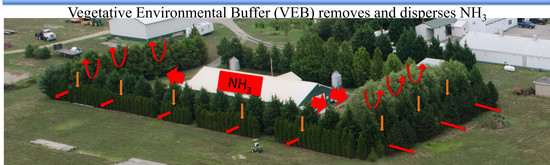
Enhanced Dispersion and Removal of Ammonia Emitted from a Poultry House with a Vegetative Environmental Buffer
Abstract
:1. Introduction
2. Materials and Methods
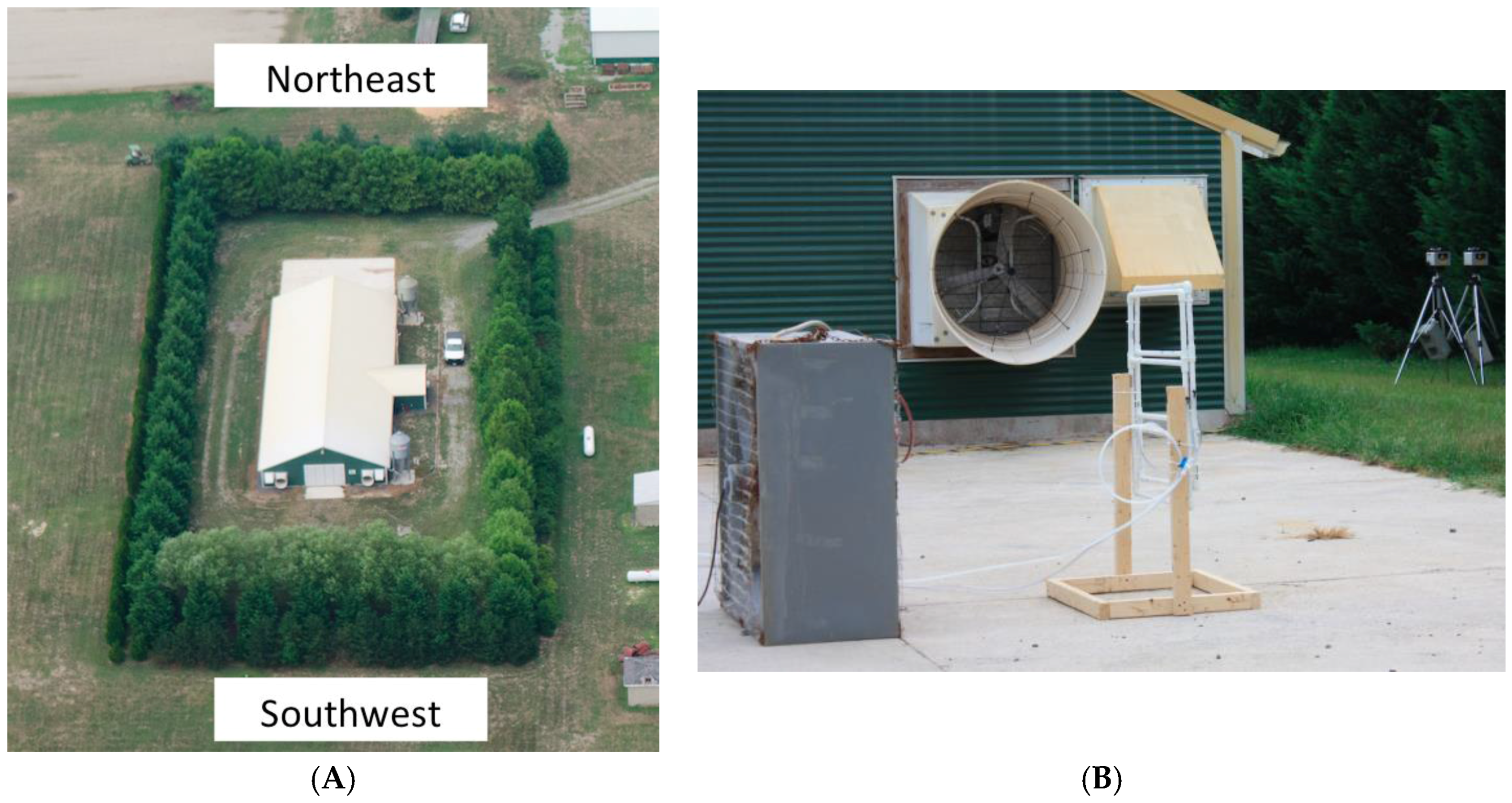
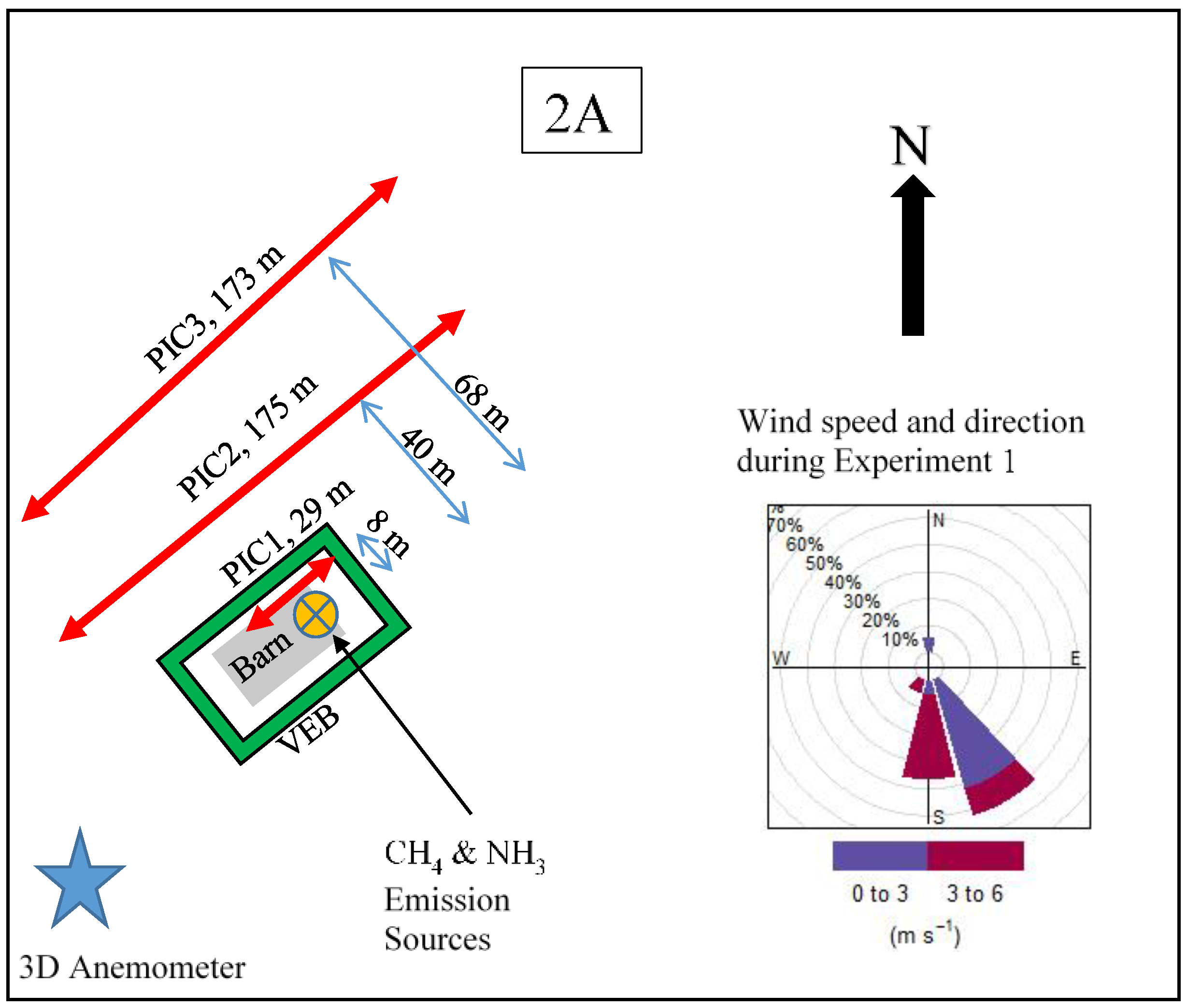
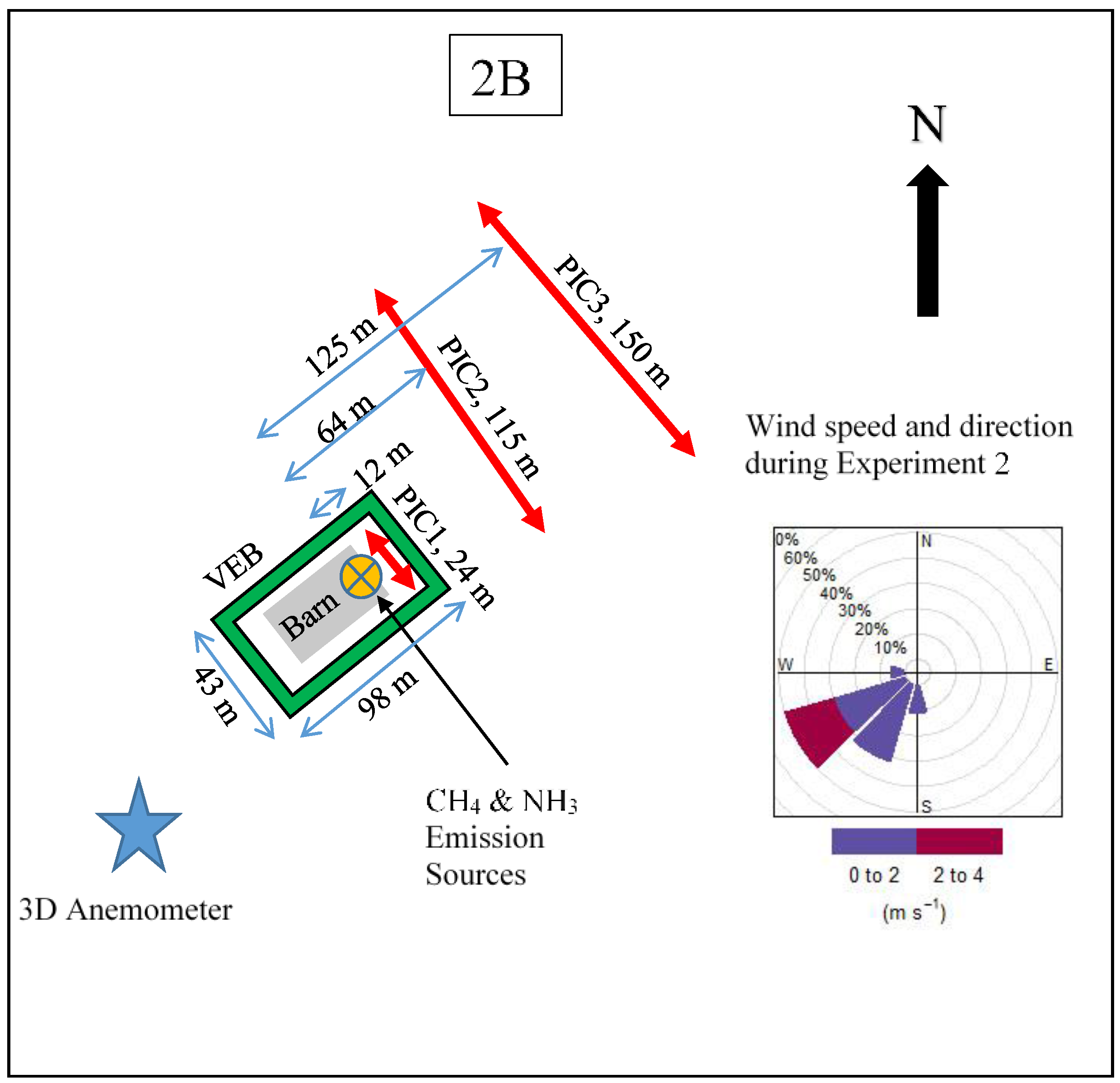
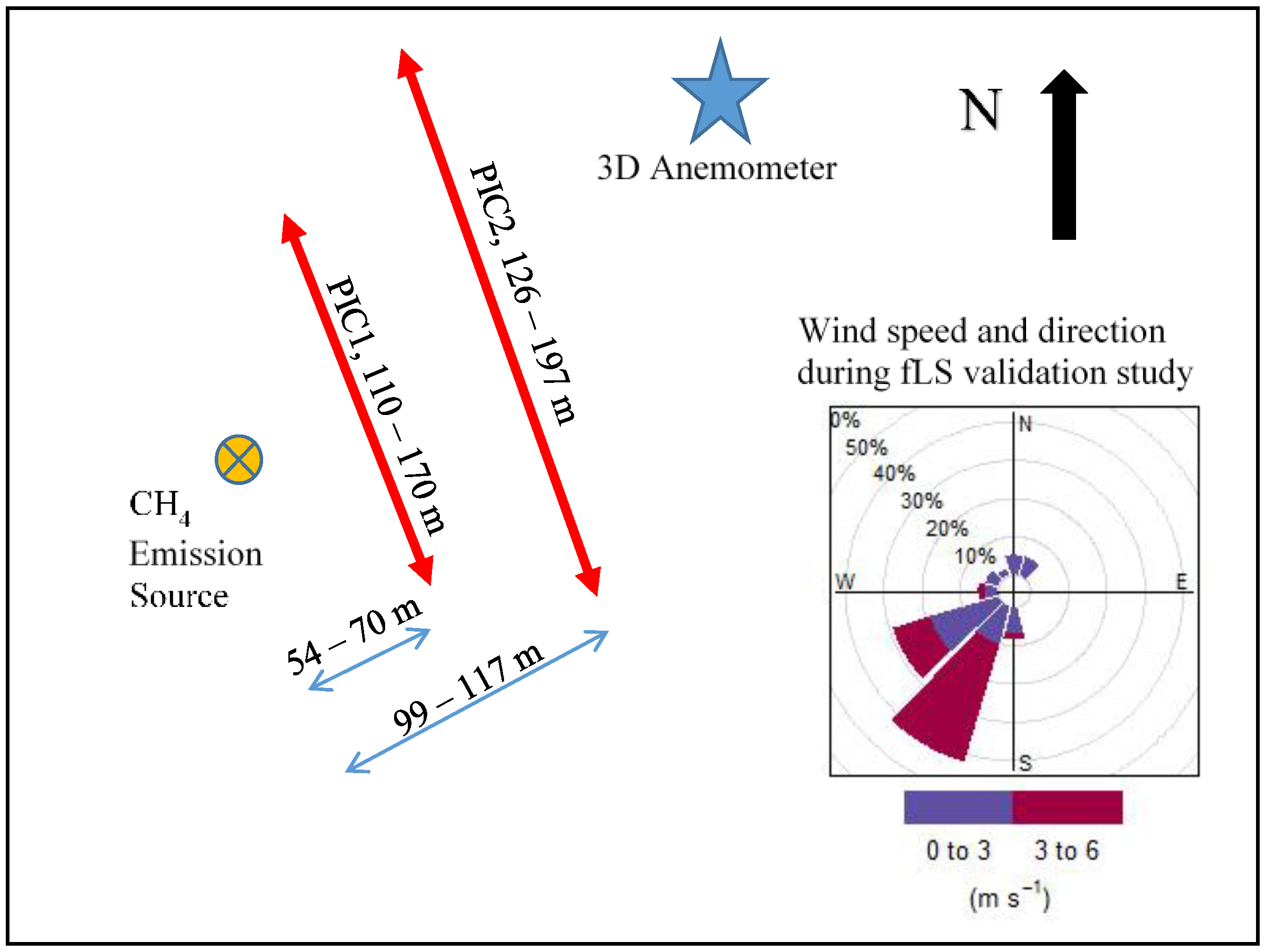
2.1. Experimental Site
2.2. Simulated Emissions
2.3. Sensors
- Obukhov stability length scale, |L| ≥ 5 m (i.e., avoid highly stable/unstable atmospheric stratification);
- friction velocity u* ≥ 0.1 m/s; and,
- net changes in PICs due to release of tracer gases > resolutions of each TDLs.
2.4. Forward Lagrangian Stochastic (fLS) Technique Validation Study
3. Results and Discussion
3.1. Accuracy of the fLS Technique
3.2. Enhanced Dispersion by a VEB
3.3. Removal of NH3 by the VEB
3.4. Increase in Gas Concentrations within the VEB
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Ro, K.S.; McConnell, L.L.; Melvin, J.H.; Hunt, P.G.; Parker, D. Livestock air treatment using PVA-coated powdered activated carbon biofilter. Appl. Eng. Agric. 2008, 24, 791–798. [Google Scholar]
- Ro, K.S.; Lima, I.M.; Reddy, G.B.; Jackson, M.A.; Gao, B. Removing gaseous NH3 using biochar as an adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef]
- Jones, W.; Morning, K.; Olenchock, S.A.; Williams, T.; Hickey, J. Environmental study of poultry confnement building. Am. Ind. Hyg. Assoc. J. 1984, 45, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Adrizal, A.; Patterson, P.H.; Hulet, R.M.; Bates, R.M.; Myers, C.A.B.; Martin, G.P.; Shockey, R.L.; Van der Grinten, M.; Anderson, D.A.; Thompson, J.R. Vegetative buffers for fan emissions from poultry farms: 2. ammonia, dust and foliar nitrogen. J. Environ. Sci. Health Part B 2008, 43, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic effects of dust and ammonia on the occupantional health effects of poultry production workers. J. Agromed. 2002, 2, 57–76. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Air Emissions from Animal Feeding Operations: Current Knowledge, Future Needs; NRC: Washington, DC, USA, 2003. [Google Scholar]
- SJVAPCD 1-Hour Extreme Ozone Attainment. Available online: http://www.valleyair.org/Air_Quality_Plans/AQ_plans_Ozone_Final.htm (accessed on 9 February 2018).
- SJVAPCD 8-Hour Ozone Plan. Available online: http://www.valleyair.org/Air_Quality_Plans/AQ_Final_Adopted_Ozone2007.htm (accessed on 9 February 2018).
- Belt, S.V.; VanDerGrinten, M.; Malone, G.; Patterson, P.H.; Shockey, R.L. Windbreak Plant Species for Odor Management around Poulty Production Facilities; National Plant Materials Center: Beltsville, MD, USA, 2007.
- Belt, S.V. Utilizing Warm-Season Grasses to Mitigate Poultry Tunnel Fan Emissions on the Delmarva Peninsula; National Plant Materials Center: Beltsville, MD, USA, 2010.
- Tyndall, J.; Colletti, J. Mitigating swine odor with strategically designed shelterbelt systems: A review. Agrofor. Syst. 2007, 69, 45–65. [Google Scholar] [CrossRef]
- Parker, D.B.; Malone, G.W.; Walter, W.D. Vegetative environmental buffers and exhaust fan deflectors for reducing downwind odor and VOCs from tunnel-ventilated swine barns. Trans. ASABE 2012, 55, 227–240. [Google Scholar] [CrossRef]
- Malone, G.; Van Wicklen, G.; Collier, S.; Hansen, D. Efficacy of Vegetative Environmental Buffers to Capture Emissions from Tunnel Ventilated Poulty Houses; Raleigh: Potomac, MD, USA, 2006; pp. 875–878. [Google Scholar]
- Malone, B. Using trees to reduce dust and odour emissions from poultry farms. In Proceedings of the Poutry Information Exchange, Surfers Paradise, Queensland, Australia, 14 April 2004; pp. 33–38. [Google Scholar]
- Patterson, P.H.; Adriazal, A. Management strategies to reduce air emissions: Emphasis—Dust and ammonia. J. Appl. Poult. Res. 2005, 14, 638–650. [Google Scholar] [CrossRef]
- Ro, K.S.; Moore, P.A.; Szogi, A.A.; Millner, P.D. Ammonia and nitrous oxide emissions from broiler houses with downtime windrowed litter. J. Environ. Qual. 2017, 46, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Flesch, T.K.; Wilson, J.D.; Harper, L.A.; Brian, P.C. Estimating gas emissions from a farm with an inverse-despersion technique. Atmos. Environ. 2005, 39, 4863–4874. [Google Scholar] [CrossRef]
- Flesch, T.K.; Wilson, J.D.; Harper, L.A.; Todd, R.W.; Cole, N.A. Determining ammonia emissions from a cattle feedlot with an inverse dispersion technique. Agric. For. Meteorol. 2007, 144, 139–155. [Google Scholar] [CrossRef]
- Harper, L.A.; Flesch, T.K.; Powell, J.M.; Coblentz, W.K.; Jokela, W.E.; Martin, N.P. Ammonia emissions from dairy production in Wisconsin. J. Dairy Sci. 2009, 92, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.A.; Flesch, T.K.; Wilson, J.D. Ammonia emissions from broiler production in the San Joaquin Valley. Poult. Sci. 2010, 89, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.S.; Johnson, M.H.; Hunt, P.G.; Flesch, T.K. Measuring trace gas emission from multi-distributed sources using vertical radial plume mapping (VRPM) and backward Lagrangian stochastic (bLS) techniques. Atmosphere 2011, 2, 553–566. [Google Scholar] [CrossRef]
- Ro, K.S.; Johnson, M.H.; Stone, K.C.; Hunt, P.G.; Flesch, T.K.; Todd, R.W. Measuring gas emissions from animal waste lagoons with an inverse-dispersion technique. Atmos. Environ. 2013, 66, 101–106. [Google Scholar] [CrossRef]
- Ro, K.S.; Stone, K.C.; Johnson, M.H.; Hunt, P.G.; Flesch, T.K.; Todd, R.W. Optimal Sensor Locations for the Backward Lagrangian Stochastic Technique in Measuring Lagoon Gas Emission. J. Environ. Qual. 2014, 43, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.A.; Flesch, T.K.; Weaver, K.H.; Wilson, J.D. The effect of biofuel production on swine farm ammonia and methane emissions. J. Environ. Qual. 2010, 39, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.A.; Denmead, O.T.; Flesch, T.K. Micrometeorological techniques for measurement of greenhouse gas emissions from ruminant animal production. Anim. Feed Sci. Technol. 2011, 166–167, 227–239. [Google Scholar] [CrossRef]
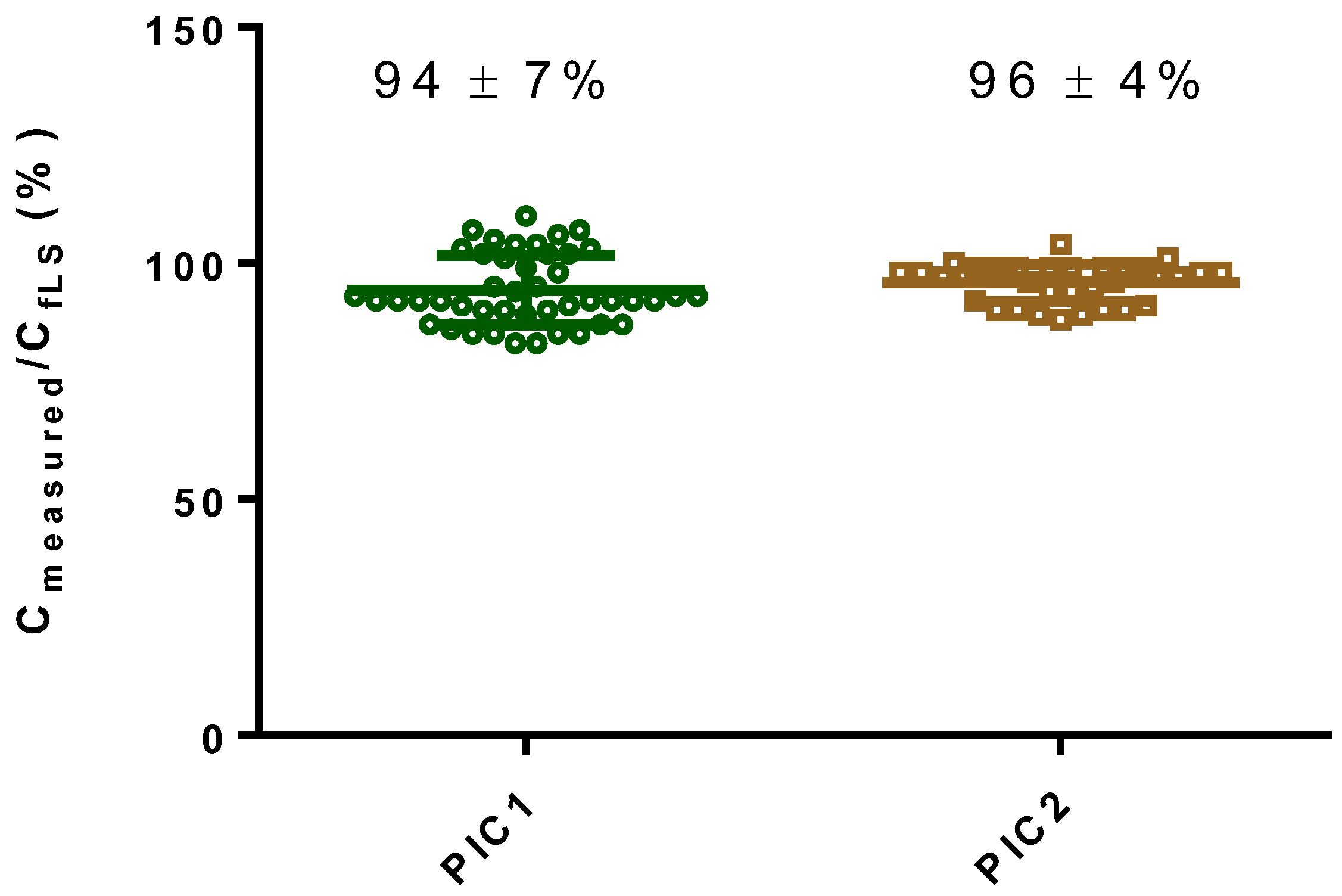

| Date | Wind Speed (m/s) | Friction Velocity u* (m/s) | Percent Accuracy for PIC1 (%) Cmeasured/CfLS | Percent Accuracy for PIC2 (%) Cmeasured/CfLS | Number of Runs per Day |
|---|---|---|---|---|---|
| 14 March 2014 | 3.4 ± 0.5 | 0.33 ± 0.07 | 105 ± 3 | 90 ± 1 | 21 |
| 20 March 2014 | 1.7 ± 0.7 | 0.22 ± 0.06 | 96 ± 4 | 96 ± 2 | 7 |
| 1 April 2014 | 2.8 ± 0.4 | 0.25 ± 0.04 | 92 ± 4 | 99 ± 3 | 16 |
| 10 April 2014 | 3.2 ± 0.4 | 0.33 ± 0.06 | 89 ± 4 | 98 ± 1 | 37 |
| All Runs | 2.8 ± 0.8 | 0.29 ± 0.07 | 94 ± 7 | 96 ± 4 | 86 |
| PICs | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| Cmeasured/CfLS† | ΔCmeasured/ΔCfLS ‡ | Cmeasured/CfLS† | ΔCmeasured/ΔCfLS ‡ | |
| Average of PIC1 | 1.67 ± 0.54 a (14) | 2.21 ± 0.87 c (14) | 1.49 ± 0.36 a (14) | 1.83 ± 0.70 c (14) |
| Averageof PIC2 | 0.94 ± 0.06 b (13) | 0.71 ± 0.22 d (13) | 0.91 ± 0.15 b (5) | 0.78 ± 0.72 d (6) |
| Averageof PIC3 | 0.93 ± 0.07 b (13) | 0.55 ± 0.23 d (13) | 0.93 ± 0.06 b (10) | 0.56 ± 0.27 d (10) |
| Average of PIC2 and PIC3 | 0.93 ± 0.06 | 0.63 ± 0.23 | 0.92 ± 0.10 | 0.63 ± 0.34 |
| Location of PICs | Experiment 1 | Experiment 2 |
|---|---|---|
| PIC1 | 0.97 ± 0.13 a,† (14) | 1.00 ± 0.08 a (14) |
| Overall average for PIC1 in Experiments 1 and 2 | 0.99 ± 0.11 | |
| PIC2 | 0.64 ± 0.39 a (8) | 0.94 ± 0.60 a (5) |
| PIC3 | 0.68 ± 0.30 a (6) | 0.90 ± 0.68 a (8) |
| Average of PIC2 and PIC3 | 0.66 ± 0.34 | 0.92 ± 0.62 |
| Overall average of PIC2 and PIC3 in Experiments 1 and 2 | 0.78 ± 0.50 | |
| Location of PICs | Experiment 1 | Experiment 2 |
|---|---|---|
| PIC1 | 2.31 ± 1.00 a (14) | 1.90 ± 0.61 a (14) |
| Average of PIC1 for Experiments 1 and 2 | 2.10 ± 0.84 | |
| PIC2 | 0.55 ± 0.35 b (8) | 0.38 ± 0.17 b (13) |
| PIC3 | 0.46 ± 0.13 b (6) | 0.65 ± 0.27 b (10) |
| Average of PIC2 and PIC3 | 0.51 ± 0.27 | 0.50 ± 0.27 |
| Overall average of PIC2 & PIC3 for Experiments 1 and 2 | 0.51 ± 0.26 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ro, K.S.; Li, H.; Hapeman, C.J.; Harper, L.A.; Flesch, T.K.; Downey, P.M.; McConnell, L.L.; Torrents, A.; Yao, Q. Enhanced Dispersion and Removal of Ammonia Emitted from a Poultry House with a Vegetative Environmental Buffer. Agriculture 2018, 8, 46. https://doi.org/10.3390/agriculture8040046
Ro KS, Li H, Hapeman CJ, Harper LA, Flesch TK, Downey PM, McConnell LL, Torrents A, Yao Q. Enhanced Dispersion and Removal of Ammonia Emitted from a Poultry House with a Vegetative Environmental Buffer. Agriculture. 2018; 8(4):46. https://doi.org/10.3390/agriculture8040046
Chicago/Turabian StyleRo, Kyoung S., Hong Li, Cathleen J. Hapeman, Lowry A. Harper, Thomas K. Flesch, Peter M. Downey, Laura L. McConnell, Alba Torrents, and Qi Yao. 2018. "Enhanced Dispersion and Removal of Ammonia Emitted from a Poultry House with a Vegetative Environmental Buffer" Agriculture 8, no. 4: 46. https://doi.org/10.3390/agriculture8040046