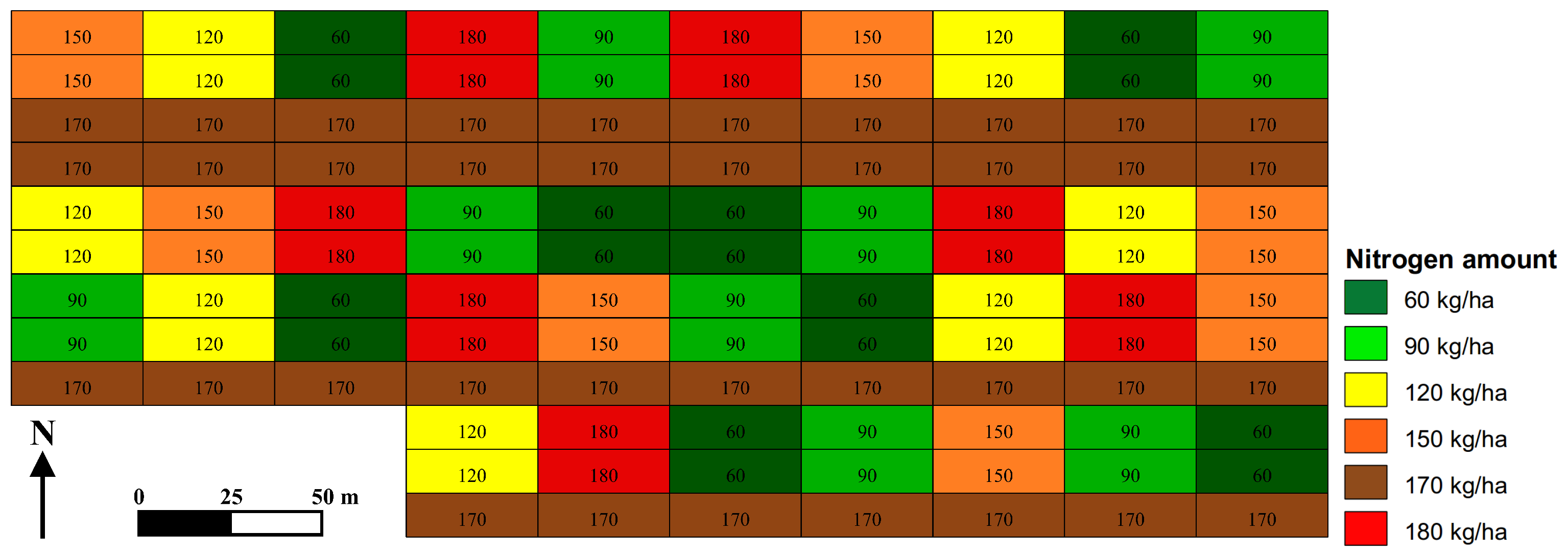
2.1. Experimental Site
2.2. Sensor Set-Up
2.3. Spectrometry
2.4. Fluorescence
2.5. Data Analysis
3. Results
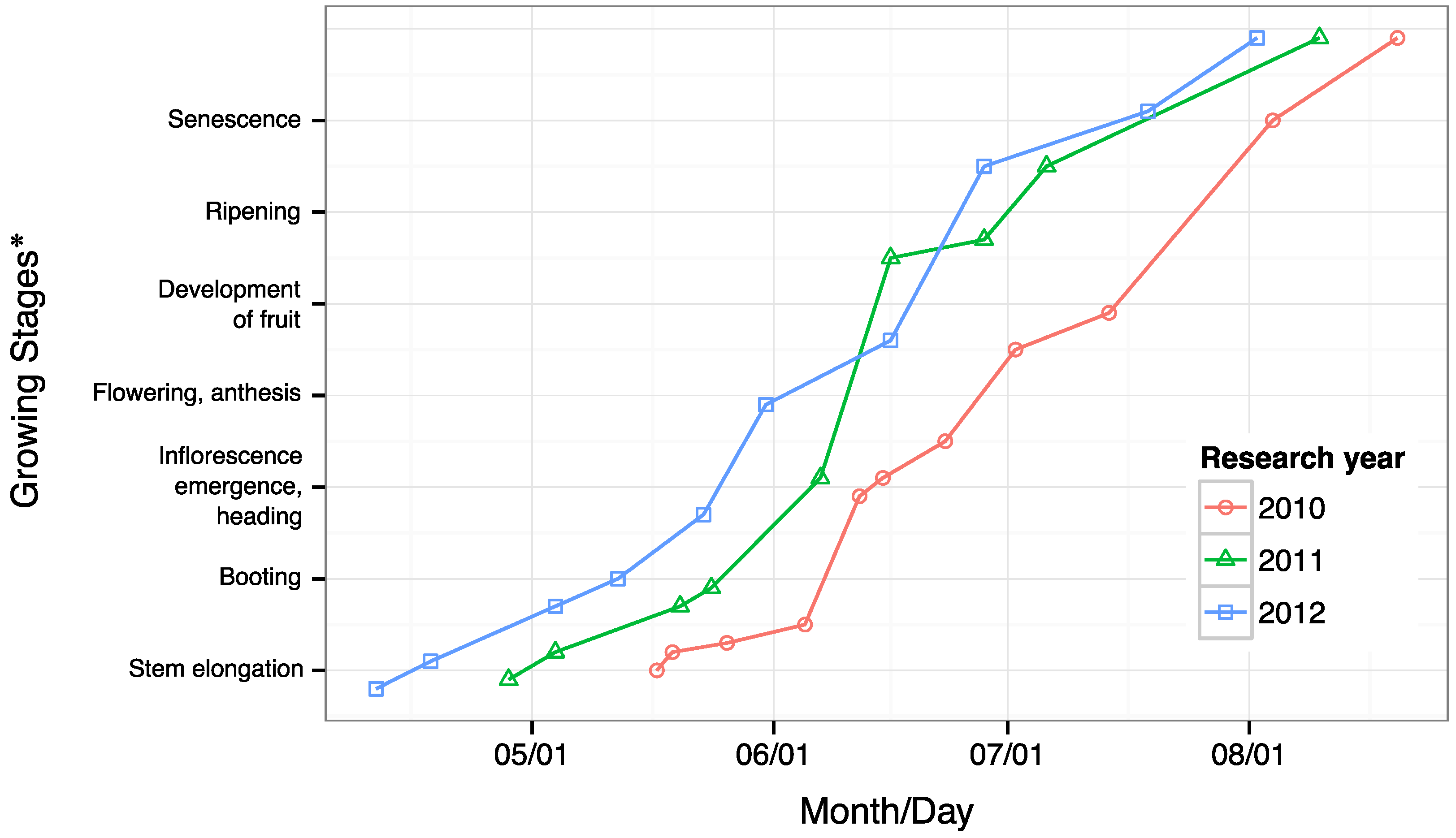
3.1. Field Conditions
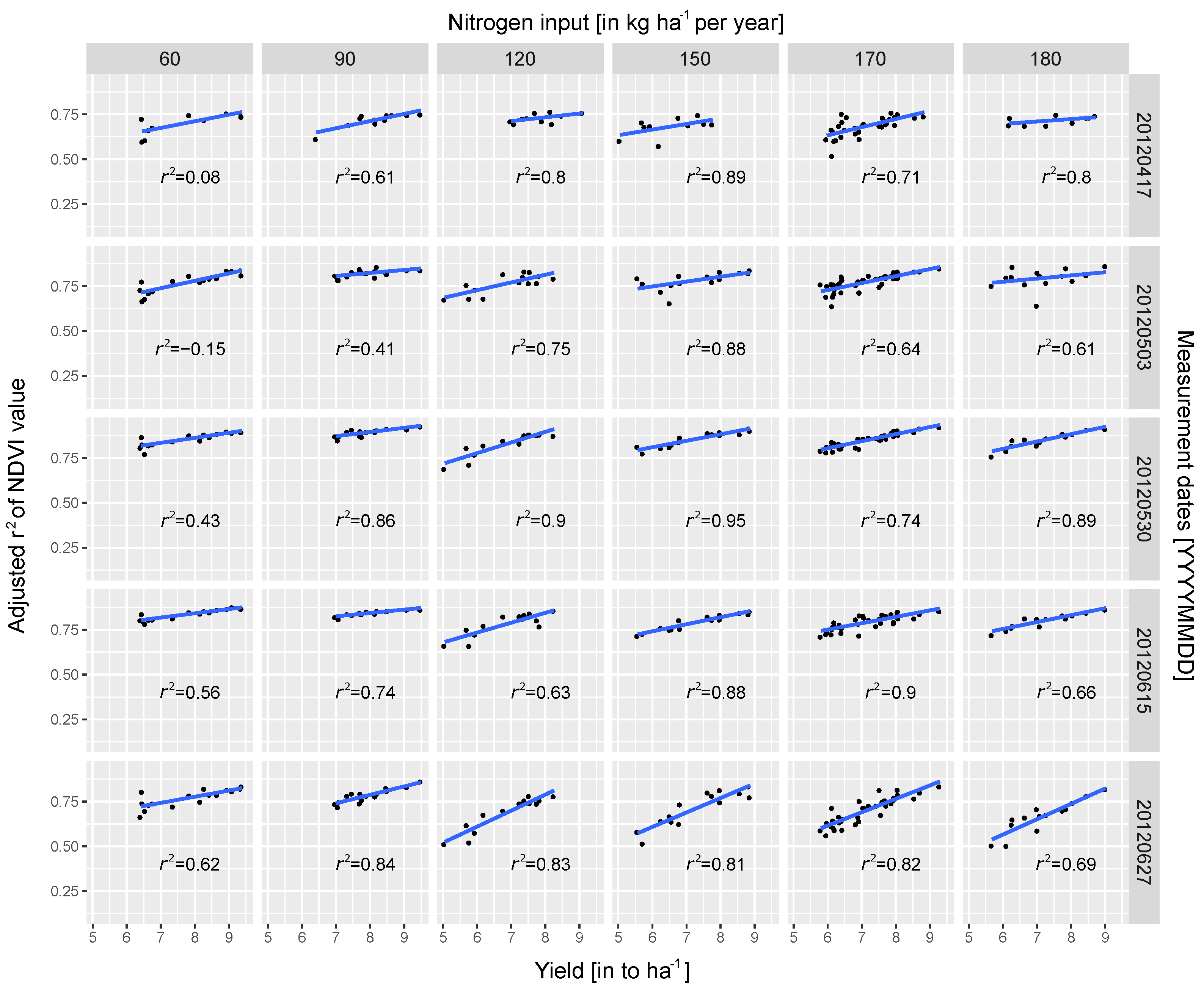
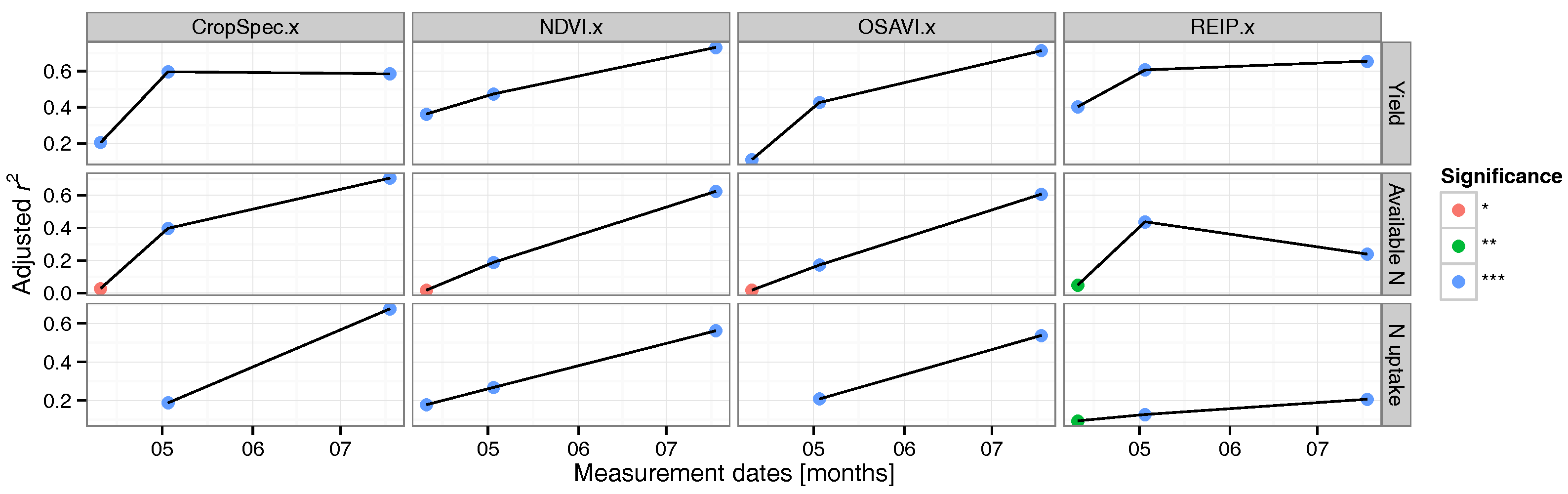
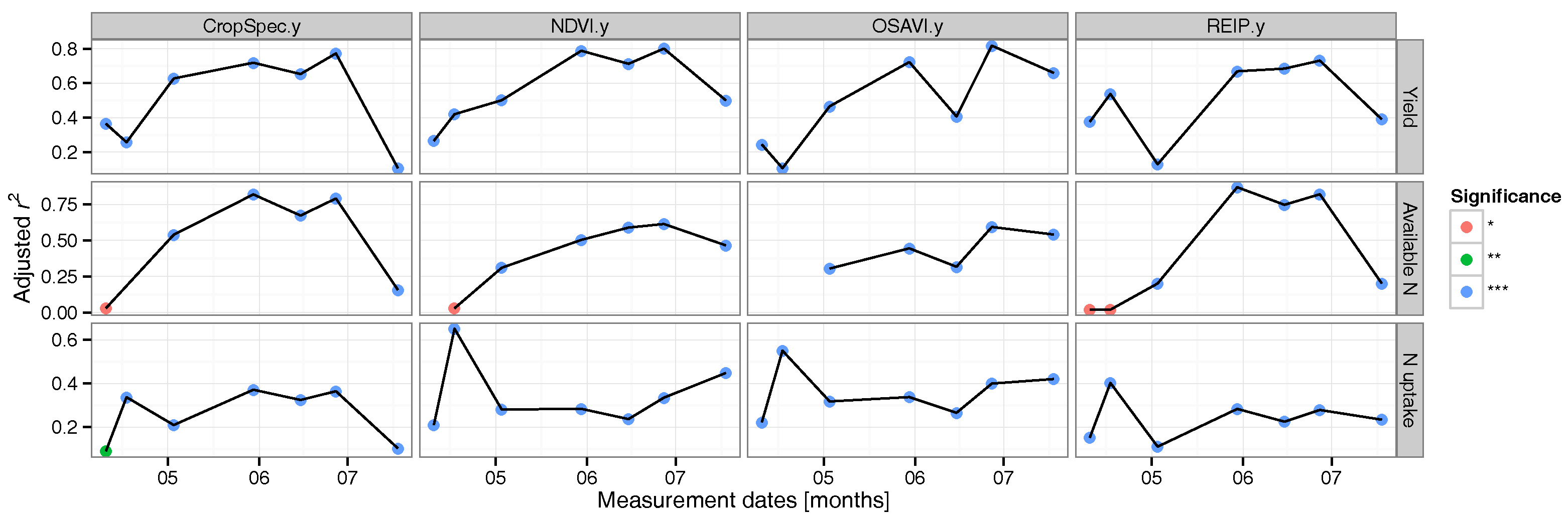
3.2. Regression Analysis
3.3. Data Validation
- Model 1:
- Model 2:
- Model 3:
- Model 4:
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.J.; Powlson, D.S.; Poulton, P.R.; Jenkinson, D.S. Unused fertiliser nitrogen in arable soils—Its contribution to nitrate leaching. J. Sci. Food Agric. 1989, 46, 407–419. [Google Scholar] [CrossRef]
- Reusch, S. ENtwicklung eines Reflexionsoptischen Sensors zur Erfassung der Stickstoffversorgung Landwirtschaftlicher Kulturpflanzen (Development of an Optical Reflectance Sensor for Recording the Nitrogen Supply of Agricultural Crops). Ph.D. Thesis, Department of Agricultural Systems Engineering, University of Kiel, Kiel, Germany, 1997. [Google Scholar]
- Sheffield, C. Selecting band combinations from multispectral data. Photogramm. Eng. Remote Sens. 1985, 51, 681–687. [Google Scholar]
- Guyot, G.; Baret, F.; Jacquemoud, S. Imaging spectroscopy for vegatation studies. In Imaging Spectroscopy: Fundamentals and Prospective Applications; Springer: Dordrecht, The Netherlands, 1992; pp. 145–165. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Bürling, K.; Cerovic, Z.G.; Cornic, G.; Ducruet, J.M.; Noga, G.; Hunsche, M. Fluorescence-based sensing of drought-induced stress in the vegetative phase of four contrasting wheat genotypes. Environ. Exp. Bot. 2013, 89, 51–59. [Google Scholar] [CrossRef]
- Thomas, J.; Gausman, H. Leaf Reflectance vs. Leaf Chlorophyll and Carotenoid Concentrations for Eight Crops. Agron. J. 1977, 69, 799–802. [Google Scholar] [CrossRef]
- Broge, N.H.; Mortensen, J.V. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sens. Environ. 2002, 81, 45–57. [Google Scholar] [CrossRef]
- Yao, X.; Yao, X.; Jia, W.; Tian, Y.; Ni, J.; Cao, W.; Zhu, Y. Comparison and intercalibration of vegetation indices from different sensors for monitoring above-ground plant nitrogen uptake in winter wheat. Sensors 2013, 13, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.E.; Ramig, R.E.; Allmaras, R.R.; Smith, C.M. Nitrogen-Sulfur Relations in Soft White Winter Wheat. II. Initial and Residual Effects of Sulfur Application on Nutrient Concentration, Uptake, and N/S Ratio. Agron. J. 1975, 67, 224–228. [Google Scholar] [CrossRef]
- Corp, L.A.; McMurtrey, J.E.; Middleton, E.M.; Mulchi, C.L.; Chappelle, E.W.; Daughtry, C.S.T. Fluorescence sensing systems: In vivo detection of biophysical variations in field corn due to nitrogen supply. Remote Sens. Environ. 2003, 86, 470–479. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2011, 32, 451–464. [Google Scholar] [CrossRef]
- Christensen, L.; Bennedsen, B.; Jørgensen, R.; Nielsen, H. Modelling nitrogen and phosphorus content at early growth stages in spring barley using hyperspectral line scanning. Biosyst. Eng. 2004, 88, 19–24. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Witzenberger, A.; Hack, H.; van den Boom, T. Erläuterungen zum BBCH-Dezimal-Code für die Entwicklungsstadien des Getreides - mit Abbildungen. Gesunde Pflanzen 1989, 41, 384–388. [Google Scholar]
- Reusch, S.; Jasper, J.; Link, A. Estimating crop biomass and nitrogen uptake using Cropspec, a newly developed active crop-canopy reflectance sensor. In Proceedings of the 10th International Conference on Positron Annihilation (ICPA), Denver, CO, USA, 18–21 July 2010; p. 381. [Google Scholar]
- Sharabian, V.R.; Noguchi, N.; Han-Ya, I.; Ishi, K. Evaluation of an active remote sensor for monitoring winter wheat growth status. Eng. Agric. Environ. Food 2013, 6, 118–127. [Google Scholar] [CrossRef]
- Reckleben, Y. Sensors for nitrogen fertilization—Experiences of 12 years practical use (in German). J. Kulturpflanzen 2013, 2, 42–47. [Google Scholar]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the Great Plains with ERTS. In Third ERTS Symposium; Freden, S.C., Becker, M.A., Eds.; National Aeronautics and Space Administration (NASA SP-351): Washington, DC, USA, 1973; Vol. 1, pp. 309–317. [Google Scholar]
- Moriondo, M.; Maselli, F.; Bindi, M. A simple model of regional wheat yield based on NDVI data. Eur. J. Agron. 2007, 26, 266–274. [Google Scholar] [CrossRef]
- Quebrajo, L.; Pérez-Ruiz, M.; Rodriguez-Lizana, A.; Agüera, J. An approach to precise nitrogen management using hand-held crop sensor measurements and winter wheat yield mapping in a mediterranean environment. Sensors 2015, 15, 5504–5517. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, E. Optical sensing-techniques for site-specific application of agricultural chemicals (in German). Ph.D. thesis, Department of Agricultural Systems Engineering, University of Kiel, Kiel, Germany, 2002. [Google Scholar]
- Laudien, R.; Bareth, G.; Doluschitz, R. Comparison of remote sensing based analysis of crop diseases by using high resolution multispectral and hyperspectral data–case study: Rhizoctonia solani in sugar beet. In Proceedings of the 12th International Conference on Geoinformatics–Geospatial Information Research: Bridging the Pacific and Atlantic, Gävle, Sweden, 7–9 June 2004; pp. 670–676. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Miller, J.; Morales, A.; Berjón, A.; Agüera, J. Hyperspectral indices and model simulation for chlorophyll estimation in open-canopy tree crops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar] [CrossRef]
- Peteinatos, G.G.; Korsaeth, A.; Berge, T.W.; Gerhards, R. Using Optical Sensors to Identify Water Deprivation, Nitrogen Shortage, Weed Presence and Fungal Infection in Wheat. Agriculture 2016, 6, 24. [Google Scholar] [CrossRef]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Baret, F.; Champion, I.; Guyot, G.; Podaire, A. Monitoring wheat canopies with a high spectral resolution radiometer. Remote Sens. Environ. 1987, 22, 367–378. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.; Abkar, A. Leaf Area Index derivation from hyperspectral vegetation indices and the red edge position. Int. J. Remote Sens. 2009, 30, 6199–6218. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D. LAI assessment of wheat and potato crops by VENμS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.d.J.; Guevara-Gonzalez, R.G.; Millan-Almaraz, J.R. Instrumentation in developing chlorophyll fluorescence biosensing: A review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef] [PubMed]
- Martinon, V.; Fadailli, E.M.; Evain, S.; Zecha, C.W. Multiplex: An innovative optical sensor for diagnosis, mapping and management of nitrogen on wheat. In Proceedings of the 8th European Conference on Precision Agriculture (ECPA), Prague, Czech Republic, 11–14 July 2011; Stafford, J.V., Ed.; Czech Centre for Science and Society: Ampthill, UK; Prague, Czech Republic, 2011; pp. 547–561. [Google Scholar]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Moise, N.; Agati, G.; Latouche, G.; Ben Ghozlen, N.; Meyer, S. New portable optical sensors for the assessment of winegrape phenolic maturity based on berry fluorescence. J. Food Compos. Anal. 2008, 21, 650–654. [Google Scholar] [CrossRef]
- Beleites, C.; Sergo, V. hyperSpec: A Package to Handle Hyperspectral Data Sets in R.’, R Package Version 098-20150304, 2016. Available online: https://cran.r-project.org/web/packages/hyperSpec/hyperSpec.pdf (accessed on 20 September 2017).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zhang, X.; Wang, S.; Sun, H.; Chen, S.; Shao, L.; Liu, X. Contribution of cultivar, fertilizer and weather to yield variation of winter wheat over three decades: A case study in the North China Plain. Eur. J. Agron. 2013, 50, 52–59. [Google Scholar] [CrossRef]
- Sidhu, B.; Beri, V. Effect of crop residue management on the yields of different crops and on soil properties. Biol. Wastes 1989, 27, 15–27. [Google Scholar] [CrossRef]
- Hansen, P.; Schjoerring, J. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Ferwerda, J.G.; Skidmore, A.K.; Mutanga, O. Nitrogen detection with hyperspectral normalized ratio indices across multiple plant species. Int. J. Remote Sens. 2005, 26, 4083–4095. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H. Imaging of the Blue, Green, and Red Fluorescence Emission of Plants: An Overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]


| Type | Manufacturer | Sensor Model | Wavelength Range | Wavelength Accuracy | Classification |
|---|---|---|---|---|---|
| Spectrometry | Analytical Spectral Devices | FieldSpec Handheld | 325–1075 nm | 1 nm | Passive |
| tec5 AG | HandySpec Field® | 360–1000 nm | 10 nm | Passive | |
| Fluorescence | Force-A | Multiplex Research | Blue-Green (BGF), Red (RF) and Far-Red (FRF) | – | Active |
| Sensor | Operating System | Connectivity | Ease of Use |
|---|---|---|---|
| FieldSpec HandHeld | Windows NT | Serial cable to PC | Complex (–) |
| HandySpec Field® | All | SD Card | Easy (0) |
| Multiplex Research | All | SD Card | Simple (+) |
| Parameter | Field | Year | Annual Nitrogen Application in kg ha | |||||
|---|---|---|---|---|---|---|---|---|
| 60 | 90 | 120 | 150 | 170 | 180 | |||
| Yield | LW | 2010 | 3.6 | 3.9 | 4.4 | 4.5 | 4.9 | 4.5 |
| IT | 2011 | 7.2 | 6.7 | 6.7 | 7.1 | 7.3 | 7.3 | |
| LW | 2012 | 6.2 | 6.5 | 7.5 | 7.8 | 7.9 | 7.9 | |
| Protein | LW | 2010 | 11.2 | 11.9 | 12.3 | 14.1 | 14.8 | 15.5 |
| IT | 2011 | 13.5 | 13.5 | 14.3 | 13.9 | 14.7 | 14.7 | |
| LW | 2012 | 9.1 | 10.1 | 11.4 | 12.7 | 13.6 | 13.2 | |
| Date | 17 April 2012 | 3 April 2012 | 30 May 2012 | 15 June 2012 | 27 June 2012 | 18 July 2012 |
|---|---|---|---|---|---|---|
| Model 1 | 0.11 | 0.38 | 0.62 | 0.57 | 0.51 | 0.74 |
| Model 2 | 0.12 | 0.48 | 0.63 | 0.58 | 0.52 | 0.76 |
| Model 3 | 0.22 | 0.52 | 0.64 | 0.59 | 0.54 | 0.76 |
| Model 4 | 0.53 | 0.53 | 0.80 | 0.76 | 0.78 | 0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zecha, C.W.; Link, J.; Claupein, W. Fluorescence and Reflectance Sensor Comparison in Winter Wheat. Agriculture 2017, 7, 78. https://doi.org/10.3390/agriculture7090078
Zecha CW, Link J, Claupein W. Fluorescence and Reflectance Sensor Comparison in Winter Wheat. Agriculture. 2017; 7(9):78. https://doi.org/10.3390/agriculture7090078
Chicago/Turabian StyleZecha, Christoph W., Johanna Link, and Wilhelm Claupein. 2017. "Fluorescence and Reflectance Sensor Comparison in Winter Wheat" Agriculture 7, no. 9: 78. https://doi.org/10.3390/agriculture7090078




