Reproductive Effects of Medicinal Plant (Azadirachta indica) Used as Forage and for Ethnoveterinary Practices: New Insights from Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Neem-Seed Oil Extraction
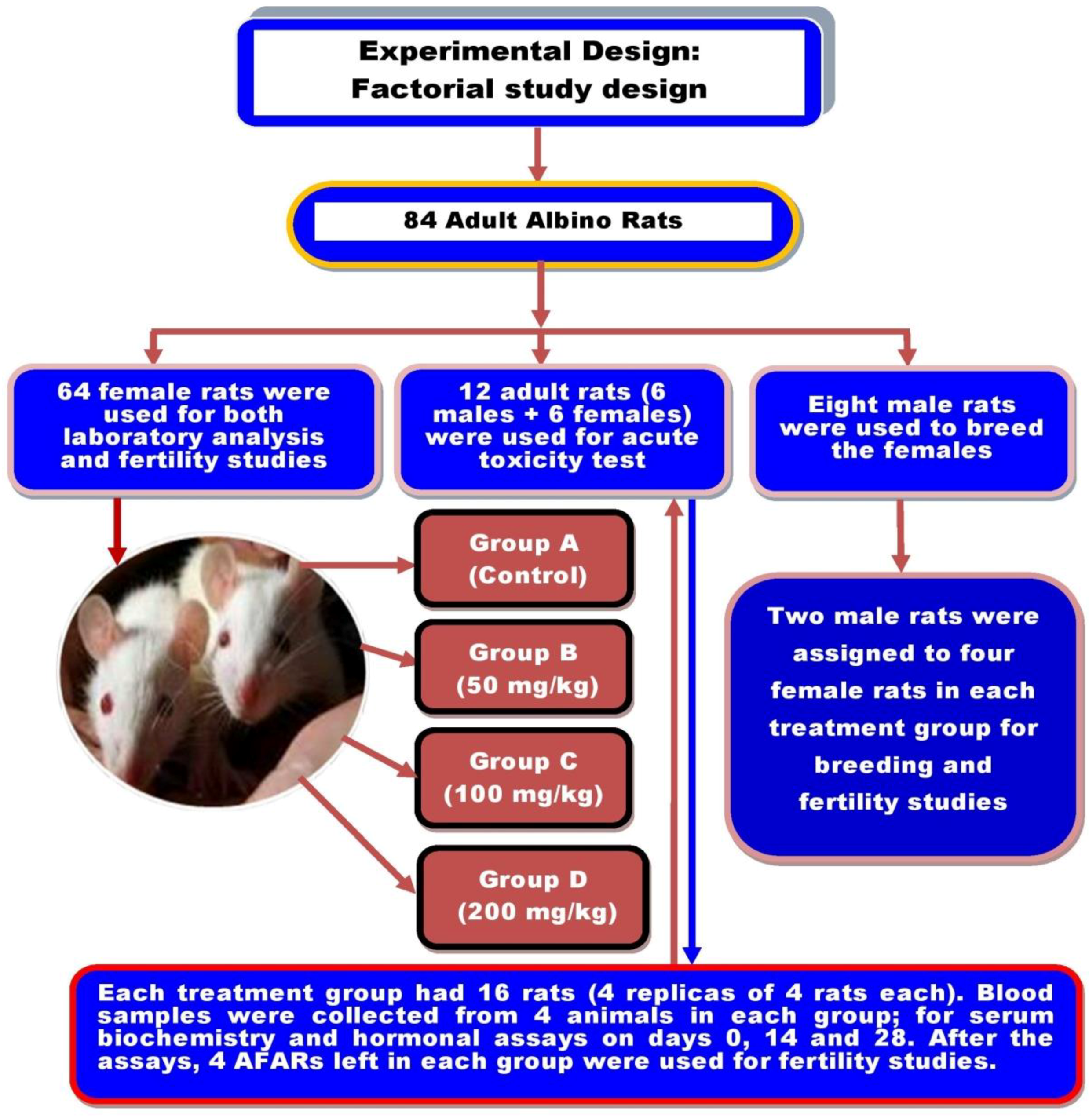
2.3. Experimental Animals and Study Design
2.4. Toxicity Test
2.5. Blood Sample Collection
2.6. Hormonal Assays
2.7. Serum Biochemistry Assays
2.8. Histopathological Examination
2.9. Fertility Studies
2.10. Statistical Analysis
3. Results
3.1. Neem Seed Oil Extraction and Toxicity Testing
3.2. Hormonal Profiles
3.2.1. Mean Serum Estradiol
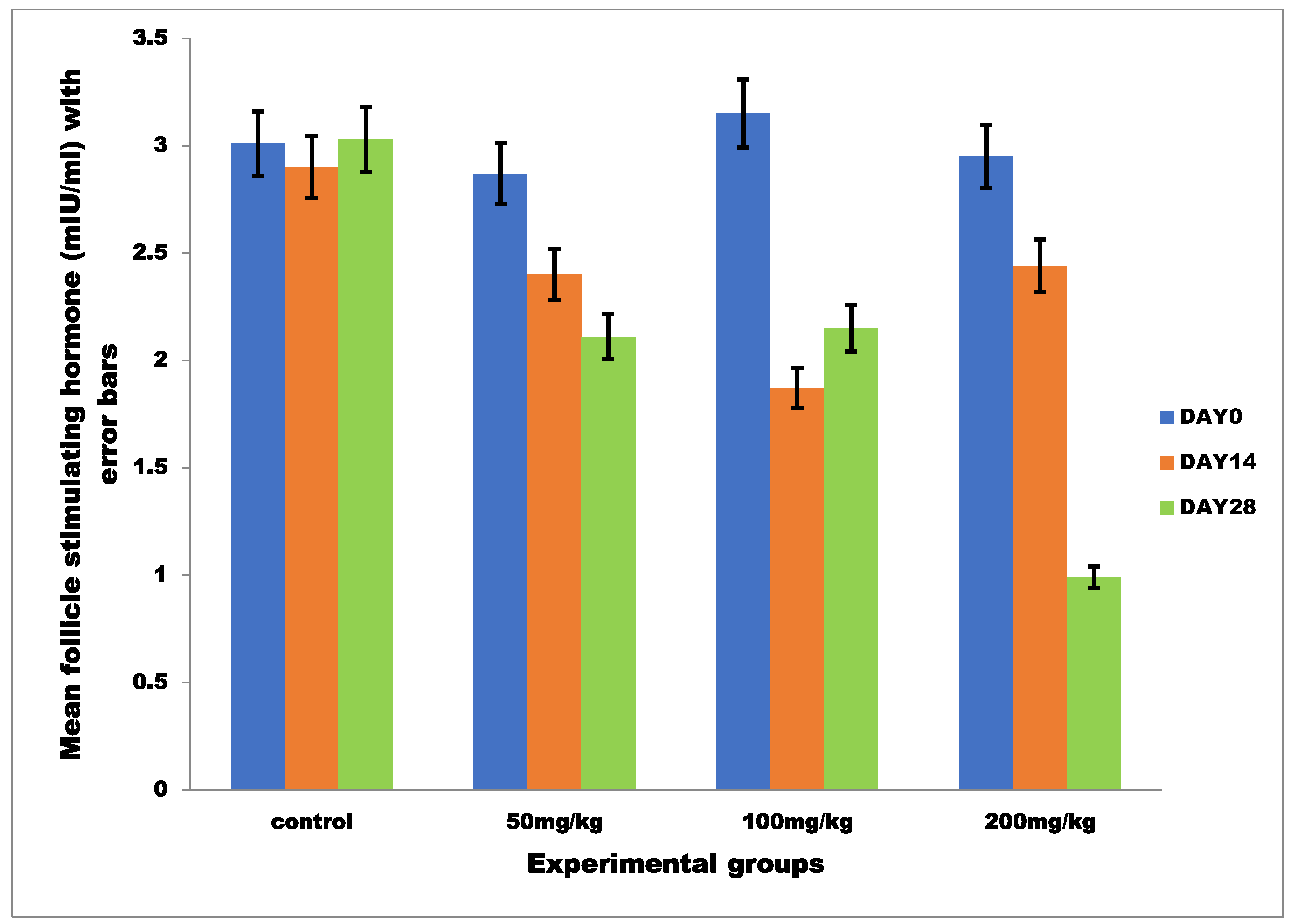
3.2.2. Mean Serum Follicle Stimulating Hormone
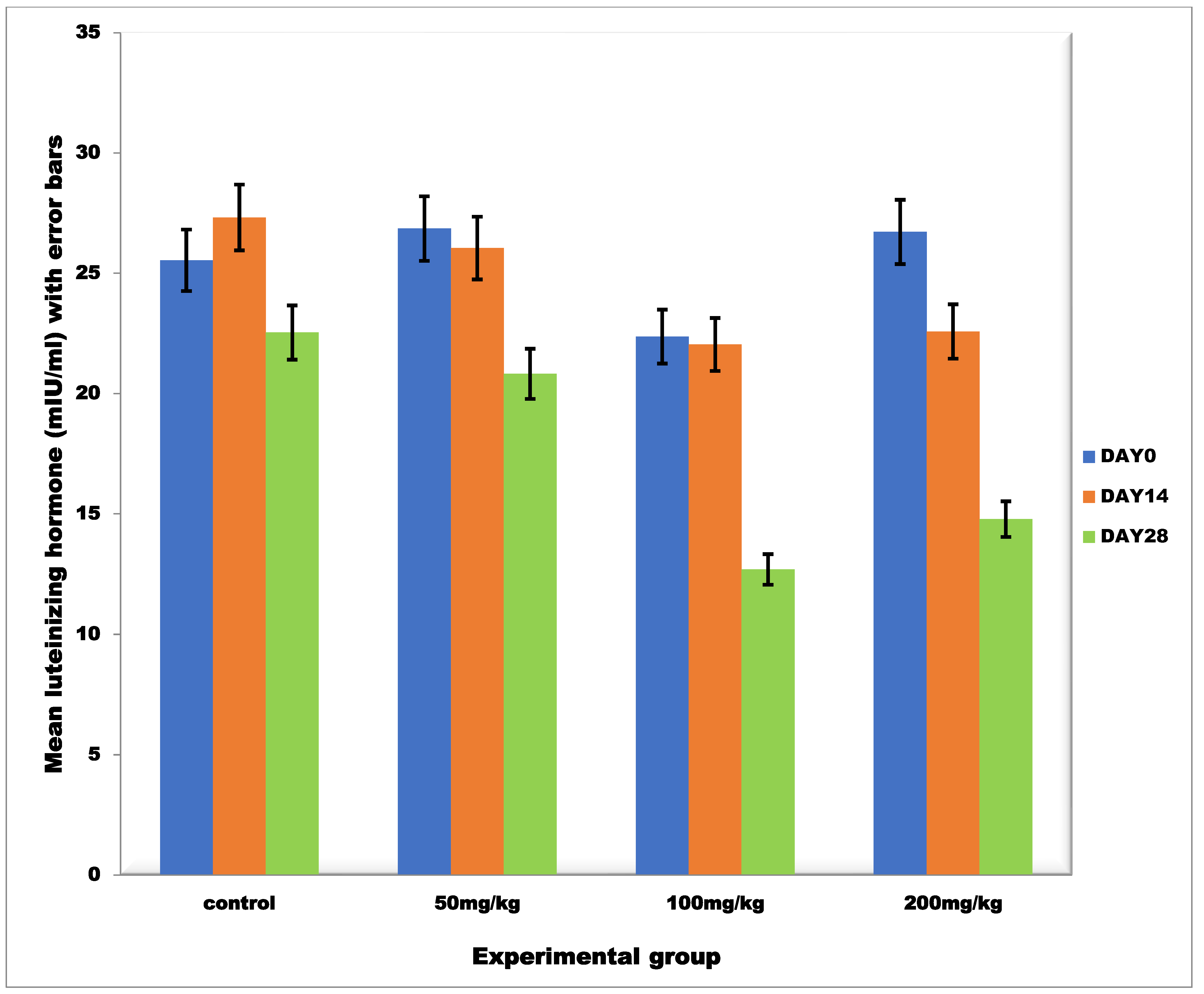
3.2.3. Mean Serum Luteinizing Hormone
3.3. Serum Biochemistry
3.3.1. Mean Serum Total Protein
3.3.2. Lipid Profile
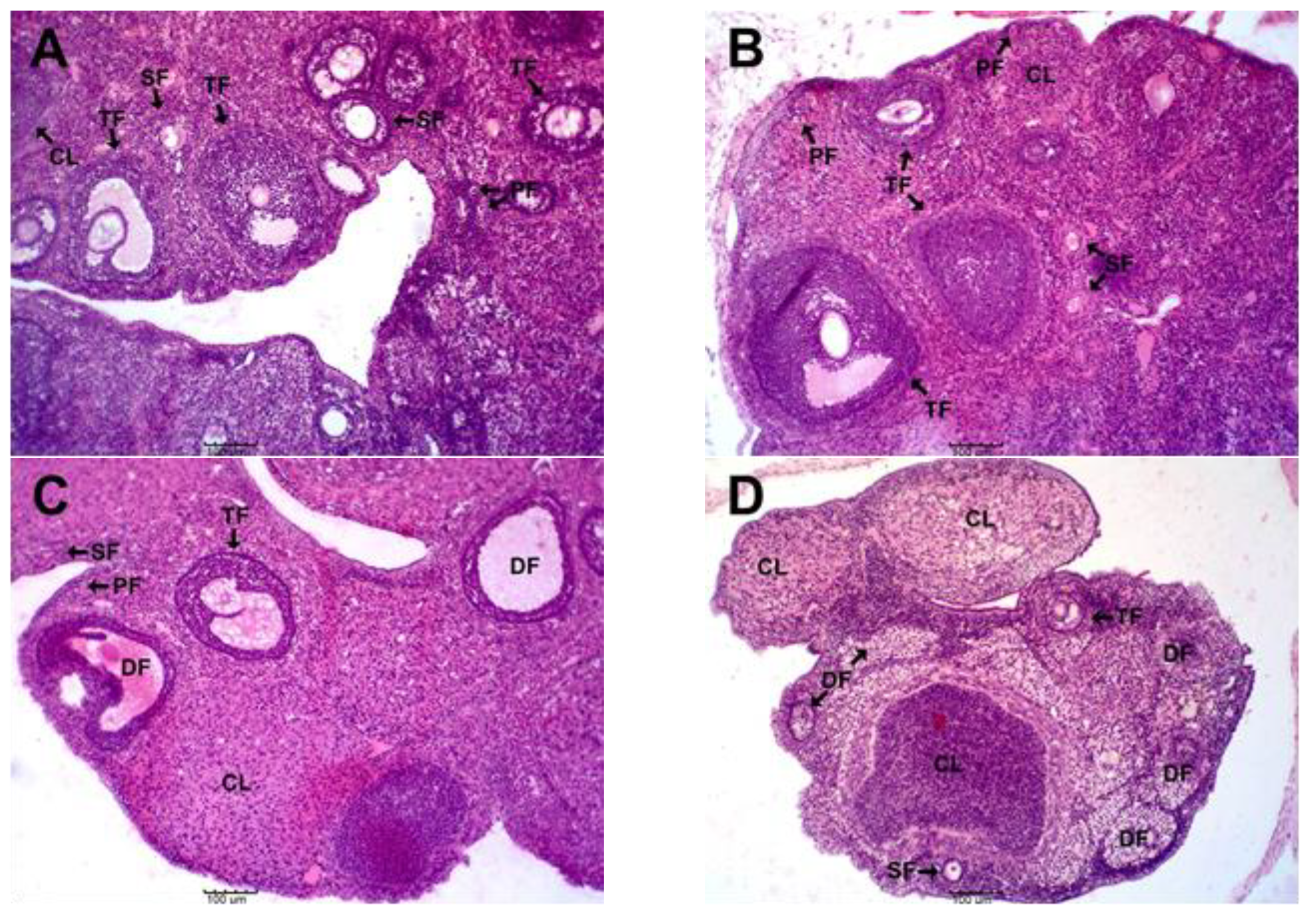
3.4. Effects of MSEAI on Ovarian Histology
3.5. Effects of the MSEAI on Reproductive and Fertility Parameters
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, M.A.; Khan, A.H.; Adnan, M.; Ullah, H. Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, J.; Zahra, L.; Hossein, A. Medicinal plants: Past history and future perspective. J. HerbMed Pharmacol. 2018, 7, 1–7. [Google Scholar]
- Oyedemi, B.O.; Oyedemi, S.O.; Chibuzor, J.V.; Ijeh, I.I.; Coopoosamy, R.M.; Aiyegoro, A.O. Pharmacological Evaluation of Selected Medicinal Plants Used in the Management of Oral and Skin Infections in Ebem-Ohafia District, Abia State, Nigeria. Sci. World J. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Susana, O.M.; Asafo-Agyei, T.; Archer, M.A.; Junior, P.A.A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D.; Agyare, C. Medicinal Plants for Treatment of Prevalent Diseases. In Pharmacognosy-Medicinal Plants; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Pratik, D.; Nisha, N.; Ahmed, I.A.Y.; Katalin, K.; Rita, B.; György, T. Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions. Plants 2020, 9, 535. [Google Scholar] [CrossRef]
- Adjorlolo, L.K.; Timpong-Jones, E.C.; Boadu, S.; Adogla-Bessa, T. Potential contribution of neem (Azadirachta indica) leaves to dry season feeding of ruminants in West Africa. Livest. Res. Rural. Dev. 2016, 28, 75. [Google Scholar]
- Lawal-Adebowale, O.A. Dynamics of Ruminant Livestock Management in the Context of the Nigerian Agricultural System. In Livestock Production; Javed, K., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Distel, R.A.; Arroquy, J.I.; Lagrange, S.; Villalba, J.J. Designing Diverse Agricultural Pastures for Improving Ruminant Production Systems. Front. Sustain. Food Syst. 2020, 4, 596869. [Google Scholar] [CrossRef]
- Farahna, M.; Bedri, S.; Khalid, S.; Idris, M.; Pillai, C.R.; Khalil, E.A. Anti-plasmodial effects of Azadirachta indica in experimental cerebral malaria: Apoptosis of cerebellar Purkinje cells of mice as a marker. N. Am. J. Med. Sci. 2010, 2, 518–525. [Google Scholar] [CrossRef]
- Bedri, S.; Khalil, E.A.; Khalid, S.A.; Alzohairy, M.A.; Mohieldein, A.; Aldebasi, Y.H.; Etet, P.F.S.; Farahna, M. Azadirachta indica ethanolic extract protects neurons from apoptosis and mitigates brain swelling in experimental cerebral malaria. Malar. J. 2013, 12, 298. [Google Scholar] [CrossRef]
- Bharat, P.; Sagar, R.; Sulav, R.; Ankit, P. Investigations of antioxidant and bacterial activity of leaf extracts of Azadirachta indica. Afr. J. Biotechnol. 2015, 14, 3159–3163. [Google Scholar] [CrossRef]
- Brahim, M.; Ismahan, L.; Mohamed, L.O. Larvicidal Activity and Influence of Azadirachtin (Neem Tree Extract) on the Longevity and Fecundity of Mosquito Species. Acta Zool. Bulg. 2017, 69, 429–435. [Google Scholar]
- Sirsat, S.D.; Visha, P.; Nanjappan, K. Effects of dietary chitosan and Neem leaf meal supplementation on intestinal bacterial count in broiler chickens. Int. J. Microbiol. Appl. Sci. 2018, 7, 3016–3022. [Google Scholar] [CrossRef]
- Habluetzel, A.; Pinto, B.; Tapanelli, S.; Nkouangang, J.; Saviozzi, M.; Chianese, G.; Lopatriello, A.; Tenoh, A.R.; Yerbanga, R.S.; Taglialatela-Scafati, O.; et al. Effects of Azadirachta indica seed kernel extracts on early erythrocytic schizogony of Plasmodium berghei and pro-inflammatory response in inbred mice. Malar. J. 2019, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.J.; Simon-Oke, I.A.; Oladokun, O.I. Antiplasmodial Activity of Ethanolic Extract of Neem Leaf (Azadirachta indica) in Albino Mice Infected with Plasmodium berghei. Int. Arch. Clin. Pharmacol. 2020, 7, 24. [Google Scholar] [CrossRef]
- Tiwari, M.; Gupta, A.; Prasad, S.; Tripathi, A.; Yadav, P.K.; Pandey, A.N.; Premkumar, K.V.; Pandey, A.K.; Shrivastav, T.G.; Chaube, S.K. Neem (Azadirachta indica L.) and Oocyte Quality. Glob. J. Reprod. Med. 2017, 1, 555553. [Google Scholar]
- Seriana, I.; Akmal, M.; Darusman, X.; Wahyumi, S. Neem leaves extract (Azadirachta indica A. Juss) on male reproductive system: A mini-review. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 1st International Seminar on Natural Resources and Environmental Management 2019; Bogor, Indonesia, 15 August 2019, IOP Publishing: Bristol, UK, 2019; Volume 399, p. 399. [Google Scholar] [CrossRef]
- Oguejiofor, C.F.; Eke, I.G.; Anya, K.O. Antifertility effects of Azadirachta indica methanol seed extract on canine spermatozoa in vitro. Asian Pac. J. Reprod. 2020, 9, 135–141. [Google Scholar]
- Chaube, S.K.; Shrivastav, T.G.; Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.K. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 2014, 3, 464. [Google Scholar] [CrossRef]
- Kgari, R.D.; Muller, J.C.; Dzama, K.; Makgahlela, M.L. Evaluation of female fertility in dairy cattle enterprises—A review. S. Afr. J. Anim. Sci. 2020, 50. [Google Scholar] [CrossRef]
- Surgeone, G.A. Rodent Control in Livestock and Poultry Facilities. Available online: https://www.thepoultrysite.com/articles/rodent-control-in-livestock-and-poultry-facilities (accessed on 2 May 2022).
- Fisher, P.; Campbell, K.J.; Howald, G.R.; Warburton, B. Anticoagulant Rodenticides, Islands and Animal Welfare Accountancy. Animals 2019, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.M.; Parkes, A.; Wight, D.; Petticrew, M.; Hart, G.J. Limits to modern contraceptive use among young women in developing countries: A systematic review of qualitative research. Reprod. Health 2009, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Muralidhar, S. Contraceptive methods: Needs, options and utilization. J. Obstet. Gynaecol. India 2011, 61, 626–634. [Google Scholar] [CrossRef]
- Tsui, A.O.; Brown, W.; Li, Q. Contraceptive Practice in Sub-Saharan Africa. Popul. Dev. Rev. 2017, 43, 166–191. [Google Scholar] [CrossRef]
- Ajayi, A.I.; Adeniyi, O.V.; Akpan, W. Use of traditional and modern contraceptives among childbearing women: Findings from a mixed methods study in two southwestern Nigerian states. BMC Public Health 2018, 18, 604. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, L.A.; Stephens, M.J.; Nsarko, N.E.; Akosah, E.; Baumgartner, J.N.; Ohemeng-Dapaah, S.; Watt, M.H. Side effect concerns and their impact on women’s uptake of modern family planning methods in rural Ghana: A mixed methods study. BMC Women’s Health 2020, 20, 57. [Google Scholar] [CrossRef]
- Lee, M. The barriers to using modern contraceptive methods among rural young married women in Moshi Rural District, the Kilimanjaro region, Tanzania. Afr. J. Reprod. Health 2021, 25, 99–107. [Google Scholar]
- Varshney, S.; Verma, S.; Arya, R. A review on scientific validity on medicinal plants used as female contraceptives. Int. J. Ayurveda Res. 2016, 7, 277–285. [Google Scholar] [CrossRef]
- Rinzin, K.; Population Dynamics and Health Status of Free-Roaming Dogs in Bhutan. Ph.D thesis, Murdoch University, Perth, Australia. Available online: https://researchrepository.murdoch.edu.au/id/eprint/27867/ (accessed on 10 December 2021).
- Njoga, U.J.; Ajibo, F.E.; Njoga, E.O. The use of contraceptives for control of stray dog population and spread of rabies virus in Nigeria. Anim. Res. Int. 2020, 17, 3809–3820. [Google Scholar]
- Mshelbwala, P.P.; Weese, J.S.; Sanni-Adeniyi, O.A.; Chakma, S.; Okeme, S.S.; Mamun, A.A.; Rupprecht, C.E.; Magalhaes, R.J.S. Rabies epidemiology, prevention and control in Nigeria: Scoping progress towards elimination. PLoS Negl. Trop. Dis. 2021, 15, e0009617. [Google Scholar] [CrossRef]
- Institute for Laboratory Animal Research; National Research Council (NRC). The Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 56–82. [Google Scholar]
- Attia, I.B.; Paolo, Z.; Flaminia, C.M.; Piras, A.; Rosa, A.; Chaieb, M.; Rescigno, A. Chemical Composition and Antioxidant Potential Differences between Cynomorium coccineum L. Growing in Italy and in Tunisia: Effect of Environmental Stress. Diversity 2018, 10, 53. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–871. [Google Scholar] [CrossRef]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; Saunders Co.: Philadelphia, WA, USA, 1995; p. 38. [Google Scholar]
- Johnson, A.M.; Rohlfs, E.M.; Silverman, L.M. Proteins. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; Saunders Company: Philadelphia, PA, USA, 1999; pp. 477–540. [Google Scholar]
- Deeg, R.; Ziegenhorn, J. Kinetic enzymatic method for automated determination of total cholesterol in serum. Clin Chem. 1983, 29, 1798–1802. [Google Scholar] [CrossRef]
- Cole, T.G.; Klotzsch, S.G.; McNamara, J. Measurement of triglyceride concentration. In Handbook of Lipoprotein Testing; Rifai, N., Warnick, G.R., Dominiczak, M.H., Eds.; AACC Press: Washington, DC, USA, 1997; pp. 115–126. [Google Scholar]
- Naito, H.K. Cholesterol: Clinical Chemistry; The CV Mosby Co.: St Louis, MI, USA; Toronto Princeton: Toronto, ON, Canada, 1984; pp. 1207–1213. [Google Scholar]
- Assmann, G.; Jabs, H.U.; Kohnert, U.; Nolte, W.; Schriewer, H. LDL-cholesterol determination in blood serum following precipitation of LDL with polyvinylsulfate. Clin. Chim. Acta 1984, 140, 77–83. [Google Scholar] [CrossRef]
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to histopathological evaluation. In Drug Safety Evaluation: Methods in Molecular Biology (Methods and Protocols); Gautier, J.C., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 69–82. [Google Scholar]
- Ochiogu, I.S.; Uchendu, C.N.; Ihedioha, J.I. A new and simple method of confirmatory detection of mating in albino rats (Rattus norvegicus). Anim. Res. Int. 2006, 3, 527–530. [Google Scholar] [CrossRef]
- Amabe, O.A.; Moses, B.E.; Kebe, E.O.; Mfon, I.A.; Theresa, B.E. Hormonal and histomorohological effects sof Azadirachta indica leaf extract on the pars anterior of Wister rats. Int. J. Morphol. 2011, 29, 441–445. [Google Scholar]
- Ekaluo, U.B.; Ikpeme, E.V.; Udensi, O.; Markson, A.A.; Madunagu, B.E.; Omosun, G.; Umana, E.J. Effects of aqueous leaf extracts of neem (Azadirachta indica) on the hormonal melieu of male rats. Int. J. Cur. Res. 2010, 4, 1–3. [Google Scholar]
- Roop, J.K. Effect of fractions of Azadirachta indica A. Juss seed extract on reproduction in female albino rats. Indo Am. J. Pharm. Res. 2015, 5, 2143–2150. [Google Scholar]
- Roop, J.K.; Dhaliwal, P.K.; Guraya, S.S. Extracts of Azadirachta Indica and Melia Azadarach seeds inhibit folliculogenesis in albino rats. Braz. J. Med. Biol. Res. 2005, 38, 943–947. [Google Scholar] [CrossRef] [PubMed]
- François, C.; Petit, F.; Giton, F.; Gougeon, A.; Ravel, C.; Magre, S.; Cohen-Tannoudji, J.; Guigon, C.J. A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Sci. Rep. 2017, 7, 46222. [Google Scholar] [CrossRef] [PubMed]
- Serge, A.J. Follicle Stimulating Hormone Abnormalities. Available online: https://emedicinemedscapecom/article/118810-overview (accessed on 14 March 2021).
- Richard, B. Gonadotropins: Luteinizing Hormone and Follicle Stimulating Hormone. Available online: www.vivocolostateedu/hbooks/pathphys/endocrine/hypopit/ihfshhtml (accessed on 12 January 2021).
- Lamidi, A.A.; Ologbose, F.I. Dry season feeds and feeding: A threat to sustainable ruminant animal production in Nigeria. J. Agric. Soc. Sci. 2014, 14, 17–30. [Google Scholar]
- Avornyo, F.K.; Zougmore, R.; Partey, S.; Tengan, K. Candidate fodder trees and shrubs for sustainable ruminant production in northern Ghana. Available online: http://www.lrrd.org/lrrd30/9/favor30154.html (accessed on 30 September 2018).
- Ouédraogo, K.; Zaré, A.; Korbéogo, G.; Ouédraogo, O.; Linstädter, A. Resilience strategies of West African pastoralists in response to scarce forage resources. Pastoralism 2021, 11, 16. [Google Scholar] [CrossRef]
- Thorne, P.J.; Thornton, P.K.; Kruska, R.L.; Reynolds, L.; Waddington, S.R.; Rutherford, A.S.; Odero, A.N. Maize as Food, Feed and Fertiliser in Intensifying Crop-Livestock Systems in East and Southern Africa: An Exante Impact Assessment of Technology Interventions to Improve Smallholder Welfare; International Livestock Research Institute Impact Assessment Series 11; International Livestock Research Institute: Nairobi, Kenya, 2002; p. 123. [Google Scholar]
- Naah, J.B.S.N. Investigating criteria for valuation of forage resources by local agro-pastoralists in West Africa: Using quantitative ethnoecological approach. J. Ethnobiol. Ethnomed. 2018, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Bongaarts, J. Development: Slow down population growth. Nature 2016, 530, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, J.D.; Adamson, D.M. Family Planning in Developing Countries: An Unfinished Success Story. Available online: https://www.rand.org/pubs/issue_papers/IP176.html (accessed on 3 May 2022).
- Ndayizigiye, M.; Fawzi, M.C.S.; Lively, C.T.; Ware, N. Understanding low uptake of contraceptives in resource-limited settings: A mixed-methods study in rural Burundi. BMC Health Serv. Res. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Alicia, A.G.; Ricardo, P.D.; Geula, G.; Carlos, M.T. Progesterone Promotes Survival of the Rat Corpus Luteum in the Absence of Cognate Receptors. Biol. Reprod. 2003, 68, 151–158. [Google Scholar]
- Prasad, B.; Parmar, D.; Sharma, N.C. A study on serum FSH, LH and prolactin levels among infertile women. Int. J. Med. Res. Health Sci. 2015, 4, 876–878. [Google Scholar] [CrossRef]
- Sheena, L.P.R.; Phil, G.K.; John, L.Y.; Yee, L.; Frank, A.; Arun, D. Granulosa cell apoptosis in the ovarian follicule—A changing view. Front. Endocrimiol. 2018, 9, 61. [Google Scholar]
- Casarini, L.; Riccetti, L.; De-Pascali, F.; Nicoli, A.; Tagliavini, S.; Trenti, T.; La Sala, G.B.; Simoni, M. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol. Cell. Endocrinol. 2016, 15, 103–114. [Google Scholar] [CrossRef]
- Casarini, L.; Riccetti, L.; De Pascali, F.; Gilioli, L.; Marino, M.; Vecchi, E.; Morini, D.; Nicoli, A.; La Sala, G.B.; Simoni, M. Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro. Int. J. Mol. Sci. 2017, 18, 926. [Google Scholar] [CrossRef]
- Geisert, R.D.; Smith, M.F.; Schmelzle, A.L.; Green, J.A. Utilizing a rat delayed implantation model to teach integrative endocrinology and reproductive biology. Adv. Physiol. Educ. 2018, 42, 56–63. [Google Scholar] [CrossRef]
- Adamu, K.M.; Aliyu-Paiko, M.; Abdullahi, F.; Mustapha, A.Y. Effects of Azadirachta indica leaf powder on some haematological parameters of the African catfish (Clarias gariepinus). Niger. J. Basic Appl. Sci. 2017, 25, 41–50. [Google Scholar] [CrossRef]
- Igwenyi, I.O.; Eze, A.C.; Aja, P.M.; Elom, S.O.; Uraku, A.J.; Awoke, J.N.; Obasi, N.A. Cholesterol-Lowering and Hepatoprotective Effect of Fruit Juice Extract of Azadirachta indica on Plasmodium berghei Infected Mice. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3367–3375. [Google Scholar]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Widayati, D.T.; Bintara, S.; Natawihardja, I.; Maharani, D. Blood biochemical profile infertile and repeat breeder Ongole cross breed cows. Pak. J. Biol. Sci 2018, 21, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid.-Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef] [PubMed]
- Ubua, J.A.; Ozung, P.O.; Inagu, P.G. Dietary Inclusion of Neem (Azadirachta indica) Leaf Meal Can Influence Growth Performance and Carcass Characteristics of Broiler Chickens. Asian J. Biol. Sci. 2019, 12, 180–186. [Google Scholar] [CrossRef]
- Tóthová, C.; Mihajlovičová, X.; Nagy, O. The Use of Serum Proteins in the Laboratory Diagnosis of Health Disorders in Ruminants. In Ruminants—The Husbandry, Economic and Health Aspects; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Gómez-Cantarino, S.; Agulló-Ortuño, M.T.; de Dios-Aguado, M.; Ugarte-Gurrutxaga, M.I.; Bouzas-Mosquera, C. Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations. Int. J. Environ. Res. Public Health 2020, 17, 6275. [Google Scholar] [CrossRef]
- Moman, R.N.; Gupta, N.; Varacallo, M. Physiology, Albumin. In StatPearls; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459198/ (accessed on 8 February 2022).
- Aoi, W.; Marunaka, Y. Importance of pH homeostasis in metabolic health and diseases: Crucial role of membrane proton transport. Biomed Res. Int. 2014, 2014, 598986. [Google Scholar] [CrossRef]
- Anonymous. Protein Fuctions in the Body. Available online: https://pressbooks-dev.oer.hawaii.edu/humannutrition/chapter/proteins-functions-in-the-body/ (accessed on 7 August 2022).
- Vierafertility. Why pH Regulations is So Important for a Health Pregnancy. Available online: https://www.vierafertility.com/blog/why-ph-regulation-is-so-important-for-a-healthy-pregnancy/ (accessed on 7 August 2022).
- Mishra, A.K.; Kumar, A.; Swain, D.K.; Yadav, S.; Nigam, R. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World 2018, 11, 852–858. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The Semen pH Affects Sperm Motility and Capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef]
- Vijeyata, V.; Ashok, P. Contraceptive effects of neem seed oil and its active fractions on female albino rabbits. Asian J. Pharm. Clin. Res. 2018, 11, 421–424. [Google Scholar]
- Mukherjee, S.; Talwar, G.P. Termination of pregnancy in rodents by oral administration of Praneem, a purified neem seed extract. Am. J. Reprod. Immunol. 1996, 35, 51–56. [Google Scholar] [CrossRef]
- Talwar, G.P.; Shah, S.; Mukherjee, S.; Chabra, R. Induced termination of pregnancy by purified extracts of Azadirachta indica (neem): Mechanisms involved. Am. J. Reprod. Immunol. 1977, 37, 485–491. [Google Scholar] [CrossRef] [PubMed]



| Crude Fraction | Weight (g) | Yield (%) |
|---|---|---|
| Hexan | 55.11 | 18.37 |
| Chloroform | 14.54 | 4.85 |
| Methanol | 31.72 | 10.57 |
| Total Oil Yield | 101.37 | 33.79 |
| Days | Experimental Groups | |||
|---|---|---|---|---|
| Control | 50 mg/kg | 100 mg/kg | 200 mg/kg | |
| Day 0 | 7.4 ± 0.14 | 7.3 ± 0.13 x | 7.3 ± 0.13 x | 7.5 ± 1.26 x |
| Day 14 | 7.5 ± 0.17 a | 6.95 ± 0.09 by | 6.9 ± 0.21 by | 6.5 ± 0.15 by |
| Day 28 | 7.6 ± 0.08 | 7.5 ± 0.17 x | 7.5 ± 0.19 x | 7.5 ± 0.21 x |
| Biochemical Parameters | Treatment Days | Experimental Groups | |||
|---|---|---|---|---|---|
| Control (Gourp-A) | 50 mg/kg (Group-B) | 100 mg/kg (Group-C) | 200 mg/kg (Group-D) | ||
| TC(mg/dL) | Day 0 | 70.1 ± 1.5 | 71.7 ± 4.4 | 72.8 ± 3.6 | 70.6 ± 4.8 |
| Day 14 | 72.0 ± 4.9 | 74.4 ± 5.7 | 72.0 ± 5.4 | 72.9 ± 4.2 | |
| Day 28 | 73.9 ± 2.7 | 78.2 ± 4.4 | 73.4 ± 1.8 | 73.9 ± 2.5 | |
| HDL (mg/dL) | Day 0 | 40.6 ± 5.5 | 46.9 ± 5.2 | 44.9 ± 6.8 | 43.4 ± 4.9 |
| Day 14 | 41.4 ± 7.5 | 45.7 ± 7.0 | 42.6 ± 5.9 | 44.6 ± 2.9 | |
| Day 28 | 43.7 ± 6.3 | 46.6 ± 6.0 | 48.1 ± 5.8 | 49.7 ± 4.1 | |
| LDL (mg/dL) | Day 0 | 29.5 ± 5.4 | 24.8 ± 1.1 | 27.9 ± 3.8 | 27.3 ± 2.3 |
| Day 14 | 30.6 ± 5.4 | 28.6 ±3.7 | 29.4 ± 0.9 | 28.2 ± 1.6 | |
| Day 28 | 30.2 ± 4.8 | 31.6 ± 5.3 | 25.3 ± 5.1 | 24.2 ± 5.0 | |
| TG (mg/dL) | Day 0 | 1.28 ± 1.39 | 1.23 ± 1.32 | 1.19 ± 1.54 | 1.29 ± 1.65 |
| Day 14 | 1.34 ± 1.49 | 1.19 ± 1.41 | 1.22 ± 2.11 | 1.26 ± 2.03 | |
| Day 28 | 1.3 ± 3.86 | 1.27 ± 2.15 | 1.14 ± 1.29 | 1.34 ± 2.51 | |
| Fertility Parameters | Treatment Groups | |||
|---|---|---|---|---|
| Control | 50 mg/kg | 100 mg/kg | 200 mg/kg | |
| Percentage abortion | 0 | 0 | 25 | 100 |
| Percentage resorption | 0 | 28.9 | 3.1 | 0 |
| LFB (%) | 100 | 100 | 100 | 0 |
| PISI (%) | 100 | 72 | 73 | 0 |
| GI | 9 | 34 | 44 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njoga, U.J.; Jaja, I.F.; Onwuka, O.S.; Ilo, S.U.; Eke, I.G.; Abah, K.O.; Oguejiofor, C.F.; Ochiogu, I.S. Reproductive Effects of Medicinal Plant (Azadirachta indica) Used as Forage and for Ethnoveterinary Practices: New Insights from Animal Models. Challenges 2022, 13, 40. https://doi.org/10.3390/challe13020040
Njoga UJ, Jaja IF, Onwuka OS, Ilo SU, Eke IG, Abah KO, Oguejiofor CF, Ochiogu IS. Reproductive Effects of Medicinal Plant (Azadirachta indica) Used as Forage and for Ethnoveterinary Practices: New Insights from Animal Models. Challenges. 2022; 13(2):40. https://doi.org/10.3390/challe13020040
Chicago/Turabian StyleNjoga, Ugochinyere J., Ishmael F. Jaja, Osita S. Onwuka, Stanley U. Ilo, Ifeanyi G. Eke, Kenneth O. Abah, Chike F. Oguejiofor, and Izuchukwu S. Ochiogu. 2022. "Reproductive Effects of Medicinal Plant (Azadirachta indica) Used as Forage and for Ethnoveterinary Practices: New Insights from Animal Models" Challenges 13, no. 2: 40. https://doi.org/10.3390/challe13020040







