SOC Stock Changes and Greenhouse Gas Emissions Following Tropical Land Use Conversions to Plantation Crops on Mineral Soils, with a Special Focus on Oil Palm and Rubber Plantations
Abstract
:1. Introduction
2. Materials and Methods
3. Effects of Land Use Change on SOC Stocks
3.1. SOC Stocks under Different Ecosystems and Land Use Conversions
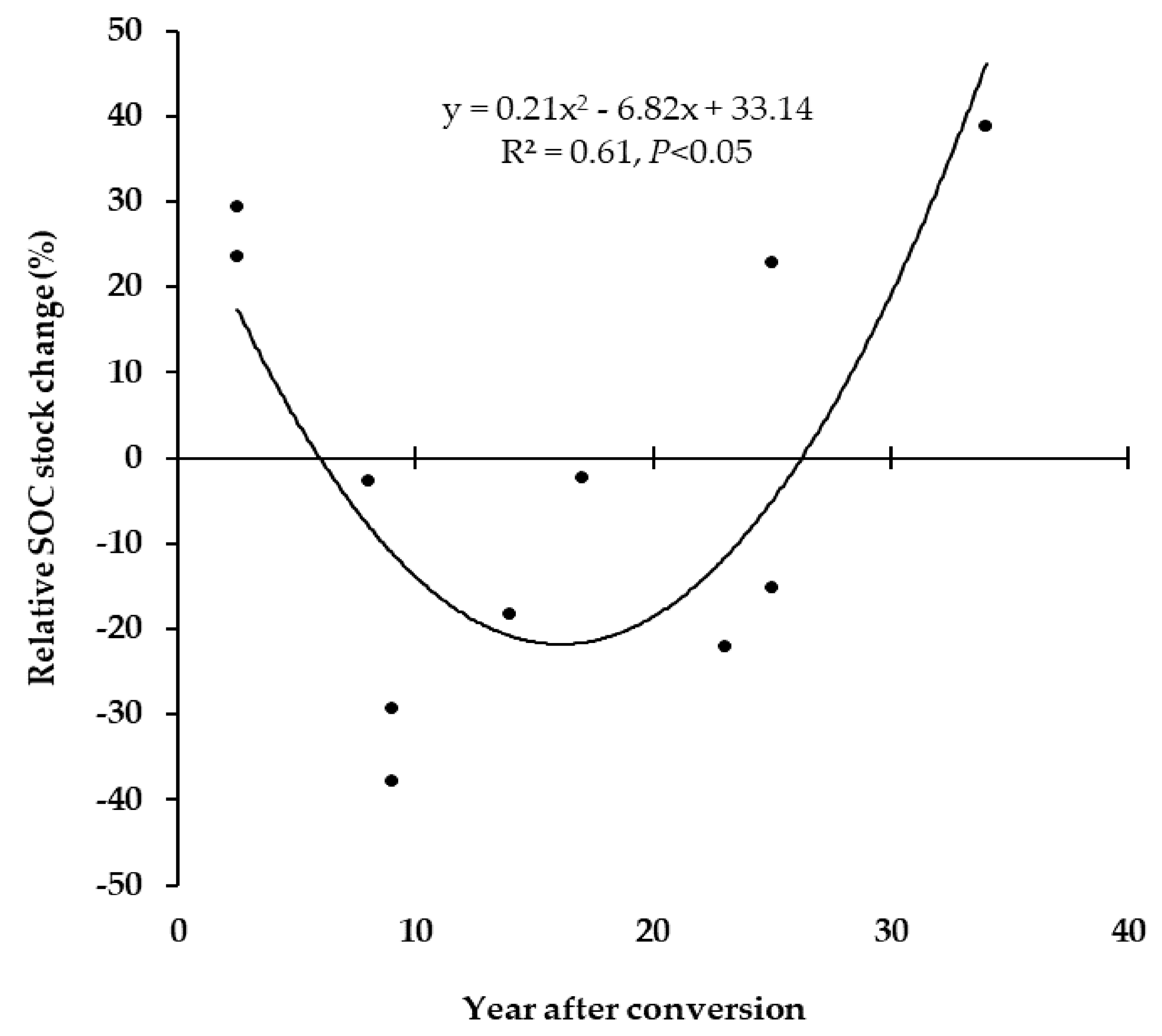
3.2. Time Trend in SOC Changes Following Land Use Change
3.3. Magnitude of SOC Losses Due to Soil Erosion
4. Effect of Different Land Uses on GHG Fluxes from Tropical Mineral Soils
Changes in Total GHG Emissions Following Land Use Change
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNCTAD. Palm Oil. 2016. Available online: http://unctad.org/en/PublicationsLibrary/INFOCOMM_cp08_PalmOil_fr.pdf (accessed on 12 March 2017).
- Wahid, M.B.; Akmar-Abdullah, S.N.; Henson, I.E. Oil palm—Achievements and potential. Plant Prod. Sci. 2005, 8, 288–297. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R.; Bhatia, S. Palm oil: Addressing issues and towards sustainable development. Renew. Sustain. Energy Rev. 2009, 13, 420–427. [Google Scholar] [CrossRef]
- UNEP. Oil Palm Plantations: Threats and Opportunities for Tropical Ecosystems. Global Environmental Alert Service (GEAS). Taking the Pulse of the Planet: Connecting Science with Policy (December Issue). Available online: www.unep.org/geas (accessed on 2 October 2015).
- UNEP. UNEP and Roundtable on Sustainable Palm Oil Sign New Agreement. Available online: http://web.unep.org/newscentre/unep-and-roundtable-sustainable-palm-oil-sign-new-agreement (accessed on 12 March 2017).
- Weicke, B.; Sikkema, R.; Dornburg, V.; Faaij, A. Exploring land use changes and the role of palm oil production in Indonesia and Malaysia. Land Use Policy 2011, 28, 193–206. [Google Scholar] [CrossRef]
- UNCTAD. Infocomm Commodity Profile, Palm Oil. Available online: http://www.unctad.info/en/Infocomm/AACP-Products/Palm-oil/ (accessed on 2 October 2015).
- Gunarso, P.; Hartoyo, M.E.; Agus, F.; Killeen, T.J. Oil palm and land use change in Indonesia, Malaysia and Papua New Guinea. Reports from the Technical Panels of the 2nd Greenhouse Gas Working Group of the Roundtable on Sustainable Palm Oil (RSPO). Available online: www.rspo.org (accessed on 2 October 2015).
- HCS. High Carbon Stock Scientific Study. The Sustainable Palm Oil manifesto. Sime Darby Plantation, Bd: Kuala Lumpur, Malaysia. Available online: http://www.simedarbyplantation.com/sustainability/beliefs-progress/high-carbon-stock (accessed on 1 May 2018).
- Cheah, L.W.; Gan, H.H.; Goh, K.J. Production, Stock and Management of Carbon in Oil Palm Plantations on Mineral Soils. In Proceedings of the International Palm Oil Conference on Green Opportunities from the Golden Crop: Agriculture, Biotechnology and Sustainability, Kuala Lumpur, Malaysia, 19–21 November 2013; p. 16. [Google Scholar]
- Khasanah, N.; van Noordwijk, M.; Ningsiha, H.; Rahayua, S. Carbon neutral? No change in mineral SOC stock under oil palm plantations derived from forest or non-forest in Indonesia. Agric. Ecosyst. Environ. 2015, 211, 195–206. [Google Scholar] [CrossRef]
- Koh, L.P.; Miettinen, J.; Liew, S.C.; Ghazoul, J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. USA 2011, 108, 5127–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramananthan, S. Organic Soils of Malaysia. Their Characteristics, Mapping, Classification and Management for Oil Palm Cultivation; Malaysian Palm Oil Council: Kelana Jaya, Selangor, Malaysia, 2016; 156p. [Google Scholar]
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.; Evers, S.; Vane, C.; Sjögersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2017, 289, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Natural Resources and Conservation Service, USDA: Washington, DC, USA, 2014; 360p. [Google Scholar]
- Lucey, J.; Hill, J.; van de Meer, P.; Reynolds, G.; Agus, F. Change in Carbon Stocks Arising from Land-Use Conversion to Oil Palm Plantations. A Science-for-Policy Paper for the Oil Palm Research-Policy Partnership Network; British Foreign and Commonwealth Office: London, UK, 2014. [Google Scholar]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Dalal, R.C.; Thornton, C.M.; Cowie, B.A. Turnover of organic carbon and nitrogen in soil assessed from δ13C and δ15N changes under pasture and cropping practices and estimates of greenhouse gas emissions. Sci. Total Environ. 2013, 465, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Turner Review no.18. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- IPCC. Special Report to the IPCC. Summary for Policy Makers. Land Use, Land Use Change and Forestry. Intergovernmental Panel on Climate Change; Watson, R.T., Noble, I.R., Bolin, B., Ravindranath, N.H., Verardo, D.J., Dokken, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Powers, J.S.; Corre, M.D.; Twine, T.E.; Veldkamp, E. Geographic bias of field observations of SOC stocks with tropical land-use changes precludes spatial extrapolation. Proc. Natl. Acad. Sci. USA 2011, 108, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Van Straaten, O.; Corre, M.D.; Wolf, K.; Tchienkoua, M.; Cuellar, E.; Matthews, R.B.; Veldkamp, E. Conversion of lowland tropical forests to tree cash crop plantations loses up to one-half of stored soil organic carbon. Proc. Natl. Acad. Sci. USA 2015, 112, 9956–9960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of SOC by converting tropical forest to plantations: Erosion and decomposition estimated by δ13C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef] [PubMed]
- Bahr, E.; Chamba Zaragocin, D.; Makeschin, F. Soil nutrient stock dynamics and land-use management of annuals, perennials and pastures after slash-and-burn in the southern Ecuadorian Andes. Agric. Ecosyst. Environ. 2014, 188, 275–288. [Google Scholar] [CrossRef]
- Law, M.C.; Balasundram, S.K.; Husni, M.H.A.; Ahmed, O.H.; Harun, M.H. Spatial variability of soil organic carbon in oil palm. Int. J. Soil Sci. 2009, 4, 93–130. [Google Scholar]
- Bruun, T.B.; Egay, K.; Mertz, O.; Magid, J. Improved sampling methods document decline in soil organic carbon stocks and concentrations of permanganate oxidisable carbon after transition from swidden to oil palm cultivation. Agric. Ecosyst. Environ. 2013, 178, 127–134. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Gas Inventories Volume 4 Agriculture, Forestry and Other Land Use, Forest Land; National Greenhouse Gas Inventories Programme: Kanagawa, Japan, 2006. [Google Scholar]
- Tanaka, S.; Tachibe, S.; Wasli, M.E.B.; Lat, J.; Seman, L.; Kendawang, J.J.; Iwasaki, K.; Sakurai, K. Soil characteristics under cash crop farming in upland areas of Sarawak, Malaysia. Agric. Ecosyst. Environ. 2009, 129, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.H.C.; Gan, H.H.; Goh, K.J. Soil nutrient changes in Ultisols under oil palm in Johor, Malaysia. J. Oil Palm Environ. 2011, 2, 93–104. [Google Scholar]
- Bakar, R.A.; Darus, S.Z.; Kulaseharan, S.; Jamaluddin, N. Effects of ten year application of empty fruit bunches in an oil palm plantation on soil chemical properties. Nutr. Cycl. Agroecosyst. 2011, 89, 341–349. [Google Scholar] [CrossRef]
- Moradi, A.; Teh, B.S.; Goh, K.J.; Hanif, M.H.A.; Ishak, C.F. Chapter 11: Soil organic carbon sequestration due to different oil palm residue mulches. In Advances in Tropical Soil Science; Universiti Putra Malaysia Press: Serdang, Malaysia, 2013; pp. 169–186. [Google Scholar]
- Aholoukpè, H.; Dubos, B.; Flori, A.; Deleporte, P.; Amadji, G.; Chotte, J.L. Estimating aboveground biomass of oil palm: Allometric equations for estimating frond biomass. For. Ecol. Manag. 2013, 292, 122–129. [Google Scholar] [CrossRef]
- Aholoukpè, H.; Amadji, G.I.; Blavet, D.; Chotte, J.I.; Deleporte, P.; Dubos, B.; Flori, A.; Jourdan, C. Effect of management of pruned fronds of oil palm on soil carbon stocks and soil physico-chemical properties in Beninese smallholder plantations. Biotechnol. Agron. Soc. Environ. 2016, 20, 171–182. [Google Scholar]
- Khalid, H.; Zin, Z.Z.; Anderson, J.M. Quantification of oil palm biomass and nutrient value in a mature plantation. 1. Above-ground biomass. J. Oil Palm Res. 1999, 11, 23–32. [Google Scholar]
- Khalid, H.; Zin, Z.Z.; Anderson, J.M. Soil nutrient dynamics and palm growth performance in relation to residue management practices following replanting of oil palm plantation. J. Oil Palm Res. 2000, 12, 25–45. [Google Scholar]
- Carron, M.P.; Auriac, Q.; Snoeck, D.; Villenave, C.; Blanchart, E.; Robeyre, F.; Marichal, R.; Darminto, M.; Caliman, J.P. Do the impact of organic residues on soil quality extend beyond the deposition area under oil palm? Eur. J. Soil Biol. 2016, 75, 54–61. [Google Scholar] [CrossRef]
- Comte, I.; Colin, F.; Whalen, J.K.; Grünberger, O.; Caliman, J. Chapter three—Agricultural practices in oil palm plantations and their impact on hydrological changes, nutrient fluxes and water quality in Indonesia: A review. Adv. Agron. 2012, 116, 71–124. [Google Scholar]
- Comte, I.; Colin, F.; Grünberger, O.; Follain, S.; Whalen, J.K.; Caliman, J.P. Landscape-scale assessment of soil response to long-term organic and mineral fertilizer application in an industrial oil palm plantation, Indonesia. Agric. Ecosyst. Environ. 2013, 169, 58–68. [Google Scholar] [CrossRef]
- Yamada, H.; Tanaka, R.; Sulaiman, O.; Hashim, R.; Hamid, Z.A.A.; Yahya, M.K.A.; Kosugi, A.; Arai, T.; Murata, Y.; Nirasawa, S.; et al. Old oil palm trunk: A promising source of sugars for bioethanol production. Biomass Bioenergy 2010, 34, 1608–1613. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2003; 562p. [Google Scholar]
- Chiew, Y.L.; Shimada, S. Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer—A case study of Malaysia. Biomass Bioenergy 2013, 51, 109–124. [Google Scholar] [CrossRef]
- Frazão, L.A.; Paustian, K.; Cerri, C.E.P.; Cerri, C.C. SOC stocks under oil palm plantations in Bahia state, Brazil. Biomass Bioenergy 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Stichnothe, H.; Schuchardt, F. Comparison of different treatment options for palm oil production waste on a life cycle basis. Int. J. Life Cycle Assess. 2010, 15, 907–915. [Google Scholar] [CrossRef]
- Stichnothe, H.; Schuchardt, F. Life cycle assessment of two palm oil production systems. Biomass Bioenergy 2011, 35, 3976–3984. [Google Scholar] [CrossRef]
- Ross, S.M.; Dykes, A. Soil conditions, erosion and nutrient loss on steep slopes under mixed dipterocarp forest in Brunei Darussalam. In Tropical Rainforest Research—Current Issues; Edwards, D.S., Booth, W.E., Choy, S.C., Eds.; Springer: Dordrecht, The Netherlands, 1996; Volume 74, pp. 259–270. [Google Scholar]
- Moradi, A.; Sung, C.T.B.; Goh, K.J.; Hanif, A.H.M.; Ishak, C.F. Effect of four soil and water conservation practices on soil physical processes in a non-terraced oil palm plantation. Soil Till. Res. 2014, 145, 62–71. [Google Scholar] [CrossRef]
- Moradi, A.; Teh, B.S.; Goh, K.J.; Hanif, M.H.A.; Fauziah, C.I. Evaluation of four soil conservation practices in a non-terraced oil palm plantation. Agron. J. 2012, 104, 1727–1740. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion and global carbon budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef]
- Foster, G.R.; Young, R.A.; Neibling, W.H. Sediment composition for nonpoint source pollution analyses. Trans. ASAE 1985, 28, 133–146. [Google Scholar] [CrossRef]
- Rose, C.W.; Dalal, R.C. Erosion and runoff of nitrogen. In Advances in Nitrogen Cycling in Agricultural Ecosystems; Wilson, J.R., Ed.; C.A.B. International: Wallingford, UK, 1988; pp. 212–235. [Google Scholar]
- Sidle, R.C.; Ziegler, A.D.; Negishi, J.N.; Nik, A.R.; Siew, R.; Turkelboom, F. Erosion processes in steep terrain—Truths, myths, and uncertainties related to forest management in Southeast Asia. For. Ecol. Manag. 2006, 224, 199–225. [Google Scholar] [CrossRef]
- Davidson, E.A.; Ackerman, H. Changes in SOC inventories following cultivation of previously untilled soils. Biogeochemistry 1993, 20, 161–193. [Google Scholar] [CrossRef]
- Yu, B.; Hashim, G.M.; Eusof, Z. Estimating the r-factor with limited rainfall data: A case study from peninsular Malaysia. J. Soil Water Conserv. 2001, 56, 101–105. [Google Scholar]
- Oliveira, P.T.S.; Wendland, E.; Nearing, M.A. Rainfall erosivity in Brazil: A review. CATENA 2013, 100, 139–147. [Google Scholar] [CrossRef]
- Gharibreza, M.; Raj, J.K.; Yusoff, I.; Othman, Z.; Tahir, W.Z.W.M.; Ashraf, M.A. Land use changes and soil redistribution estimation using 137 Cs in the tropical Bera Lake catchment, Malaysia. Soil Till. Res. 2013, 131, 1–10. [Google Scholar] [CrossRef]
- Boye, A.; Albrecht, A. Soil erodibility, control and SOC losses under short-term tree fallows in western Kenya. In Soil Erosion and Carbon Dynamics; Feller, C., Roose, E.J., Stewart, B.A., Barthes, B., Lal, R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 181–196. [Google Scholar]
- Bilgo, A.; Serpentie, G.; Masser, D.; Fournier, J.; Hien, V. Carbon, nitrogen, and fine particles removed by water erosion on crops, fallows, and mixed plots in Sudanese savannas (Burkina Faso). In Soil Erosion and Carbon Dynamics; Roose, E.J., Feller, C., Barthes, B., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 125–142. [Google Scholar]
- Lacoste, M.; Viaud, V.; Michot, D.; Walter, C. Landscape-scale modelling of erosion processes and SOC dynamics under land-use and climate change agroecosystems. Eur. J. Soil Sci. 2015, 66, 780–791. [Google Scholar] [CrossRef]
- Muller-Nedebock, D.; Chaplot, V. SOC losses by sheet erosion: A potentially critical contribution to the global carbon cycle. Earth Surf. Process. Landf. 2015, 40, 1803–1813. [Google Scholar] [CrossRef]
- Gachene, C.K.K.; Jarvis, N.J.; Linner, H.; Mbuvi, J.P. Soil erosion effects on soil properties in a highland area in central Kenya. Soil Sci. Soc. Am. J. 1997, 61, 559–564. [Google Scholar] [CrossRef]
- Ishizuka, S.; Tsuruta, H.; Murdiyarso, D. An intensive field study on CO2 CH4, and N2O emissions from soils at four land-use types in Sumatra, Indonesia. Glob. Biogeochem. Cycles 2002, 16, 22:1–22:11. [Google Scholar] [CrossRef]
- Ishizuka, S.; Iswandi, A.; Nakajima, Y.; Yonemura, S.; Sudo, S.; Tsuruta, H.; Murdiyarso, D. The variation of greenhouse gas emissions from soils of various land-use/cover types in Jambi province, Indonesia. Nutr. Cycl. Agroecosyst. 2005, 71, 17–32. [Google Scholar] [CrossRef]
- Werner, C.; Zheng, X.H.; Tang, J.W.; Xie, B.H.; Liu, C.Y.; Kiese, R.; Butterbach-Bahl, K. N2O, CH4 and CO2 emissions from seasonal tropical rainforests and a rubber plantation in Southwest China. Plant Soil 2006, 289, 335–353. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattanio, J.H.; Ackerman, I.L.; Erickson, H.E.; Keller, M. Land use change and biogeochemical controls of nitrogen oxide emissions from soils in eastern Amazonia. Glob. Biogeochem. Cycles 1999, 13, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Verchot, L.V.; Davidson, E.A.; Cattanio, J.H.; Ackerman, I.L. Land-use change and biogeochemical controls of methane fluxes in soils of Eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Feigl, B.J.; Neill, C.; Garcia, D.; Piccolo, M.C.; Cerri, C.C.; Tian, H. Nitrous oxide emissions from forests and pastures of various ages in the Brazilian Amazon. J. Geophys. Res. 2001, 106, 34179–34188. [Google Scholar] [CrossRef] [Green Version]
- Weitz, A.M.; Veldkamp, E.; Keller, M.; Neff, J.; Crill, P.M. Nitrous oxide, nitric oxide, and methane fluxes from soils following clearing and burning of tropical secondary forest. J. Geophys. Res. 1998, 103, 28047–28058. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.; Asner, G.P.; Kitayama, K. Substrate, climate and land use controls over soil N dynamics and N-oxide emissions in Borneo. Biogeochemistry 2004, 70, 27–58. [Google Scholar] [CrossRef]
- Singh, J.S.; Raghubanshi, A.S.; Reddy, V.S.; Singh, S.; Kashyap, A.K. Methane flux from irrigated paddy and dryland rice fields, and from seasonally dry tropical forest and savanna soils of India. Soil Biol. Biochem. 1998, 30, 135–139. [Google Scholar] [CrossRef]
- Awasthi, K.D.; Sitaula, B.K.; Singh, B.R.; Bajracharya, R.M. Fluxes of methane and carbon dioxide from soil under forest, grazing land, irrigated rice and rainfed field crops in a watershed of Nepal. Biol. Fertil. Soils 2005, 41, 163–172. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E.; Livesley, S.J.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Hassler, E.; Corre, M.D.; Veldkamp, A.; Tjoa, M.; Damris, S.R.; Utami, E. Soil fertility controls soil-atmosphere carbon dioxide and methane fluxes in a tropical landscape converted from lowland forest to rubber and oil palm plantations. Biogeosciences 2015, 12, 5831–5852. [Google Scholar] [CrossRef]
- Osher, L.J.; Matson, P.A.; Amundson, R. Effect of land use change on SOC in Hawaii. Biogeochemistry 2003, 65, 213–232. [Google Scholar] [CrossRef]
- Powers, J.S. Changes in SOC and nitrogen after contrasting land-use transitions in Northeastern Costa Rica. Ecosystems 2004, 7, 134–146. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Zhao, Y.; Zhao, W.; Qi, Z. Chemical degradation of a Ferralsol (Oxisol) under intensive rubber (Hevea brasiliensis) farming in tropical China. Soil Till. Res. 2007, 93, 109–116. [Google Scholar] [CrossRef]
- Galdos, M.V.; Cerri, C.C.; Cerri, C.E.P. SOC stocks under burned and unburned sugarcane in Brazil. Geoderma 2009, 153, 347–352. [Google Scholar] [CrossRef]
- Ziegler, A.D.; Phelps, J.; Yuen, J.Q.; Webb, E.L.; Lawrence, D.; Fox, J.M.; Bruun, T.B.; Leisz, S.J.; Ryan, C.M.; Dressler, W. Carbon outcomes of major land-cover transitions in SE Asia: Great uncertainties and REDD+ policy implications. Glob. Chang. Biol. 2012, 18, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Frazão, L.A.; Paustian, K.; Cerri, C.E.P.; Cerri, C.C. SOC stocks and changes after oil palm introduction in the Brazilian Amazon. Glob. Chang. Biol. 2013, 5, 384–390. [Google Scholar] [CrossRef]
- Nelson, P.N.; Banabas, M.; Nake, S.; Goodrick, I.; Webb, M.J.; Gabriel, E. Soil fertility changes following conversion of grassland to oil palm. Soil Res. 2014, 52, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.L.C.; Cherubin, M.R.; Pavinato, P.S.; Cerri, C.E.P.; Six, J.; Davies, C.A.; Cerri, C.C. SOC, nitrogen and phosphorus changes under sugarcane expansion in Brazil. Sci. Total Environ. 2015, 515–516, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Goodrick, I.; Nelson, P.N.; Banabas, M.; Wurster, C.M.; Bird, M.I. SOC balance following conversion of grassland to oil palm. Glob. Chang. Biol. Bioenergy 2015, 7, 263–272. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options, a review. Aust. J. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Myhre, G.D.; Shindell, F.M.; Bréon, W.; Collins, J.; Fuglestvedt, J.; Huang, D.; Koch, J.F.; Lamarque, D.; Lee, B.; Mendoza, T.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the IPCC; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Doschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 659–740. [Google Scholar]




| Previous Land Use | Converted Land Use | Age of New Land Use (year) (Mean ± SD) | SOC Stock of Previous Land Use (t/ha) (Mean ± SD) (A) | SOC Stock of Converted Land Use (t/ha) (Mean ± SD) (B) | Relative Change in SOC Stock (%) *,# (Mean ± SD) [(B − A)/A] × 100 | Number of Data Considered |
|---|---|---|---|---|---|---|
| Primary forest | Oil palm plantation | 14.4 ± 7 | 57.7 ± 21.7 | 43.2 ± 20.4 | −23.3 ± 21.4 | n = 40 |
| Secondary forest | Oil palm plantation | 15.4 ± 10.3 | 56.1 ± 20.4 | 56.4 ± 26.6 | −1.2 ± 26.1 | n = 11 |
| Pasture/grassland | Oil palm plantation | 25 | 61.8 ± 55.8 | 54.8 ± 54.5 | −11.7 ± 27.0 | n = 3 |
| Slash-and-burn Agriculture | Oil palm plantation | 11 ± 3.7 | 60 ± 12.4 | 41.9 ± 15.6 | −30.6 ± 19.8 | n = 5 |
| Cropland | Oil palm plantation | 20 | 33.1 | 69.0 | 108.5 | n = 1 |
| Primary forest | Rubber plantation | 23.2 ± 15 | 74.7 ± 27.8 | 62.8 ± 34.7 | −14.3 ± 36.8 | n = 34 |
| Secondary forest | Rubber plantation | 29.1 ± 22.2 | 70.3 ± 10.3 | 58.8 ± 10.6 | −16.3 ± 8.5 | n = 8 |
| Pasture/grassland | Young Rubber/tree plantation | 9 ± 5 | 54.8 ± 8.3 | 61.2 ± 14.7 | 10. 8 ± 10.2 | n = 3 |
| Pasture/grassland | Old Rubber/Broadleaf-tree plantation | 19 ± 14 | 58.7 ± 37.3 | 49.0 ± 31.8 | −15.9 ± 8.3 | n = 3 |
| Primary forest | Perennial tree plantation | 35.4 ± 22.5 | 55.9 ± 22.3 | 41.1 ± 19.4 | −25.8 ± 15.9 | n = 23 |
| Secondary forest | Tree/perennial plantation | 27.8 ± 20.6 | 75.9 ± 32.8 | 69.2 ± 26.5 | −7.4 ± 6.7 | n = 6 |
| Primary forest | Pasture | 33.6 ± 24.3 | 76 ± 25.6 | 63 ± 29.6 | −19.9 ± 18.5 | n = 9 |
| Secondary forest | Grass/Cropland | 24.9 ± 18.7 | 66.5 ± 43.4 | 53.6 ± 36 | −16.1 ± 21 | n = 8 |
| Primary forest | Crop and fallow land | 29 ± 21 | 96.4 ± 46.3 | 75.5 ± 45.7 | −23.1 ± 14.2 | n = 9 |
| Pasture/grassland | Cropland | 27.6 ± 20.9 | 102.5 ± 19.7 | 80.7 ± 17.3 | −24.3 ± 11.3 | n = 5 |
| Previous Land Use | Converted Land Use | Net SOC Stock Change (t C/ha/year) * (Mean ± SD) | Net SOC-CO2e Flux (CO2e t/ha/year) * (Mean ± SD) | Net N2O-Flux (N2O kg/ha/year) * (Mean ± SD) | Net CH4-Flux (CH4 kg/ha/year) * (Mean ± SD) |
|---|---|---|---|---|---|
| Primary forest | Oil palm | 1.17 ± 2.39 | 4.29 ± 8.75 (n = 40) | na | 0.33 |
| Secondary forest | Oil palm | −0.52 ± 2.09 | −1.92 ± 7.66 (n = 11) | 0.095 ± 1.42 (n = 2) | 0.86 ± 1.14 (n = 3) |
| Pasture/grassland | Oil palm | 0.28 ± 0.37 | 1.03 ± 1.35 (n = 3) | na | na |
| Cropland—Slash-and-burn Agriculture | Oil palm | 1.51 ± 0.79 | 5.54 ± 2.9 (n = 5) | na | na |
| Primary forest | Rubber | 0.78 ± 1.61 | 2.87 ± 5.91 (n = 34) | −0.23 ± 0.04 (n = 2) | 1.78 ± 1.41(n = 2) |
| Secondary forest | Rubber | 0.62 ± 0.38 | 2.26 ± 1.39 (n = 8) | −0.65 ± 0.29 (n = 3) | 1.52 ± 0.71 (n = 3) |
| Secondary forest | Old Rubber, >8–10 years, after tapping | na | na | 1.56 | 3.48 |
| Pasture/grassland | Young Rubber/other tree plantation | −1.01 ± 1.40 | −3.69 ± 5.12 (n = 3) | na | na |
| Pasture/grassland | Old Rubber/Broad-leaf tree plantation | 0.87 ± 0.72 | 3.19 ± 2.65 (n = 3) | na | na |
| Secondary forest | Other perennial tree plantation | 0.45 ± 0.43 | 1.63 ± 1.58 (n = 6) | −0.47 | 0.25 |
| Primary forest | Other perennial tree plantation | 0.65 ± 0.92 | 2.39 ± 3.36 (n = 23) | na | 3.10 |
| Primary forest | Secondary forest | na | na | 0.25 ± 0.10 (n = 2) | −0.02 ± 0.67 (n = 2) |
| Primary forest | Paddy rice | na | na | na | 70.48 ± 90.26 (n = 2) |
| Primary forest | Pasture, (degraded, old) | na | na | −2.11 ± 2.01(n = 4) | na |
| Primary forest | Pasture and cropland | 0.58 ± 0.35 | 2.11 ± 1.29 (n = 9) | 9.38 ± 6.38 (n = 2) | 4.89 ± 7.46 (n = 6) |
| Primary forest | Crop and fallow | 1.31 ± 1.52 | 4.81 ± 5.58 (n = 9) | na | na |
| Pasture/grassland | Cropland | 1.32 ± 1.07 | 4.85 ± 3.93 (n = 4) | na | na |
| Secondary forest | Grasslands | na | na | −1.19 ± 0.17 (n = 3) | na |
| Secondary forest/Woodland savannah | Cultivated pasture, cropland | 0.67 ± 0.85 | 2.44 ± 3.1 (n = 8) | 2.84 ± 5.56 (n = 5) | 0.30 ± 1.88 (n = 4) |
| Previous Land Use | Converted Land Use | SOC Flux (Mean ± SD) | N2O Flux (Mean ± SD) | CH4 Flux (Mean ± SD) | Total GHG Emissions (Mean ± SD) |
|---|---|---|---|---|---|
| CO2-Equivalent (kg/ha/year) | |||||
| Primary forest | Oil palm plantation | 4290 ± 8750 | na | 9.24 | * |
| Secondary forest | Oil palm plantation | −1920 ± 7660 | 25.18 ± 377 | 24.17 ± 32 | −1870 ± 8068 |
| Primary forest | Rubber plantation | 2870 ± 5910 | −60.95 ± 11 | 49.84 ± 40 | 2859 ± 5961 |
| Secondary forest | Rubber plantation | 2260 ± 1390 | −171.37 ± 77 | 42.47 ± 20 | 2131 ± 1487 |
| Secondary forest | Other perennial tree plantation | 1630 ± 1580 | −124.55 | 7.0 | 1512 ± 1580 |
| Primary forest | Pasture, cropland and fallow | 2110 ± 1290 | 2485.7 ± 1690 | 136.88 ± 209 | 4733 ± 3189 |
| Secondary forest/Woodland savannah | Cultivated pasture, cropland | 2440 ± 3100 | 753.13 ± 1474 | 8.33 ± 53 | 3201 ± 4627 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugam, S.; Dalal, R.C.; Joosten, H.; Raison, R.J.; Joo, G.K. SOC Stock Changes and Greenhouse Gas Emissions Following Tropical Land Use Conversions to Plantation Crops on Mineral Soils, with a Special Focus on Oil Palm and Rubber Plantations. Agriculture 2018, 8, 133. https://doi.org/10.3390/agriculture8090133
Shanmugam S, Dalal RC, Joosten H, Raison RJ, Joo GK. SOC Stock Changes and Greenhouse Gas Emissions Following Tropical Land Use Conversions to Plantation Crops on Mineral Soils, with a Special Focus on Oil Palm and Rubber Plantations. Agriculture. 2018; 8(9):133. https://doi.org/10.3390/agriculture8090133
Chicago/Turabian StyleShanmugam, Sanjutha, Ram C. Dalal, Hans Joosten, R. J. Raison, and Goh Kah Joo. 2018. "SOC Stock Changes and Greenhouse Gas Emissions Following Tropical Land Use Conversions to Plantation Crops on Mineral Soils, with a Special Focus on Oil Palm and Rubber Plantations" Agriculture 8, no. 9: 133. https://doi.org/10.3390/agriculture8090133






