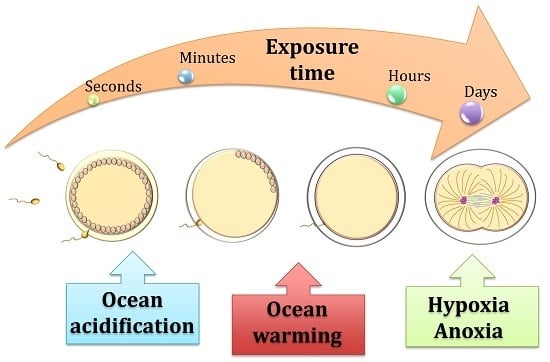
Life under Climate Change Scenarios: Sea Urchins’ Cellular Mechanisms for Reproductive Success
Abstract
:1. Threats to the Life Cycle Of Echinoids under Ocean Acidification
Gametogenesis, Spawning and Fertilization of Sea Urchins
2. Synergy between pHi and Ca2+ Homeostasis at Fertilization
2.1. Sperm Activation, Chemoattraction, and Acrosomal Reaction
2.2. Egg Fertilization, Cortical Reaction, and Metabolic Activation
2.3. Cytokinesis
3. Main Mechanisms of pHi Regulation in Sea Urchins
4. Evidence of Subcellular Alterations Due to Ocean Acidification Exposure
5. Conclusions and Future Perspectives for Research
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR | acetylcholine receptor |
| Cai2+ | intracellular calcium |
| Ca2+-CaMK | Ca2+/calmodulin kinase |
| cADPR | cyclic ADP ribose |
| CG | cortical granules |
| CGSP1 | cortical granule serine protease 1 |
| CO2 | carbon dioxide |
| DAG | diacylglicerol |
| ECM | extracellular matrix |
| ER | endoplasmic reticulum |
| ERK | extracellular regulated kinase |
| FE | fertilization envelope |
| FSP | fucose sulphated polysaccharide |
| H2O2 | hydrogen peroxide |
| HCO3- | bicarbonate |
| HL | hyaline layer |
| IP3 | inositol triphosphate |
| IP3R | IP3-receptors |
| MAPs | microtubule-associated proteins |
| NAADP | nicotinic acid adenine dinucleotide phosphate |
| NADP | nicotinamide adenine dinucleotide phosphate |
| NBC | Na+-HCO3−co-transporter |
| NHE | Na+-H+ exchanger |
| OA | ocean acidification |
| OVOP | ovoperoxidase |
| pHi | intracellular pH |
| PIP2 | polyphosphatidylinositol 4,5-biphosphate |
| PKC | protein kinase C |
| PLCγ | phospholipase C |
| PPP | pentose phosphate pathway |
| ROS | reactive oxygen species |
| RyR | ryanodine receptor |
| sAC | soluble adenyl cyclase |
| Udx1 | urchin dual oxidase |
| VL | vitelline layer |
References
- Fitzer, S.C.; Caldwell, G.S.; Close, A.J.; Clare, A.S.; Upstill-Goddard, R.C.; Bentley, M.G. Ocean acidification induces multi-generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. J. Exp. Mar. Biol. Ecol. 2012, 418–419, 30–36. [Google Scholar]
- Movilla, J.; Calvo, E.; Pelejero, C.; Coma, R.; Serrano, E.; Fernández-Vallejo, P.; Ribes, M. Calcification reduction and recovery in native and non-native Mediterranean corals in response to ocean acidification. J. Exp. Mar. Biol. Ecol. 2012, 438, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Thiyagarajan, V.; Ko, G.W.K. Larval growth response of the Portuguese oyster (Crassostrea angulata) to multiple climate change stressors. Aquaculture 2012, 370–371, 90–95. [Google Scholar]
- Crim, R.N.; Sunday, J.M.; Harley, C.D.G. Elevated seawater CO2 concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana). J. Exp. Mar. Biol. Ecol. 2011, 400, 272–277. [Google Scholar] [CrossRef]
- Dixson, D.L.; Munday, P.L.; Jones, G.P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 2010, 13, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Findlay, H.S.; Kendall, M.A.; Spicer, J.I.; Widdicombe, S. Relative influences of ocean acidification and temperature on intertidal barnacle post-larvae at the northern edge of their geographic distribution. Estuar. Coast. Shelf Sci. 2010, 86, 675–682. [Google Scholar] [CrossRef]
- Shi, D.; Xu, Y.; Hopkinson, B.M.; Morel, F.M.M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 2010, 327, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Encyclopedia of Earth; IPCC-Report; Saundry, P., Vranes, K., Eds.; Environmental Information Coalition, National Council for Science and the Environment: Washington, DC, USA, 2007. [Google Scholar]
- Roy, T.; Bopp, L.; Gehlen, M.; Schneider, B.; Cadule, P.; Frölicher, T.; Segschneider, J.; Tjiputra, J.; Heinze, C.; Joos, F. Regional impacts of climate change and atmospheric CO2 on future ocean carbon uptake: A multimodel linear feedback analysis. J. Clim. 2011, 24, 2300–2318. [Google Scholar] [CrossRef]
- Gangstø, R.; Joos, F.; Gehlen, M. Sensitivity of pelagic calcification to ocean acidification. Biogeosciences 2011, 8, 433–458. [Google Scholar] [CrossRef] [Green Version]
- Steinacher, M.; Joos, F.; Frölicher, T.L.; Bopp, L.; Cadule, P.; Cocco, V.; Doney, S.C.; Gehlen, M.; Lindsay, K.; Moore, J.K.; et al. Projected 21st century decrease in marine productivity: A multi-model analysis. Biogeosciences 2010, 7, 979–1005. [Google Scholar] [CrossRef] [Green Version]
- Frölicher, T.L.; Joos, F. Reversible and irreversible impacts of greenhouse gas emissions in multi-century projections with the NCAR global coupled carbon cycle-climate model. Clim. Dyn. 2010, 35, 1439–1459. [Google Scholar] [CrossRef]
- Steinacher, M.; Joos, F.; Frölicher, T.L.; Plattner, G.K.; Doney, S.C. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 2009, 6, 515–533. [Google Scholar] [CrossRef] [Green Version]
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 105, 18860–18864. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Ortega-Martínez, O.; Thorndyke, M. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 2010, 19, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Thorndyke, M.C. Impact of CO2-driven ocean acidification on invertebrates early life-history—What we know, what we need to know and what we can do. Biogeosciences 2009, 6, 3109–3131. [Google Scholar] [CrossRef]
- Mercier, A.; Hamel, J. Chapter 2 Gametogenesis. In Advances in Marine Biology; Annie, M., Jean-Francois, H., Eds.; Academic Press: Cambridge, MA, USA, 2009; Vol. 55, pp. 7–72. [Google Scholar]
- Holtmann, W.; Stumpp, M.; Gutowska, M.; Syré, S.; Himmerkus, N.; Melzner, F.; Bleich, M. Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: The role of body cavity epithelia and stereom dissolution. Mar. Biol. 2013, 160, 2631–2645. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Mortensen, A.; Dale, T.; Foss, A. Effects of carbon dioxide exposure on feed intake and gonad growth in green sea urchin, Strongylocentrotus droebachiensis. Aquaculture 2007, 266, 97–101. [Google Scholar] [CrossRef]
- Uthicke, S.; Soars, N.; Foo, S.; Byrne, M. Effects of elevated pCO2 and the effect of parent acclimation on development in the tropical Pacific sea urchin Echinometra mathaei. Mar. Biol. 2013, 160, 1913–1926. [Google Scholar] [CrossRef]
- Kurihara, H.; Yin, R.; Nishihara, G.; Soyano, K.; Ishimatsu, A. Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus. Aquat. Biol. 2013, 18, 281–292. [Google Scholar] [CrossRef]
- Bögner, D.; Bickmeyer, U.; Köhler, A. CO2-induced fertilization impairment in Strongylocentrotus droebachiensis collected in the Arctic. Helgol. Mar. Res. 2014, 68, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.; Ho, M.; Selvakumaraswamy, P.; Nguyen, H.D.; Dworjanyn, S.A.; Davis, A.R. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. Biol. Sci. 2009, 276, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Dorey, N.; Stumpp, M.; Melzner, F.; Thorndyke, M. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2013, 160, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Bögner, D.; Zwicker, S.; Bickmeyer, U.; Koehler, A. Influence of time of exposure on fertilization traits of the green sea urchin Strongylocentrotus droebachiensis from an Arctic population under CO2-induced Ocean Acidification. Unpublished work. 2016. [Google Scholar]
- Reuter, K.E.; Lotterhos, K.E.; Crim, R.N.; Thompson, C.A.; Harley, C.D.G. Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Glob. Chang. Biol. 2011, 17, 163–171. [Google Scholar] [CrossRef]
- Pennington, J.T. The ecology of fertilization of echinoid eggs: The consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 1985, 169, 417–430. [Google Scholar] [CrossRef]
- Wahle, R.; Peckham, S. Density-related reproductive trade-offs in the green sea urchin, Strongylocentrotus droebachiensis. Mar. Biol. 1999, 134, 127–137. [Google Scholar] [CrossRef]
- Meidel, S.K.; Yund, P.O. Egg longevity and time-integrated fertilization in a temperate sea urchin (Strongylocentrotus droebachiensis). Biol. Bull. 2001, 201, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Epel, D.; Kaufman, M.; Xiao, L.; Kibak, H.; Patton, C. Enhancing use of sea urchin eggs and embryos for cell and developmental studies: Method for storing spawned eggs for extended periods. Mol. Biol. Cell 1998, 9, 182A. [Google Scholar]
- Morita, M.; Suwa, R.; Iguchi, A.; Nakamura, M.; Shimada, K.; Sakai, K.; Suzuki, A. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 2010, 18, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Havenhand, J.N.; Schlegel, P. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 2009, 6, 3009–3015. [Google Scholar] [CrossRef]
- Schlegel, P.; Havenhand, J.N.; Obadia, N.; Williamson, J.E. Sperm swimming in the polychaete Galeolaria caespitosa shows substantial inter-individual variability in response to future ocean acidification. Mar. Pollut. Bull. 2014, 78, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Havenhand, J.N.; Buttler, F.-R.; Thorndyke, M.C.; Williamson, J.E. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008, 18, R651–R652. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, G.S.; Fitzer, S.; Gillespie, C.S.; Pickavance, G.; Turnbull, E.; Bentley, M.G. Ocean acidification takes sperm back in time. Invertebr. Reprod. Dev. 2011, 55, 217–221. [Google Scholar] [CrossRef]
- Schlegel, P.; Binet, M.T.; Havenhand, J.N.; Doyle, C.J.; Williamson, J.E. Ocean acidification impacts on sperm mitochondrial membrane potential bring sperm swimming behaviour near its tipping point. J. Exp. Biol. 2015, 218, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Vacquier, V.D. Egg fucose sulfate polymer, sialoglycan, and speract all trigger the sea urchin sperm acrosome reaction. Biochem. Biophys. Res. Commun. 2002, 296, 833–839. [Google Scholar] [CrossRef]
- Bögner, D.; Zwicker, S.; Bickmeyer, U.; Koehler, A. Actin polymerization patterns in eggs of Strongylocentrotus droebachiensis (O.F. Müller, 1776) exposed to acidified seawater. Unpublished work. 2016. [Google Scholar]
- Watanabe, K.; Hamaguchi, M.S.; Hamaguchi, Y. Effects of intracellular pH on the mitotic apparatus and mitotic stage in the sand dollar egg. Cell Motil. Cytoskeleton 1997, 37, 263–270. [Google Scholar] [CrossRef]
- Von Dassow, G. Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol. 2009, 19, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Foe, V.E.; von Dassow, G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J. Cell Biol. 2008, 183, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Coffman, J.A.; Denegre, J.M. Mitochondria, redox signaling and axis specification in metazoan embryos. Dev. Biol. 2007, 308, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Coffman, J.A.; McCarthy, J.J.; Dickey-Sims, C.; Robertson, A.J. Oral–aboral axis specification in the sea urchin embryo: II. Mitochondrial distribution and redox state contribute to establishing polarity in Strongylocentrotus purpuratus. Dev. Biol. 2004, 273, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Coffman, J.A.; Davidson, E.H. Oral–Aboral Axis Specification in the Sea Urchin Embryo: I. Axis Entrainment by Respiratory Asymmetry. Dev. Biol. 2001, 230, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Shimode, S.; Shirayama, Y. Sub-lethal effects of elevated concentration of CO2 on planktonic copepods and sea urchins. J. Oceanogr. 2004, 60, 743–750. [Google Scholar] [CrossRef]
- Yu, P.C.; Sewell, M.A.; Matson, P.G.; Rivest, E.B.; Kapsenberg, L.; Hofmann, G.E. Growth attenuation with developmental schedule progression in embryos and early larvae of Sterechinus neumayeri raised under elevated CO2. PLoS ONE 2013, 8, e52448. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Grünbaum, D.; Arnberg, M.; Thorndyke, M.; Dupont, S.T. Ocean acidification induces budding in larval sea urchins. Mar. Biol. 2013, 160, 2129–2135. [Google Scholar] [CrossRef]
- Byrne, M.; Ho, M.A.; Koleits, L.; Price, C.; King, C.K.; Virtue, P.; Tilbrook, B.; Lamare, M. Vulnerability of the calcifying larval stage of the Antarctic sea urchin Sterechinus neumayeri to near-future ocean acidification and warming. Glob. Chang. Biol. 2013, 19, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, M.; Hu, M.Y.; Melzner, F.; Gutowska, M.A.; Dorey, N.; Himmerkus, N.; Holtmann, W.C.; Dupont, S.T.; Thorndyke, M.C.; Bleich, M. Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proc. Natl. Acad. Sci. USA 2012, 109, 18192–18197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, S. Reproductive Disorders in Sea Urchins (Psammechinus miliaris) Caused by Environmental Pollutants. Ph.D. Thesis, Jacobs University, Bremen, Germany, 2009. [Google Scholar]
- Bookbinder, L.H.; Shick, J.M. Anaerobic and aerobic energy metabolism in ovaries of the sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 1986, 93, 103–110. [Google Scholar] [CrossRef]
- Au, D.W.T.; Lee, C.Y.; Chan, K.L.; Wu, R.S.S. Reproductive impairment of sea urchin upon chronic exposure to cadmium. Part I: Effects on gamete quality. Environ. Pollut. 2001, 111, 1–9. [Google Scholar] [CrossRef]
- Uthicke, S.; Liddy, M.; Nguyen, H.D.; Byrne, M. Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin, Echinometra sp. A. Coral Reefs 2014, 33, 831–845. [Google Scholar] [CrossRef]
- Hirohashi, N.; Kamei, N.; Kubo, H.; Sawada, H.; Matsumoto, M.; Hoshi, M. Egg and sperm recognition systems during fertilization. Dev. Growth Differ. 2008, 50, S221–S238. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Silva, A.-C.E.; Hirohashi, N.; Mourão, P.A. The structure of sulfated polysaccharides ensures a carbohydrate-based mechanism for species recognition during sea urchin fertilization. Int. J. Dev. Biol. 2008, 52, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Vilela-Silva, A.-C.E.S.; Mourão, P.A.S.; Vacquier, V.D. Structural requirements for species-specific induction of the sperm acrosome reaction by sea urchin egg sulfated fucan. Biochem. Biophys. Res. Commun. 2002, 298, 403–407. [Google Scholar] [CrossRef]
- Himmelman, J.H.; Dumont, C.P.; Gaymer, C.F.; Vallières, C.; Drolet, D. Spawning synchrony and aggregative behaviour of cold-water echinoderms during multi-species mass spawnings. Mar. Ecol. Prog. Ser. 2008, 361, 161–168. [Google Scholar] [CrossRef]
- Gaudette, J.; Wahle, R.A.; Himmelman, J.H. Spawning events in small and large populations of the green sea urchin Strongylocentrotus droebachiensis as recorded using fertilization assays. Limnol. Oceanogr. 2006, 51, 1485–1496. [Google Scholar] [CrossRef]
- Meidel, S.K.; Scheibling, R.E. Variation in egg spawning among subpopulations of sea urchins Strongylocentrotus droebachiensis: A theoretical approach. Mar. Ecol. Prog. Ser. 2001, 213, 97–110. [Google Scholar] [CrossRef]
- Levitan, D.R. Asynchronous spawning and aggregative behavior in the sea urchin Diadema antillarum (Philippi). In Echinoderm Biology, Proccedings of the Sixth International Echinoderm Conference, Victoria, Australia, 23–28 August 1987; pp. 181–186.
- Lera, S.; Pellegrini, D. Evaluation of the fertilization capability of Paracentrotus lividus sea urchin storaged gametes by the exposure to different aqueous matrices. Environ. Monit. Assess. 2006, 119, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 2004, 274, 161–169. [Google Scholar] [CrossRef]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Przeslawski, R. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 2013, 53, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Lamare, M.; Winter, D.; Dworjanyn, S.A.; Uthicke, S. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 4th ed.; Sinauer: Sunderland, MA, USA, 1994; p. 894. [Google Scholar]
- Hiramoto, Y. Microinjection of the live spermatozoa into sea urchin eggs. Exp. Cell Res. 1962, 27, 416–426. [Google Scholar] [CrossRef]
- Runnström, J.; Manelli, H. Induction of polyspermy by treatment of sea urchin eggs with mercurials. Exp. Cell Res. 1964, 35, 157–193. [Google Scholar] [CrossRef]
- Dale, B.; DeFelice, L. Polyspermy prevention: Facts and artifacts? J. Assist. Reprod. Genet. 2011, 28, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.A.; Wessel, G.M. Proteolytic cleavage of the cell surface protein p160 is required for detachment of the fertilization envelope in the sea urchin. Dev. Biol. 2004, 272, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Wessel, G.M.; Conner, S.; Laidlaw, M.; Harrison, J.; LaFleur, G.J. SFE1, a constituent of the fertilization envelope in the sea urchin is made by oocytes and contains low-density lipoprotein-receptor-like repeats. Biol. Reprod. 2000, 63, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.A.; Wessel, G.M. The cortical granule serine protease CGSP1 of the sea urchin, Strongylocentrotus purpuratus, is autocatalytic and contains a low-density lipoprotein receptor-like domain. Dev. Biol. 1999, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; de Santis, A. Maturation and fertilization of the sea urchin oocyte: An electrophysiological study. Dev. Biol. 1981, 85, 474–484. [Google Scholar] [CrossRef]
- Jaffe, L.A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 1976, 261, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Epel, D. The program of and mechanisms of fertilization in the echinoderm egg. Am. Zool. 1975, 15, 507–522. [Google Scholar] [CrossRef]
- Schomer, B.; Epel, D. Redox changes during fertilization and maturation of marine invertebrate eggs. Dev. Biol. 1998, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.-P.; Payan, P.; Sardet, C. Changes in intracellular cations following fertilization of sea urchin eggs: Na+/H+ and Na+/K+ exchanges. Exp. Cell Res. 1982, 142, 215–221. [Google Scholar] [CrossRef]
- Johnson, C.H.; Epel, D. Intracellular pH of sea urchin eggs measured by the dimethyloxazolidinedione (DMO) method. J. Cell Biol. 1981, 89, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, R.; Zucker, R.; Schatten, G. Intracellular calcium release at fertilization in the sea urchin egg. Dev. Biol. 1977, 58, 185–196. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Steinhardt, R.A. Ionic regulation of egg activation. Q. Rev. Biophys. 1982, 15, 593–666. [Google Scholar] [CrossRef] [PubMed]
- Karp, G.C.; Hajek, A.S. Uptake and incorporation of [3H]glycerol into lipids of unfertilized and fertilized sea urchin eggs. Exp. Cell Res. 1977, 104, 79–93. [Google Scholar] [CrossRef]
- Terasaki, M.; Jaffe, L.A. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J. Cell Biol. 1991, 114, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Epel, D. Changes in intracellular acidic compartments in sea urchin eggs after activation. Dev. Biol. 1983, 98, 446–448. [Google Scholar] [CrossRef]
- Sluder, G.; Begg, D.A. Control mechanisms of the cell cycle: Role of the spatial arrangement of spindle components in the timing of mitotic events. J. Cell Biol. 1983, 97, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D. Dynamic changes of the egg cortex. Dev. Biol. 1981, 84, 1–26. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Havenhand, J.N.; Gillings, M.R.; Williamson, J.E. Individual variability in reproductive Success determines winners and losers under ocean acidification: A case study with sea urchins. PLoS ONE 2012, 7, e53118. [Google Scholar] [CrossRef] [PubMed]
- Vihtakari, M.; Hendriks, I.; Holding, J.; Renaud, P.; Duarte, C.; Havenhand, J. Effects of ocean acidification and warming on sperm activity and early life stages of the mediterranean mussel (Mytilus galloprovincialis). Water 2013, 5, 1890–1915. [Google Scholar] [CrossRef]
- Nakamura, M.; Morita, M. Sperm motility of the scleractinian coral Acropora digitifera under preindustrial, current, and predicted ocean acidification regimes. Aquat. Biol. 2012, 15, 299–302. [Google Scholar] [CrossRef]
- Frommel, A.Y.; Stiebens, V.; Clemmesen, C.; Havenhand, J. Effect of ocean acidification on marine fish sperm (Baltic cod: Gadus morhua). Biogeosciences 2010, 7, 3915–3919. [Google Scholar] [CrossRef] [Green Version]
- Daye, P.G.; Glebe, B.D. Fertilization success and sperm motility of Atlantic salmon (Salmo salar L.) in acidified water. Aquaculture 1984, 43, 307–312. [Google Scholar] [CrossRef]
- Hamamah, S.; Gatti, J.-L. Role of the ionic environment and internal pH on sperm activity. Hum. Reprod. 1998, 13, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Tosti, E. Sperm activation in species with external fertilisation. Zygote 1994, 2, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Christen, R.; Schackmann, R.W.; Shapiro, B.M. Ionic regulation of sea urchin sperm motility, metabolism and fertilizing capacity. J. Physiol. 1986, 379, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Aluigi, M.; Sgro, M.; Trombino, S.; Falugi, C.; Thielecke, H. Cell signalling during sea urchin development: A model for assessing toxicity of environmental contaminants. In Echinodermata; Springer: New York, NY, USA, 2005; pp. 45–70. [Google Scholar]
- Trimmer, J.S.; Vacquier, V.D. Activation of sea urchin gametes. Annu. Rev. Cell Biol. 1986, 2, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Christen, R.; Schackmann, R.W.; Shapiro, B.M. Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. J. Biol. Chem. 1983, 258, 5392–5399. [Google Scholar] [PubMed]
- Tresguerres, M.; Barott, K.L.; Barron, M.E.; Roa, J.N. Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals. J. Exp. Biol. 2014, 217, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Vacquier, V.D. Proteins associated with soluble adenylyl cyclase in sea urchin sperm flagella. Cell Motil. Cytoskeleton 2006, 63, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Kirkman-Brown, J.C.; Sutton, K.A.; Florman, H.M. How to attract a sperm. Nat. Cell Biol. 2003, 5, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ward, G.E.; Brokaw, C.J.; Garbers, D.L.; Vacquier, V.D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 1985, 101, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Nishigaki, T.; Carneiro, J.; Yoshiro, T.; Wood, C.D.; Darszon, A. Tuning sperm chemotaxis by calcium burst timing. Dev. Biol. 2010, 344, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, M.; Yoshida, M. Activation of motility and chemotaxis in the spermatozoa: From invertebrates to humans. Reprod. Med. Biol. 2005, 4, 101–114. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Solzin, J.; Hildebrand, E.; Brown, J.E.; Helbig, A.; Hagen, V.; Beyermann, M.; Pampaloni, F.; Weyand, I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat. Cell Biol. 2003, 5, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Ghigo, D.; Moffa, F.; Massobrio, M.; Tur-Kaspa, I. Guanylate cyclase activity and sperm function. Endocr. Rev. 2002, 23, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Labarca, P.; Nishigaki, T.; Espinosa, F. Ion Channels in Sperm Physiology. Am. Physiol. Soc. 1999, 79, 481–510. [Google Scholar]
- Schulz, J.R.; Wessel, G.M.; Vacquier, V.D. The exocytosis regulatory proteins syntaxin and VAMP are shed from sea urchin sperm during the acrosome reaction. Dev. Biol. 1997, 191, 80–87. [Google Scholar] [CrossRef] [PubMed]
- García-Soto, J.; Darszon, A. High pH-induced acrosome reaction and Ca2+ uptake in sea urchin sperm suspended in Na+-free seawater. Dev. Biol. 1985, 110, 338–345. [Google Scholar] [CrossRef]
- Begg, D.A.; Rebhun, L.I. pH regulates the polymerization of actin in the sea urchin egg cortex. J. Cell Biol. 1979, 83, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012, 425, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Chan, F.; Menge, B.A.; Hofmann, G.E. Transcriptomic responses to ocean acidification in larval sea urchins from a naturally variable pH environment. Mol. Ecol. 2013, 22, 1609–1625. [Google Scholar] [CrossRef] [PubMed]
- Ciapa, B.; Philippe, L. Intracellular and extracellular pH and Ca are bound to control mitosis in the early sea urchin embryo via ERK and MPF activities. PLoS ONE 2013, 8, e66113. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, M.; Trübenbach, K.; Brennecke, D.; Hu, M.Y.; Melzner, F. Resource allocation and extracellular acid–base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat. Toxicol. 2012, 110–111, 194–207. [Google Scholar]
- Stumpp, M.; Dupont, S.; Thorndyke, M.C.; Melzner, F. CO2 induced seawater acidification impacts sea urchin larval development II: Gene expression patterns in pluteus larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Runft, L.L.; Jaffe, L.A.; Mehlmann, L.M. Egg activation at fertilization: Where it all begins. Dev. Biol. 2002, 245, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.S.; Watanabe, K.; Hamaguchi, Y. Regulation of intracellular pH in sea urchin eggs by medium containing both weak acid and base. Cell Struct. Funct. 1997, 22, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Rees, B.B.; Patton, C.; Grainger, J.L.; Epel, D. Protein synthesis increases after fertilization of sea urchin eggs in the absence of an increase in intracellular pH. Dev. Biol. 1995, 169, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Buck, W.R. Sources of calcium in sea urchin eggs during the fertilization response. Dev. Biol. 1993, 157, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Dubé, F.; Schmidt, T.; Johnson, C.H.; Epel, D. The hierarchy of requirements for an elevated intracellular pH during early development of sea urchin embryos. Cell 1985, 40, 657–666. [Google Scholar] [CrossRef]
- Payan, P.; Girard, J.-P.; Ciapa, B. Mechanisms regulating intracellular pH in sea urchin eggs. Dev. Biol. 1983, 100, 29–38. [Google Scholar] [CrossRef]
- Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Vasilev, F.; Chun, J.T. Fertilization in echinoderms. Biochem. Biophys. Res. Commun. 2012, 425, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Parrington, J.; Davis, L.C.; Galione, A.; Wessel, G. Flipping the switch: How a sperm activates the egg at fertilization. Dev. Dyn. 2007, 236, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.J.; Galione, A. Fertilization and nicotinic acid adenine dinucleotide phosphate induce pH changes in acidic Ca2+ stores in sea urchin eggs. J. Biol. Chem. 2007, 282, 37730–37737. [Google Scholar] [CrossRef] [PubMed]
- Sardet, C.; Prodon, F.; Dumollard, R.; Chang, P.; Chênevert, J. Structure and function of the egg cortex from oogenesis through fertilization. Dev. Biol. 2002, 241, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Fliegel, L. Physiological role and regulation of the Na+-H+ exchanger. Can. J. Physiol. Pharmacol. 2006, 84, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.F. Sources of calcium in egg activation: A review and hypothesis. Dev. Biol. 1983, 99, 265–276. [Google Scholar] [CrossRef]
- Roderick, H.L.; Cook, S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, J.; Philipova, R.; Huang, J.Y.; Whitaker, M. MAP kinase dependent cyclinE/cdk2 activity promotes DNA replication in early sea urchin embryos. Dev. Biol. 2009, 334, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Santella, L. The role of calcium in the cell cycle: Facts and hypotheses. Biochem. Biophys. Res. Commun. 1998, 244, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Nicotera, P.; Santella, L. Calcium signalling in the cell nucleus. Cell Calcium 1997, 22, 313–319. [Google Scholar] [CrossRef]
- Swezey, R.R.; Epel, D. The in vivo rate of glucose-6-phosphate dehydrogenase activity in sea urchin eggs determined with a photolabile caged substrate. Dev. Biol. 1995, 169, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Kyozuka, K. Reinitiation of meiosis in starfish oocytes requires an increase in nuclear Ca2+. Biochem. Biophys. Res. Commun. 1994, 203, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.W.; Rebhun, L.I. Sea urchin egg cortical granule exocytosis is followed by a burst of membrane retrieval via uptake into coated vesicles. Dev. Biol. 1983, 99, 456–472. [Google Scholar] [CrossRef]
- Epel, D.; Patton, C.; Wallace, R.W.; Cheung, W.Y. Calmodulin activates NAD kinase of sea urchin eggs: An early event of fertilization. Cell 1981, 23, 543–549. [Google Scholar] [CrossRef]
- Rangel-Mata, F.; Méndez-Márquez, R.; Martínez-Cadena, G.; López-Godínez, J.; Nishigaki, T.; Darszon, A.; García-Soto, J. Rho, Rho-kinase, and the actin cytoskeleton regulate the Na+-H+ exchanger in sea urchin eggs. Biochem. Biophys. Res. Commun. 2007, 352, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Shapiro, B.M. Respiratory burst oxidase of fertilization. Proc. Natl. Acad. Sci. USA 1989, 86, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Matese, J.C.; Black, S.; McClay, D.R. Regulated exocytosis and sequential construction of the extracellular matrix surrounding the sea urchin zygote. Dev. Biol. 1997, 186, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.E.; Kazilek, C.J. Extracellular coats on the surface of Strongylocentrotus purpuratus eggs: Stereo electron microscopy of quick-frozen and deep-etched specimens. Cell Tissue Res. 1986, 246, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Wessel, G.M. Renovation of the egg extracellular matrix at fertilization. Int. J. Dev. Biol. 2008, 52, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.A.; Wessel, G.M. Regulated proteolysis by cortical granule serine protease 1 at fertilization. Mol. Biol. Cell 2004, 15, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.; Reed, C.; Marsden, M.; Rise, M.; Wang, D.; Burke, R.D. The αBβC integrin is expressed on the surface of the sea urchin egg and removed at fertilization. Dev. Biol. 2000, 227, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Deits, T.; Shapiro, B.M. pH-induced hysteretic transitions of ovoperoxidase. J. Biol. Chem. 1985, 260, 7882–7888. [Google Scholar] [PubMed]
- Deits, T.; Farrance, M.; Kay, E.S.; Medill, L.; Turner, E.E.; Weidman, P.J.; Shapiro, B.M. Purification and properties of ovoperoxidase, the enzyme responsible for hardening the fertilization membrane of the sea urchin egg. J. Biol. Chem. 1984, 259, 13525–13533. [Google Scholar] [PubMed]
- Foerder, C.A.; Shapiro, B.M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc. Natl. Acad. Sci. USA 1977, 74, 4214–4218. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Créton, R.; Wessel, G.M. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev. Cell. 2004, 7, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Wessel, G.M. Reactive oxygen species and Udx1 during early sea urchin development. Dev. Biol. 2005, 288, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.M. The control of oxidant stress at fertilization. Science 1991, 252, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.; Hager, L.; Shapiro, B. Ovothiol replaces glutathione peroxidase as a hydrogen peroxide scavenger in sea urchin eggs. Science 1988, 242, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Wessel, G.M.; Berg, L.; Adelson, D.L.; Cannon, G.; McClay, D.R. A Molecular analysis of hyalin—A substrate for cell adhesion in the hyaline layer of the sea urchin embryo. Dev. Biol. 1998, 193, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Adelson, D.L.; Alliegro, M.C.; McClay, D.R. On the ultrastructure of hyalin, a cell adhesion protein of the sea urchin embryo extracellular matrix. J. Cell Biol. 1992, 116, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.G.; Vacquier, V.D. The apical lamina of the sea urchin embryo: Major glycoproteins associated with the hyaline layer. Dev. Biol. 1982, 89, 168–178. [Google Scholar] [CrossRef]
- Citkowitz, E. Analysis of the isolated hyaline layer of sea urchin embryos. Dev. Biol. 1972, 27, 494–503. [Google Scholar] [CrossRef]
- Moore, A.R. On the hyaline membrane and hyaline droplets of the fertilized egg of the sea urchin, Strongylocentrotus purpuratus. Protoplasma 1927, 3, 524–530. [Google Scholar] [CrossRef]
- McClay, D.R.; Fink, R.D. Sea urchin hyalin: Appearance and function in development. Dev. Biol. 1982, 92, 285–293. [Google Scholar] [CrossRef]
- Spiegel, E.; Spiegel, M. The hyaline layer is a collagen-containing extracellular matrix in sea urchin embryos and reaggregating cells. Exp. Cell Res. 1979, 123, 434–441. [Google Scholar] [CrossRef]
- Citkowitz, E. The hyaline layer: Its isolation and role in echinoderm development. Dev. Biol. 1971, 24, 348–362. [Google Scholar] [CrossRef]
- Whitaker, M.J. Cell cycle control proteins are second messenger targets at fertilization in sea-urchin eggs. J. Reprod. Fertil. Suppl. 1990, 42, 199–204. [Google Scholar] [PubMed]
- Kishimoto, T. Cell-cycle control during meiotic maturation. Curr. Opin. Cell Biol. 2003, 15, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Kuwaki, T.; Sakai, H. Phosphorylation of microtubule-associated proteins (MAPs) and pH of the medium control interaction between MAPs and actin filaments. J. Biochem. 1981, 90, 575–578. [Google Scholar] [PubMed]
- Begg, D.; Rebhun, L.; Hyatt, H. Structural organization of actin in the sea urchin egg cortex: Microvillar elongation in the absence of actin filament bundle formation. J. Cell Biol. 1982, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, L.I.; Begg, D.A.; Fisher, G. Reorganization of the cortex of sea urchin eggs as a function of activation. Cell Differ. 1982, 11, 271–276. [Google Scholar] [CrossRef]
- Santella, L.; Chun, J. Actin, more than just a housekeeping protein at the scene of fertilization. Sci. China Life Sci. 2011, 54, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Schatten, G.; Bestor, T.; Balczon, R.; Henson, J.; Schatten, H. Intracellular pH shift leads to microtubule assembly and microtubule-mediated motility during sea urchin fertilization: Correlations between elevated intracellular pH and microtubule activity and depressed intracellular pH and microtubule disassembly. Eur. J. Cell Biol. 1985, 36, 116–127. [Google Scholar] [PubMed]
- Alonso, M.T.; García-Sancho, J. Nuclear Ca2+ signalling. Cell Calcium 2011, 49, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Baltz, J.M.; Zhao, Y.; Phillips, K.P. Intracellular pH and its regulation during fertilization and early embryogenesis. Theriogenology 1995, 44, 1133–1144. [Google Scholar] [CrossRef]
- Treviño, C.L.; Orta, G.; Figueiras-Fierro, D.; de la Vega-Beltran, J.L.; Ferreira, G.; Balderas, E.; José, O.; Darszon, A. Cl− channels and transporters in sperm physiology. In Sexual Reproduction in Animals and Plants; Sawada, H., Inoue, N., Iwano, M., Eds.; Springer: Tokyo, Japan, 2014; pp. 59–84. [Google Scholar]
- Beltrán, C.; Galindo, B.E.; Rodríguez-Miranda, E.; Sánchez, D. Signal transduction mechanisms regulating ion fluxes in the sea urchin sperm. Signal Transduct. 2007, 7, 103–117. [Google Scholar] [CrossRef]
- Humphreys, B.; Jiang, L.; Chernova, M.N.; Alper, S.L. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1994, 36, C1295–C1307. [Google Scholar]
- Churchill, G.C.; Okada, Y.; Thomas, J.M.; Genazzani, A.A.; Patel, S.; Galione, A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 2002, 111, 703–708. [Google Scholar] [CrossRef]
- Heinecke, J.W.; Shapiro, B.M. Superoxide peroxidase activity of ovoperoxidase, the cross-linking enzyme of fertilization. J. Biol. Chem. 1990, 265, 9241–9246. [Google Scholar] [PubMed]
- Place, S.P.; Smith, B.W. Effects of seawater acidification on cell cycle control mechanisms in Strongylocentrotus purpuratus Embryos. PLoS ONE 2012, 7, e34068. [Google Scholar] [CrossRef] [PubMed]
- Kiyomoto, M.; Kikuchi, A.; Morinaga, S.; Unuma, T.; Yokota, Y. Exogastrulation and interference with the expression of major yolk protein by estrogens administered to sea urchins. Cell Biol. Toxicol. 2008, 24, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.D.; Myers, R.L.; Sexton, T.L.; Jackson, C. Cell movements during the initial phase of gastrulation in the sea urchin embryo. Dev. Biol. 1991, 146, 542–557. [Google Scholar] [CrossRef]
- Hardin, J.D.; Cheng, L.Y. The mechanisms and mechanics of archenteron elongation during sea urchin gastrulation. Dev. Biol. 1986, 115, 490–501. [Google Scholar] [CrossRef]
- Hoshi, M. Exogastrulation induced by heavy water in sea urchin larvae. Cell Differ. 1979, 8, 431–436. [Google Scholar] [CrossRef]
- Takahashi, T.; Hoshi, M.; Asahina, É. Exogastrulation induced by chilling in sea urchin larvae. Dev. Growth Differ. 1977, 19, 131–137. [Google Scholar] [CrossRef]
- Okazaki, K. Exogastrulation induced by calcium deficiency inthe sea urchin, Pseudocentrotus depressus*. Embryologia 1956, 3, 23–36. [Google Scholar] [CrossRef]
- Epel, D. Does ADP regulate respiration following fertilization of sea urchin eggs? Exp. Cell Res. 1969, 58, 312–318. [Google Scholar] [CrossRef]
- Coffman, J.A.; Coluccio, A.; Planchart, A.; Robertson, A.J. Oral–aboral axis specification in the sea urchin embryo: III. Role of mitochondrial redox signaling via H2O2. Dev. Biol. 2009, 330, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Henson, J.; Begg, D.; Kaminer, B.; Sardet, C. Characterization of sea urchin egg endoplasmic reticulum in cortical preparations. Dev. Biol. 1991, 148, 398–401. [Google Scholar] [CrossRef]
- Izant, J.G. The role of calcium ions during mitosis. Chromosoma 1983, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, E.; Howard, L.; Spiegel, M. Elongated microvilli support the sea urchin embryo concentrically within the perivitelline space until hatching. Dev. Genes Evol. 1989, 198, 85–91. [Google Scholar] [CrossRef]
- Mabuchi, I. Cleavage furrow: Timing of emergence of contractile ring actin filaments and establishment of the contractile ring by filament bundling in sea urchin eggs. J. Cell Sci. 1994, 107, 1853–1862. [Google Scholar] [PubMed]
- Schweitzer, J.K.; D’Souza-Schorey, C. Finishing the job: Cytoskeletal and membrane events bring cytokinesis to an end. Exp. Cell Res. 2004, 295, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bögner, D.; Koehler, A. Ocean Acidification-induced fertilization disruptions in Psammechinus miliaris. Unpublished work. 2016. [Google Scholar]


| Stage | Studied Effect [Reference] | Observations |
|---|---|---|
| Gametogenesis | - ⇑ HCO3− compensation mechanisms and dissolution of carbonate structures [18] - ⇓ Food intake and gonadal growth [19] - ⇓ Ability to spawn [20]- Delayed maturation and spawning at low pH [21] | Temperature and photoperiod regulate the gametogenesis cycle; future studies could focus on the effects of climate change variables on the hormone system behind gametogenesis regulation in different taxa. |
| Fertilization rate | - ⇓ Proportion of fertilized eggs [22,23] - ⇓ Fecundity (reversible effect) [24] - ⇓ Fertilization success at longer exposure periods before fertilization [25] - ⇑ Polyspermy rates [26] | Depending on species origin and methods used, there are inconsistent results indicating the need of a standard protocol. |
| Gamete’s viability | - Life spam under laboratory conditions [27,28,29,30] | Stated under laboratory conditions; more studies are required including other environmental variables. Temperatures out of the range of the habitat conditions reduce the viability of the gametes but within that range an increase may contribute to gametes’ contact. |
| Sperm cells | - ⇓ Sperm motility (due to pH dependent activation of dynein ATPase) [31,32,33] - ⇓ Sperm swimming speed [34,35] - ⇓ Mitochondrial activity [36] | Responses depend on species. Some species show no effect or even positive impact of decreasing pH. That could indicate acclimation or adaption patterns. More studies are required to demonstrate the effects of pH, T, and oxygen depletion on sperm motility, swimming speed, mitochondrial activity, ATP generation for motility, and ionic mechanism involved on sperm activation, capacitation, and acrosomal reaction. |
| Sperm-Egg recognition and contact | - ⇓ Egg jelly induction of acrosomal reaction at low pH [37] | The pH effect on the induction of the acrosomal reaction was elegantly studied in S. purpuratus. The authors demonstrated the resilience of this process until pHe = 7 but pH changes were not achieved with CO2 manipulations. Studies are required for T and H/A effects. |
| Egg cells and early zygotes | - ⇓ pHi due to exposure to acidified seawater matrices before and after fertilization [22] (with still unstudied impact on Cai2+ sequestration and cytosolic buffer affinity for Ca2+) - ⇓ Proportion of perfect FE and HL formation [22] - ⇓ Actin polymerization in the cortex region [38] - Cleavage retardation/inhibition at low pH leading to delayed larval formation [38,39] - ⇑ Alterations of the division plane [40,41] - Embryo radialization [42,43,44] | There are few studies dealing with T and oxygen depletion impacts on eggs before and immediately after fertilization. The strong dependence of all stages after fertilization on the redox state of the zygote, the pHi and Cai2+ levels and the sensitivity of the molecular machinery to pH (and probably T and H/A too), make of these stages a great-unexplored field requiring further attention. Markers ease to be studied at these stages: cortical reaction, CGSP1 activity which decreases with acidic pH, exocytosis and endocytosis cycles, ROS formation/elimination, enzymatic activity, cytoskeleton, and mitochondrial activity. |
| Larval development | - ⇑ Larval malformations (e.g., exogastrulation, defect spiculogenesis and arms’ formation etc.) [45,46,47,48] - ⇓ Development efficiency and larval settlement [49] | Many studies use the larval stages for the demonstration of environmental effects but few of them studied the molecular mechanisms behind the processes supporting the larval development. Studies are required to elucidate the impact of oxygen depletion. |
| Phylum, Species | pH/T Levels Tested | Results | Ref. |
|---|---|---|---|
| Echinodermata | |||
| Centrostephanus rodgersii | pH = 8.10 pH = 7.80–7.60 (−0.30 until −0.5 pH units) | - Reduced mitochondrial membrane potential - Slight decrease in sperm swimming behaviour - Significant inter-individual variability | [36] |
| Heliocidaris erythrogramma | pH = 8.12 pH = 7.80–7.60 (−0.32 until −0.52 pH units) | - No effect on sperm swimming speed - Reduced proportion of motile sperm - Significant inter-individual variability | [87] |
| Heliocidaris erythrogramma | pH = 8.1 pH = 7.7 (−0.4 pH units) | - Reduced sperm swimming speed - Reduced percent sperm motility | [34] |
| Psammechinus miliaris | pH = 8.06 pH = 7.95–7.67 (−0.11 until −0.39 pH units) T = 14 °C; 17 °C; 20 °C | - Improvement of swimming speed and percent motility at reduced pH - Temperature affects swimming speed with no impact on percent motility | [35] |
| Holothuria spp. | pH = 8.03 pH = 7.77–6.55 (−0.26 until −1.48 pH units) | - Reduced sperm flagellar motility | [31] |
| Mollusca | |||
| Mytilus galloprovincialis | pH = 8.0 pH = 7.6 (−0.4 pH units) | - Reduced proportion of motile sperm - Reduced sperm swimming speed | [88] |
| Crassostrea gigas | pH = 8.21–7.81 (−0.35 pH units) | - No effect on sperm swimming speed and sperm motility | [32] |
| Annelida | |||
| Galeolaria caespitosa | pH = 8.10 pH = 7.80–7.60 (−0.30 until −0.50 pH units) | - Reduced swimming speed and percent motility | [33] |
| Cnidaria | |||
| Acropora digitifera | pH = 8.17 pH = 8.05–7.74 (−0.12 until −0.43 pH units) | - Reduced sperm flagellar motility | [89] |
| Acropora digitifera | pH = 8.03 pH = 7.77–6.55 (−0.26 until −1.48 pH units) | - Reduced sperm flagellar motility | [31] |
| Chordata | |||
| Gadus morhua | pH = 8.08 pH = 7.55 (−0.53 pH units) | - No effect on sperm swimming speed and sperm motility | [90] |
| Salmo salar | pH = 6.8 pH = 6.0−3.4 * (−0.8 until −3.4 pH units) | - Reduced sperm motility | [91] |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bögner, D. Life under Climate Change Scenarios: Sea Urchins’ Cellular Mechanisms for Reproductive Success. J. Mar. Sci. Eng. 2016, 4, 28. https://doi.org/10.3390/jmse4010028
Bögner D. Life under Climate Change Scenarios: Sea Urchins’ Cellular Mechanisms for Reproductive Success. Journal of Marine Science and Engineering. 2016; 4(1):28. https://doi.org/10.3390/jmse4010028
Chicago/Turabian StyleBögner, Desislava. 2016. "Life under Climate Change Scenarios: Sea Urchins’ Cellular Mechanisms for Reproductive Success" Journal of Marine Science and Engineering 4, no. 1: 28. https://doi.org/10.3390/jmse4010028
APA StyleBögner, D. (2016). Life under Climate Change Scenarios: Sea Urchins’ Cellular Mechanisms for Reproductive Success. Journal of Marine Science and Engineering, 4(1), 28. https://doi.org/10.3390/jmse4010028