Turnover of Minerals and Organics in the Postharvest Herbage of Annuals and Perennials: Winter Wheat and Goldenrod
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sources
2.2. Soils
2.3. Processing Plant Samples of Goldenrod
2.4. Processing Plant Samples of Wheat in Two Crop Rotation Models
2.5. Mineral Concentrations in Soils and Plant Tissues
2.6. Nitrogen Compounds
2.7. Weight Loss of Wheat Straw Samples by Standing Infusion in Water
2.8. Statistical Treatments
3. Results and Discussion
3.1. Turnover of Organics and Minerals in the Goldenrod Colony
3.2. Mineral Preservation by the Postharvest Biomass of Crop Rotation Model Wheat-Maize (Model A)
3.3. Balancing Minerals in the Crop Rotation Model Wheat-Wheat (Model B)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. World Fertilizer Trends and Outlook to 2018; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates; United States Environmental Protection Agency: Washington, DC, USA, 2017.
- Meyer, S.; Bergmeier, E.; Becker, T.; Wesche, K.; Krause, B.; Leuschner, C. Detecting long-term losses at the plant community level—Arable fields in Germany revisited. Appl. Veg. Sci. 2015, 18, 432–442. [Google Scholar] [CrossRef]
- Bringezu, S.; Schütz, H.; Pengue, W.; O′Brien, M.; Garcia, F.; Sims, R.; Howarth, R.W.; Kauppi, L.; Swilling, M.; Herrick, J. Assessing Global Land Use: Balancing Consumption with Sustainable Supply; United Nations Environment Programme: Nairobi, Kenya, 2014. [Google Scholar]
- Dubovyk, O.; Menz, G.; Conrad, C.; Lamers, J.; Lee, A.; Khamzina, A. Spatial targeting of land rehabilitation: A relational analysis of cropland productivity decline in arid Uzbekistan. Erdkunde 2013, 67, 167–181. [Google Scholar] [CrossRef]
- Houyou, Z.; Bielders, C.L.; Benhorma, H.A.; Dellal, A.; Boutemdjet, A. Evidence of strong land degradation by wind erosion as a result of rainfed cropping in the Algerian steppe: A case study at Laghouat. Land Degrad. Dev. 2016, 27, 1788–1796. [Google Scholar] [CrossRef]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 402647. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. The mineral preservation in Giant Goldenrod (Solidago gigantea Ait.): A model for perennial seed crops? In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers: New York, NY, USA, 2018; Volume 23, pp. 101–130. [Google Scholar]
- Sharpley, A.N.; Weld, J.L.; Beegle, D.B.; Kleiman, P.J.A.; Gburek, W.J.; Moore, P.A., Jr.; Mullins, G. Development of phosphorus indices for nutrient management planning strategies in the United States. J. Soil Water Conser. 2003, 58, 137–152. [Google Scholar]
- Janssens, I.A.; Freibauer, A.; Ciais, P.; Smith, P.; Nabuurs, G.-J.; Folberth, G.; Schlamadinger, B.; Hutjes, R.W.A.; Ceulemans, R.; Schulze, E.-D.; et al. Europe′s terrestrial biosphere absorbs 7 to 12% of European anthropogenic CO2 emissions. Science 2003, 300, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, T.; Welp, G.; Holbeck, B.; Amelung, W. Long-term development of organic carbon contents in arable soil of North Rhine—Westphalia, Germany, 1979–2015. Eur. J. Soil Sci. 2016, 67, 616–623. [Google Scholar] [CrossRef]
- Batello, C.; Wade, L.; Cox, S.; Pogna, N.; Bozzini, A.; Choptiany, J. Perennial Crops for Food Security; FAO: Rome, Italy, 2014. [Google Scholar]
- Bell, L.W.; Byrne, F.; Ewing, M.A.; Wade, L.J. A preliminary whole-farm economic analysis of perennial wheat in an Australian dryland farming system. Agric. Syst. 2008, 96, 166–174. [Google Scholar] [CrossRef]
- Kane, D.A.; Rogé, P.; Snapp, S.S. A systematic review of perennial staple crops literature using topic modeling and bibliometric analysis. PLoS ONE 2016, 11, e0155788. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.S.; Van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Progress in breeding perennial grains. Crop Pasture Sci. 2010, 61, 513–521. [Google Scholar] [CrossRef]
- Curwen-McAdams, C.; Jones, S.S. Breeding perennial grain crops based on wheat. Crop Sci. 2017, 57, 1172–1188. [Google Scholar] [CrossRef]
- Perennial Staple Crops of the World. Available online: https://permaculturenews.org/2012/02/25/perennial-staple-crops-of-the-world/ (accessed on 22 October 2018).
- Zhang, Y.; Li, Y.; Jiang, L.; Tian, C.; Lib, J.; Xiao, Z. Potential of perennial crop on environmental sustainability of agriculture. Procedia Environ. Sci. 2011, 10, 1141–1147. [Google Scholar] [CrossRef]
- Tsitsin, N.V.; Lubimova, V.F. New species and forms of cereals derived from hybridization between wheat and couch grass. Am. Nat. 1959, 93, 181–191. [Google Scholar] [CrossRef]
- Farmers Get Guidance on Growing New Perennial Grains. Available online: http://news.cornell.edu/stories/2018/03/farmers-get-guidance-growing-new-perennial-grains (accessed on 16 July 2018).
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; DeHaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for multifunctionality in perennial grain crops. BioScience 2018, 68, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Culman, S.W.; Snapp, S.S.; Ollenburger, M.; Basso, B.; DeHaan, L.R. Soil and water quality rapidly responds to the perennial grain Kernza wheatgrass. Agron. J. 2013, 105, 735–744. [Google Scholar] [CrossRef]
- Rogé, P.; Snapp, S.; Kakwera, M.N.; Mungai, L.; Jambo, I.; Peter, B. Ratooning and perennial staple crops in Malawi. A review. Agron. Sustain. Dev. 2016, 36, 50. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Zhang, J.; Ting, Z.; Huang, W.; Xu, P.; Tao, D.; Fu, B.; Hu, F. The Progression of Perennial Rice Breeding and Genetics: Research in China; Perennial Crops for Food Security: Rome, Italy, 2014; pp. 27–38. [Google Scholar]
- Bell, L.W.; Wade, L.J.; Ewing, M.A. Perennial wheat: A review of environmental and agronomic prospects for development in Australia. Crop Pasture Sci. 2010, 61, 679–690. [Google Scholar] [CrossRef]
- Perennial Wheat. Available online: http://www.canr.msu.edu/foodsystems/uploads/files/E-3208.pdf (accessed on 16 July 2018).
- Toliver, D.K.; English, B.C.; Tyler, D.D.; Lee, J.; Menard, R.J.; Walton, J.C. Soil organic carbon changes for switchgrass farms in East Tennessee, USA. Soil Syst. 2018, 2, 25. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P. Perennial grains food security for the future. Issues Sci. Technol. 2010, 26, 41–47. [Google Scholar]
- Van Beilen, J.B.; Poirier, Y. Prospects for biopolymer production in plants. Adv. Biochem. Eng. Biotechnol. 2007, 107, 133–151. [Google Scholar] [PubMed]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Lemaître, T.; Christ, A.; Azzopardi, M.; Kato, Y.; Sato, F.; Morot-Gaudry, J.-F.; Le Dily, F.; Masclaux-Daubresse, C. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol. 2008, 147, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendoza, M.; Velasco-Arroyo, B.; Santamaria, M.E.; González-Melendi, P.; Martinez, M.; Diaz, I. Plant senescence and proteolysis: Two processes with one destiny. Gen. Molec. Biol. 2016, 39, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ospina, L.A.A. Autophagy, Senescence and Nitrogen Remobilization in Barley. Ph.D. Thesis, Paris-Sud University, Orsay, France, 2014. [Google Scholar]
- Von Fircks, Y.; Ericsson, T.; Sennerby-Forsse, L. Seasonal variation of macronutrients in leaves, stems and roots of Salix dasyclados Wimm. grown at two nutrient levels. Biomass Bioenergy 2001, 21, 321–334. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Grigal, F.D.; Ohman, L.F.; Brander, R.B. Seasonal dynamics of tall shrubs in northeastern Minnesota: Biomass and nutrient element changes. For. Sci. 1976, 22, 195–208. [Google Scholar]
- Beale, C.V.; Long, S.P. Seasonal dynamics of nutrient accumulation and partitioning in the perennial C4-grasses Miscanthus × giganteus and Spartina cynosuroides. Biomass Bioenergy 1997, 12, 419–428. [Google Scholar] [CrossRef]
- Brink, G.E.; Sistani, K.R.; Oldham, J.L.; Pederson, G.A. Maturity effects on mineral concentration and uptake in annual ryegrass. J. Plant Nutr. 2006, 29, 1143–1155. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Yang, M.; Yang, H.; Zhang, Q. Stoichiometric characteristics of carbon, nitrogen, and phosphorus in leaves of differently aged lucerne (Medicago sativa) stands. Front. Plant Sci. 2015, 6, 1062. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Amasino, R. Nitrogen recycling and flowering time in perennial bioenergy crops. Front. Plant Sci. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Worley, E.; Ma, Q.; Li, J.; Torres-Jerez, I.; Li, G.; Zhao, P.X.; Xu, Y.; Tang, Y.; Udvardi, M. Nitrogen remobilization and conservation, and underlying senescence-associated gene expression in the perennial switchgrass Panicum virgatum. New Phytol. 2016, 211, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud, F.; Vitte, G.; Machet, J.-M.; Beaudoin, N.; Catterou, M.; Mary, B. The fate of cumulative applications of 15N-labelled fertiliser in perennial and annual bioenergy crops. Agric. Ecosyst. Environ. 2016, 223, 76–86. [Google Scholar] [CrossRef]
- Alcoverro, T.; Manzanera, M.; Romero, J. Nutrient mass balance of the seagrass Posidonia oceanica: The importance of nutrient retranslocation. Mar. Ecol. Prog. Ser. 2000, 194, 13–21. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Hamel, C. Impact of arbuscular mycorrhizal fungi on N and P cycling in the root zone. Canad. J. Soil Sci. 2004, 84, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.-P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Tukey, H.B., Jr.; Wittwer, S.H.; Bukovac, M.J. Absorption of radionuclides by above-ground plant parts and movement within the plants. Agric. Food Chem. 1961, 9, 106–113. [Google Scholar] [CrossRef]
- Tukey, H.B., Jr. Leaching of metabolites from above-ground plant parts and its implications. Bull. Torrey Bot. Club 1966, 93, 385–401. [Google Scholar] [CrossRef]
- Maie, N.; Jaffé, R.; Miyoshi, T.; Childers, D.L. Quantitative and qualitative aspects of dissolved organic carbon leached from senescent plants in an oligotrophic wetland. Biogeochemistry 2006, 78, 285–314. [Google Scholar] [CrossRef]
- Tukey, H.B., Jr.; Tukey, H.B.; Wittwer, S.H. Loss of nutrients by foliar leaching as determined by radioisotopes. Proc. Am. Soc. Hort. Sci. 1958, 71, 496. [Google Scholar]
- Chapin, F.S., III. Effects of multiple stresses on nutrient availability and use. In Response of Plants to Multiple Stresses; Mooney, H.A., Winner, W.E., Pell, E.J., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 67–88. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Bodenbiologische Arbeitsmethoden, 2nd ed.; Springer: Berlin, Germany, 1993. [Google Scholar]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Revs. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D. Gradual accumulation of heavy metals in an industrial wheat crop from uranium mine soil and the potential use of the herbage. Agriculture 2016, 6, 51. [Google Scholar] [CrossRef]
- Huang, H.; Guo, S.; Chen, G. Reproductive biology in an invasive plant Solidago canadensis. Front. Biol. China 2007, 2, 196–204. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Cogle, A.L.; Saffigna, P.G.; Strong, W.M. Carbon transformations during wheat straw decomposition. Soil Biol. Biochem. 1989, 21, 367–372. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singha, B.P.; Cowie, A.L.; Fang, Y.; Collins, D.; Dougherty, W.J.; Singh, B.K. Carbon and nutrient mineralisation dynamics in aggregate-size classes from different tillage systems after input of canola and wheat residues. Soil Biol. Biochem. 2018, 116, 22–38. [Google Scholar] [CrossRef]
- Corre, N.; Bouchart, V.; Ourry, A.; Boucaud, J. Mobilization of nitrogen reserves during regrowth of defoliated Trifolium repens L., and identification of potential vegetative storage proteins. J. Exp. Bot. 1996, 301, 1111–1118. [Google Scholar] [CrossRef]
- Cunningham, S.M.; Volenec, J.J. Purification and characterization of vegetative storage proteins from alfalfa (Medicago sativa L.) taproots. J. Plant Physiol. 1996, 147, 625–632. [Google Scholar] [CrossRef]
- Dierking, R.M.; Allen, D.J.; Cunningham, S.M.; Brouder, S.M.; Volenec, J.J. Nitrogen reserve pools in two Miscanthus × giganteus genotypes under contrasting N managements. Front. Plant Sci. 2017, 8, 1618. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Volenec, J.J.; Joern, B.C.; Cunningham, S.M. Seasonal changes in nonstructural carbohydrates, protein, and macronutrients in roots of alfalfa, red clover, sweetclover, and birdsfoot trefoil. Crop Sci. 1996, 36, 617–623. [Google Scholar] [CrossRef]
- Rice Straw and Wheat Straw—Potential Feedstocks for the Biobased Economy. Available online: https://library.wur.nl/WebQuery/wurpubs/reports/448025 (accessed on 5 December 2017).
- Steinmann, T.; Welp, G.; Wolf, A.; Holbeck, B.; Große-Rüschkamp, T.; Amelung, W. Repeated monitoring of organic carbon stocks after eight years reveals carbon losses from intensively managed agricultural soils in Western Germany. J. Plant Nutr. Soil Sci. 2016, 179, 355–366. [Google Scholar] [CrossRef]
- Early, R. Manage stubble load to save soil moisture. Farming Ahead 2004, 146, 31–32. [Google Scholar]
- Cereal Stubble Management On-Farm Demonstrations and Case Studies 2009. Available online: http://murrumbidgeelandcare.org.au/files/Stubble-Report-2009-results.pdf (accessed on 10 November 2017).
- Kanders, M.J.; Berendonk, C.; Fritz, C.; Watson, C.; Wichern, F. Catch crops store more nitrogen below-ground when considering rhizodeposits. Plant Soil 2017, 417, 287–299. [Google Scholar] [CrossRef]
- Wallace, J.M.; Williams, A.; Liebert, J.A.; Ackroyd, V.J.; Vann, R.A.; Curran, W.S.; Keene, C.L.; VanGessel, M.J.; Ryan, M.R.; Mirsky, S.B. Cover crop-based, organic rotational no-till corn and soybean production systems in the Mid-Atlantic United States. Agriculture 2017, 7, 34. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Liang, Y.; Niu, J.; Xiao, Y.; Gu, Y.; Ma, L.; Meng, D.; Zhang, Y.; Huang, W.; et al. Maize growth responses to soil microbes and soil properties after fertilization with different green manures. Appl. Microbiol. Biotechnol. 2017, 101, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Yang, X.; Huang, S. Contributions of wheat and maize residues to soil organic carbon under long-term rotation in north China. Sci. Rep. 2015, 5, 11409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.S.; Mubeen, U. Wheat straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Xu, K.; Liu, M.; Chen, J.-D.; Gu, H.-Y.; Dai, Q.-G.; Ma, K.-Q.; Jiang, F.; He, L. Effects of wheat straw returning into paddy soil on dissolved organic carbon contents and rice grain yield. Yingyong Shengtai Xuebao 2015, 26, 430–436. [Google Scholar] [PubMed]
- George, J.F.; Smeins, F.E. Decomposition of common curlymesquite herbage on Edwards Plateau Rangeland, Texas. J. Range Manag. 1982, 35, 104–106. [Google Scholar] [CrossRef]
- Straw Decomposition. Available online: https://eprints.nwisrl.ars.usda.gov/1147/1/93.pdf (accessed on 24 October 2018).
- Halsall, D.M.; Gibson, A.H. Comparison of two Cellulomonas strains and their interaction with Azospirillum brasilense in degradation of wheat straw and associated nitrogen fixation. Appl. Environ. Microbiol. 1986, 51, 855–861. [Google Scholar] [PubMed]
- Lynch, J.M.; Harper, S.H.T. Straw as a substrate for cooperative nitrogen fixation. J. Gen. Microbiol. 1983, 129, 251–253. [Google Scholar] [CrossRef]
- Schnitzer, M.; Monreal, C.M.; Powell, E.E. Wheat straw biomass: A resource for high-value chemicals. J. Environ. Sci. Health 2014, 49, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Susic, M. Replenishing humic acids in agricultural soils. Agronomy 2016, 6, 45. [Google Scholar] [CrossRef]
- Knicker, H.; Fründ, R.; Lüdemann, H.-D. The chemical nature of nitrogen in native soil organic matter. Naturwissenschaften 1993, 80, 219–221. [Google Scholar] [CrossRef] [Green Version]
- Sonnenberg, L.B.; Johnson, J.D.; Christman, R.F. Chemical degradation of humic substances for structural characterization. In Aquatic Humic Substances: Influence on Fate and Treatment of Pollutants; American Chemical Society: Washington, DC, USA, 1989; pp. 3–23. [Google Scholar]
- Woods, G.C.; Simpson, M.J.; Koerner, P.J.; Napoli, A.; Simpson, A.J. HILIC-NMR: Toward the identification of individual molecular components in dissolved organic matter. Environ. Sci. Technol. 2011, 45, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Jia, B.; Lv, J.; Ma, Q.; Kuzyakov, Y.; Li, F.-M. Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol. Biochem. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Reid, J.B.; Goss, M.J. Suppression of decomposition of 14C-labelled plant roots in the presence of living roots of maize and perennial ryegrass. J. Soil Sci. 1982, 33, 387–395. [Google Scholar] [CrossRef]
- Cookson, W.R.; Beare, M.H.; Wilson, P.E. Effects of prior crop residue management on microbial properties and crop residue decomposition. Appl. Soil Ecol. 1998, 7, 179–188. [Google Scholar] [CrossRef]
- Rezig, F.A.M.; Elhadi, E.A.; Abdalla, M.R. Decomposition and nutrient release pattern of wheat (Triticum aestivum) residues under different treatments in desert field conditions of Sudan. Int. J. Recycl. Org. Waste Agric. 2014, 3, 69. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Wei, J.; Zhang, Y.; Zhang, L.; Chang, J.; Thompson, M.L. Decomposition dynamics and changes in chemical composition of wheat straw residue under anaerobic and aerobic conditions. PLoS ONE 2016, 11, e0158172. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J. Humus Chemistry, 2nd ed.; J. Wiley and Sons: New York, NY, USA, 1994. [Google Scholar]
- Wei, T.; Zhang, P.; Wang, K.; Ding, R.; Yang, B.; Nie, J.; Jia, Z.; Han, Q. Effects of wheat straw incorporation on the availability of soil nutrients and enzyme activities in semiarid areas. PLoS ONE 2015, 10, e0120994. [Google Scholar] [CrossRef] [PubMed]





| Soil Type | As | Ca | Cd | Cr | Cs | Cu | Fe | K | Mg |
| A | 11.1 | 44,520 | 0.35 | 31.7 | 1.91 | 32.2 | 24,170 | 3130 | 8320 |
| B | 3.62 | 1056 | 0.105 | 12.9 | 0.990 | 8.4 | 6592 | 1749 | 1364 |
| Soil Type | Mn | Ni | NO3-N | NH4-N | P | Pb | Sr | U | Zn |
| A | 750 | 31.3 | 68 | ND | 685 | 37.7 | 150 | 1.18 | 84 |
| B | 198 | 6.49 | 0 | 27 | 385 | 9.4 | ND | 0.547 | 33.1 |
| Biomass | Ca | K | Mg | N | P | Zn | |
|---|---|---|---|---|---|---|---|
| Biomass at Anthesis, 23 August | |||||||
| Whole shoots incl. stubbles | 10,070 | 81.9 (8.14) | 143 (14.2) | 13.8 (1.37) | 98.4 (9.77) | 22.9 (2.27) | 0.242 (0.024) |
| Whole roots | 2850 | 23.9 (8.39) | 35.1 (12.3) | 2.73 (0.957) | 20.9 (7.33) | 5.30 (1.86) | 0.070 (0.025) |
| 16-cm stubbles section | 1085 | 3.94 (3.63) | 12.2 (11.2) | 0.405 (0.373) | 3.79 (3.49) | 1.24 (1.15) | 0.015 (0.014) |
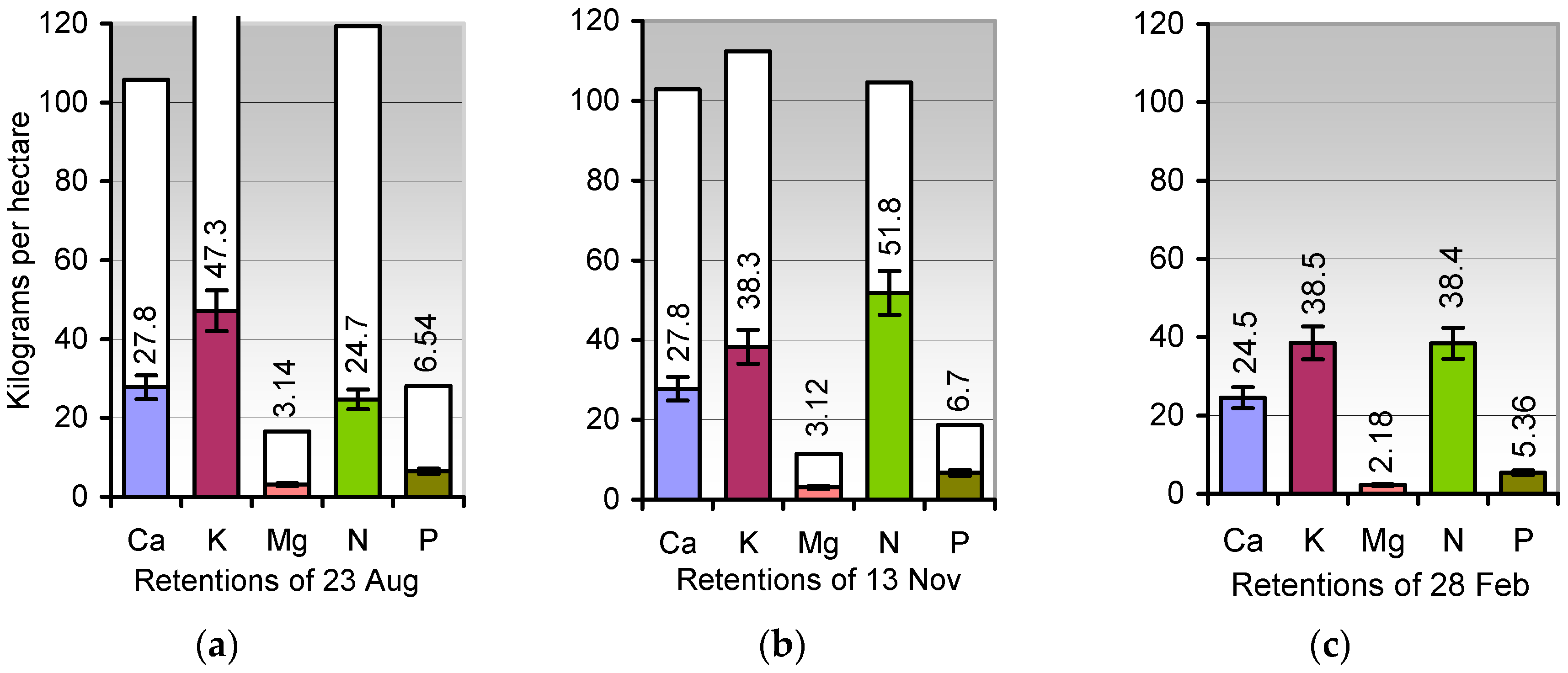
| Roots/stubbles vs. 23 August a | 3935 (30.5%) | 27.8 (26.3%) | 47.3 (26.6%) | 3.14 (19.0%) | 24.7 (20.7%) | 6.54 (23.2%) | 0.085 (27.2%) |
| Shoot Biomass at Maturity, 13 November, and Total Offtake by Harvest | |||||||
| Whole shoots incl. stubbles | 7778 | 79.0 (10.2) | 77.5 (9.97) | 8.73 (1.12) | 54.7 (7.03) | 12.2 (1.57) | 0.271 (0.035) |
| Total offtake c | 6940 | 74.9 | 74.1 | 8.36 | 52.7 | 11.9 | 0.255 |
| Remaining Postharvest Biomass, 13 November | |||||||
| Stubbles 16 cm | 838 | 4.10 (4.90) | 3.42 (4.08) | 0.368 (0.439) | 2.01 (2.40) | 0.287 (0.343) | 0.016 (0.019) |
| Whole roots | 2850 | 23.7 (8.32) | 34.9 (12.2) | 2.75 (0.964) | 49.7 (17.5) | 6.40 (2.25) | 0.083 (0.029) |
| Roots/stubbles vs. 13 November b | 3688 (34.7%) | 27.8 (27%) | 38.3 (34.1%) | 3.12 (27.2%) | 51.8 (49.6%) | 6.69 (36%) | 0.099 (28%) |
| Postharvest Biomass at Resprouting, 28 February | |||||||
| Stubbles 16 cm | 838 | 4.49 (5.36) | 1.77 (2.11) | 0.360 (0.430) | 2.10 (2.50) | 0.234 (0.279) | 0.014 (0.017) |
| Whole roots | 2850 | 20.0 (7.03) | 36.7 (12.9) | 1.82 (0.637) | 36.3 (12.8) | 5.13 (1.80) | 0.065 (0.023) |
| Roots/stubbles | 3688 | 24.5 | 38.5 | 2.18 | 38.4 | 5.36 | 0.079 |
| Biomass | Ca | K | Mg | Norg | NO3-N | P | Zn | |
|---|---|---|---|---|---|---|---|---|
| Biomass at Harvest, 23 July | ||||||||
| Straw without stubbles | 5980 ± 550 | 13.7 (2.29) | 99.3 (16.6) | 4.81 (0.805) | 53.2 (8.9) | 7.00 (1.17) | 6.82 (1.14) | 0.060 (0.010) |
| Grains | 7090 ± 670 | 3.20 (0.452) | 29.4 (4.15) | 9.14 (1.29) | 160 (22.6) | 0 (0) | 25.7 (3.62) | 0.220 (0.031) |
| Stubbles | 2772 ± 84 | 3.82 (1.38) | 62.0 (22.4) | 1.19 (0.431) | 31.6 (11.4) | 7.57 (2.73) | 2.49 (0.900) | 0.022 (0.008) |
| Spilt grains | 116 ± 9 | 0.052 (0.452) | 0.482 (4.15) | 0.149 (1.29) | 2.62 (22.6) | 0 (0) | 0.420 (3.62) | 0.004 (0.031) |
| Stubbles plus spilt grains a | 2888 ± 85 (18%) | 3.87 (18.6%) | 62.5 (32.7%) | 1.34 (8.8%) | 41.8 (16%) | 2.91 (8.2%) | 0.026 (8.5%) | |
| Biomass after Emergence of Wheat Regrowth, 19 September | ||||||||
| Stubbles | 2243 ± 115 | 4.27 (1.90) | 13.0 (5.81) | 1.13 (0.503) | 26.0 (11.6) | 0.323 (0.144) | 3.01 (1.34) | 0.028 (0.013) |
| Regrowth | 1106 ± 7 | 3.72 (3.36) | 31.5 (28.5) | 0.991 (0.896) | 24.9 (22.5) | 0.954 (0.863) | 4.12 (3.73) | 0.028 (0.025) |
| Biomass at the Outset of Winter, 1 December | ||||||||
| Stubbles | 2088 ± 107 | 4.00 (1.91) | 4.59 (2.20) | 0.992 (0.475) | 11.3 (5.4) | 0.223 (0.107) | 0.983 (0.471) | 0.018 (0.009) |
| Regrowth | 897 ± 7 | 2.58 (2.87) | 18.3 (20.4) | 0.761 (0.848) | 22.2 (24.8) | 0.850 (0.946) | 3.20 (3.57) | 0.029 (0.032) |
| Biomass at the End of Winter, 28 February | ||||||||
| Stubbles | 1867 ± 44 | 5.66 (3.03) | 2.67 (1.43) | 1.21 (0.650) | 13.8 (7.4) | 0 (0) | 1.18 (0.633) | 0.022 (0.012) |
| Regrowth | 977 ± 7 | 3.03 (3.10) | 19.3 (19.7) | 1.06 (1.08) | 27.8 (28.4) | 0.340 (0.348) | 3.72 (3.81) | 0.039 (0.040) |
| Biomass | Ca | K | Mg | Norg | NO3-N | P | Zn | |
|---|---|---|---|---|---|---|---|---|
| Biomass Monitored on 19 September Ploughed down on 28 September | ||||||||
| Stubbles | 2243 ± 115 | 4.27 (1.90) | 13.0 (5.81) | 1.13 (0.503) | 26.0 (11.6) | 0.323 (0.144) | 3.01 (1.34) | 0.028 (0.013) |
| Regrowth | 1106 ± 7 | 3.72 (3.36) | 31.5 (28.5) | 0.991 (0.896) | 24.9 (22.5) | 0.954 (0.863) | 4.12 (3.73) | 0.028 (0.025) |
| Biomass at the Outset of Winter, 1 December | ||||||||
| Stubbles | 1622 ± 86 | 7.59 (4.68) | 3.52 (2.17) | 1.18 (0.726) | 20.9 (12.9) | 0 (0) | 1.82 (1.12) | 0.022 (0.013) |
| Regrowth | 709 ± 26 | 5.18 (7.31) | 2.73 (3.85) | 1.00 (1.41) | 14.4 (20.3) | 0.013 (0.019) | 2.18 (3.07) | 0.026 (0.036) |
| Seedlings | 215 ± 28 | 0.817 (3.80) | 10.7 (49.7) | 0.376 (1.75) | 8.2 (38.0) | 1.73 (8.04) | 1.12 (5.21) | 0.009 (0.040) |
| Biomass at the End of Winter, 28 February | ||||||||
| Stubbles | 1568 ± 84 | 8.73 (5.57) | 3.45 (2.20) | 1.41 (0.900) | 23.0 (14.7) | 0 (0) | 2.05 (1.31) | 0.28 (0.18) |
| Regrowth | 669 ± 52 | 6.96 (10.4) | 2.52 (3.77) | 1.18 (1.76) | 14.4 (21.5) | 0.064 (0.096) | 2.96 (4.43) | 0.032 (0.048) |
| Seedlings | 454 ± 59 | 2.00 (4.41) | 20.2 (44.4) | 0.808 (1.78) | 14.3 (31.5) | 0.825 (1.82) | 1.78 (3.91) | 0.014 (0.030) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramss, G.; Voigt, K.-D. Turnover of Minerals and Organics in the Postharvest Herbage of Annuals and Perennials: Winter Wheat and Goldenrod. Agriculture 2018, 8, 170. https://doi.org/10.3390/agriculture8110170
Gramss G, Voigt K-D. Turnover of Minerals and Organics in the Postharvest Herbage of Annuals and Perennials: Winter Wheat and Goldenrod. Agriculture. 2018; 8(11):170. https://doi.org/10.3390/agriculture8110170
Chicago/Turabian StyleGramss, Gerhard, and Klaus-Dieter Voigt. 2018. "Turnover of Minerals and Organics in the Postharvest Herbage of Annuals and Perennials: Winter Wheat and Goldenrod" Agriculture 8, no. 11: 170. https://doi.org/10.3390/agriculture8110170





