Particle Handling Techniques in Microchemical Processes
Abstract
:1. Introduction
2. Particles Encountered in Microreactors
3. Particle Formation, Stability, and Accumulation Considerations
3.1. Nucleation Theory

3.2. Particle Inertia


3.3. Colloidal Attraction and Repulsion Physics
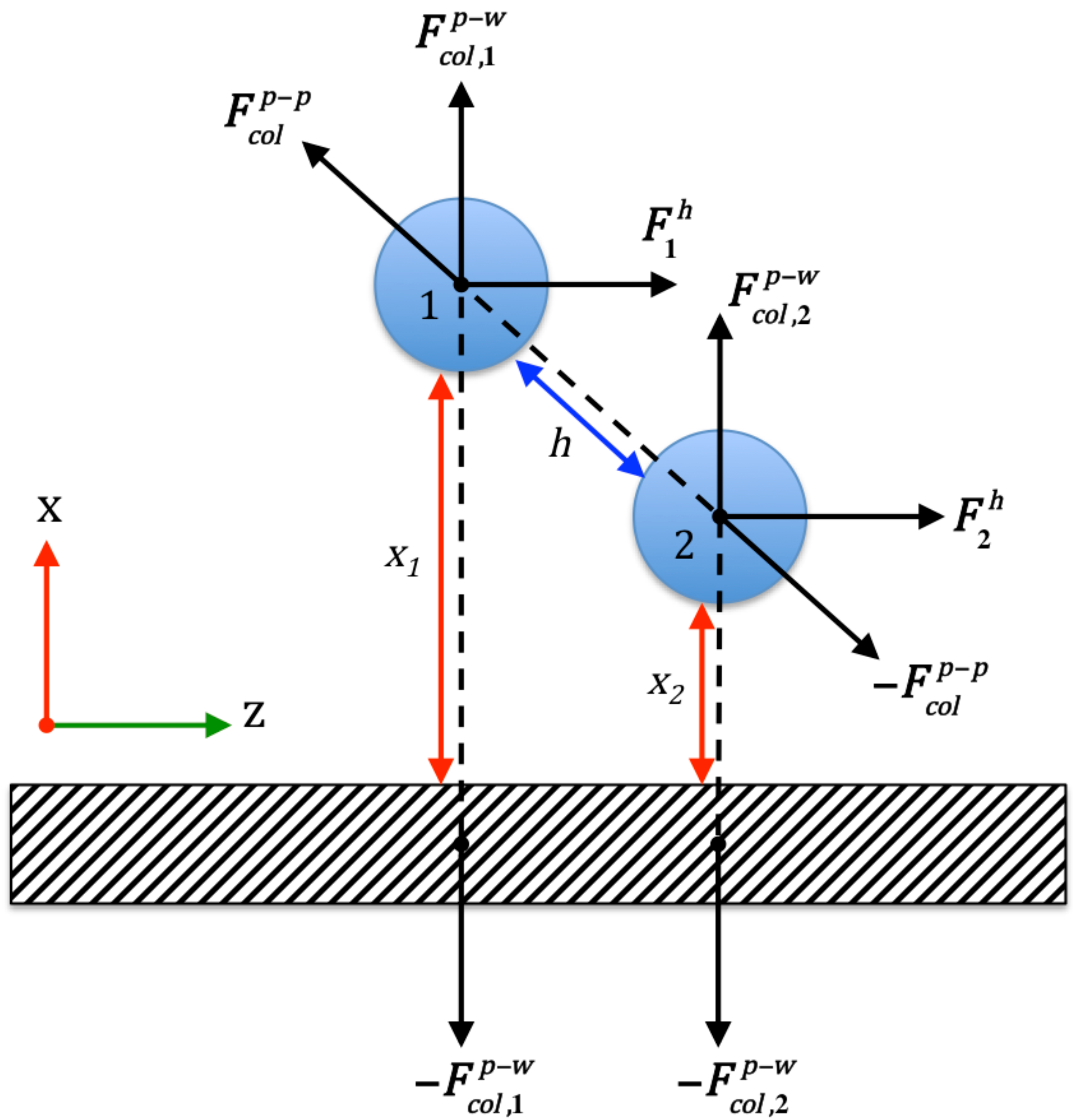





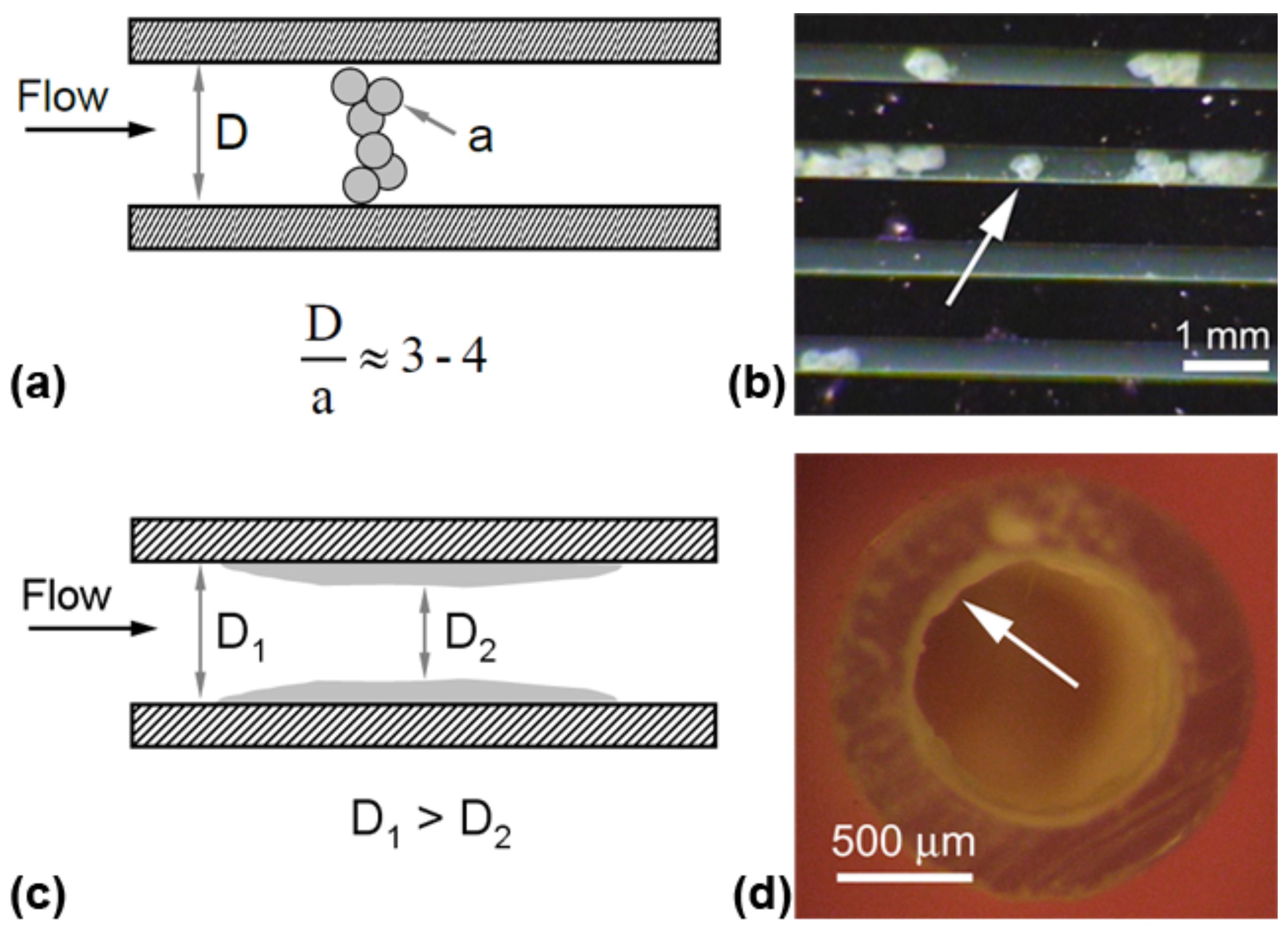
3.4. Bridging, Constriction, and Random Detachment
4. Techniques to Control Particle Transport
4.1. Passive Particle Manipulation

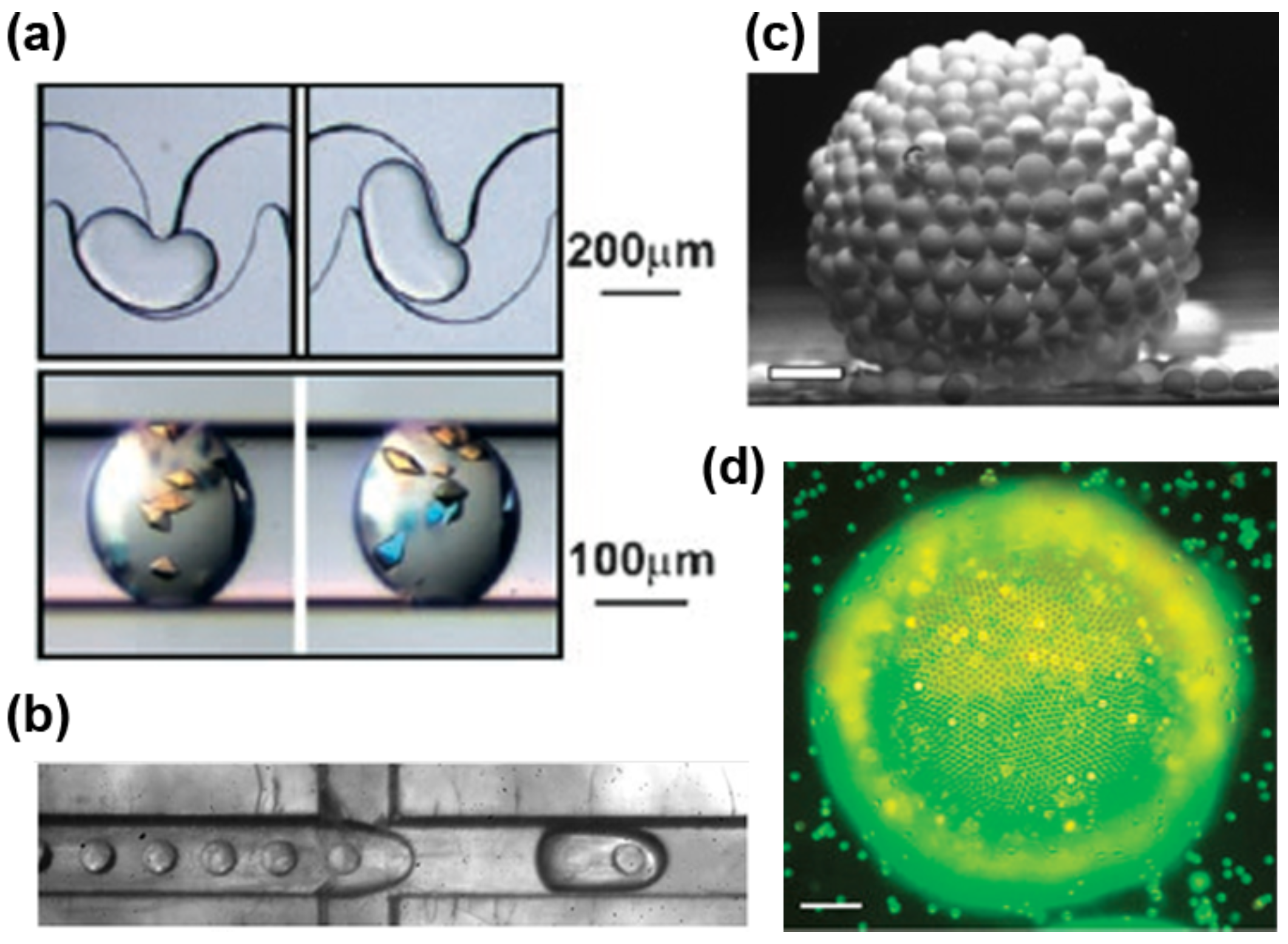
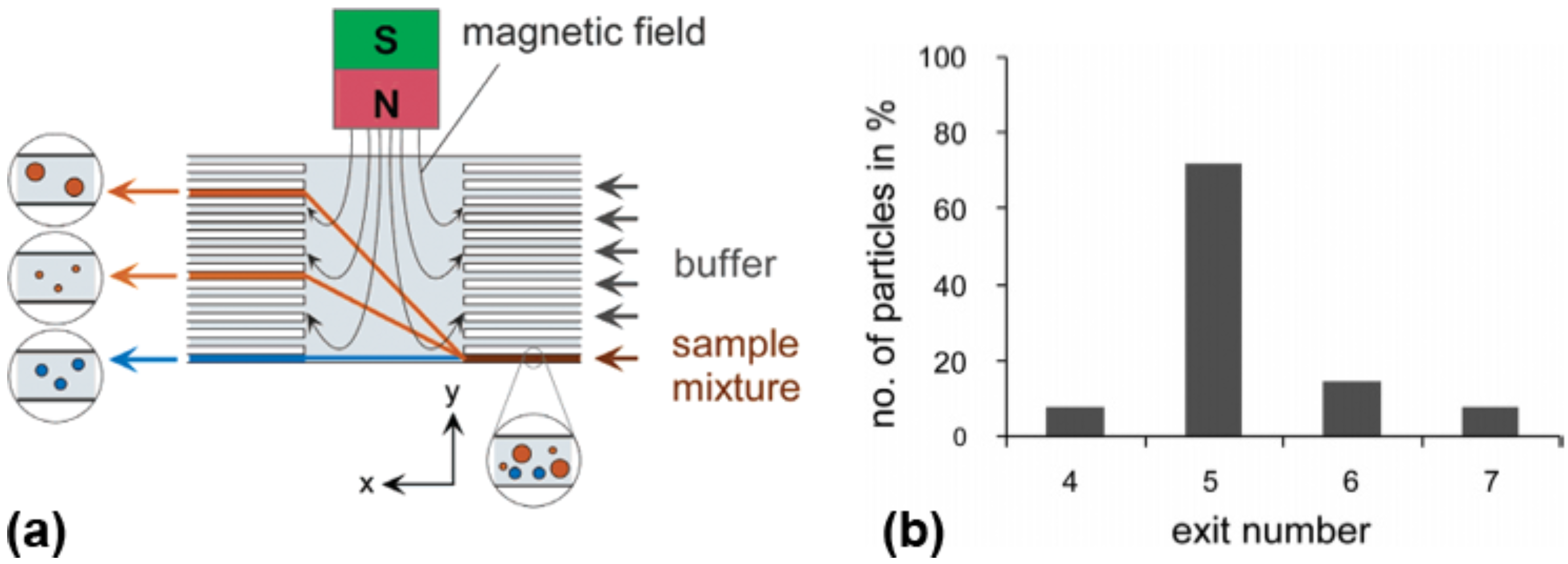
4.2. Active Particle Manipulation




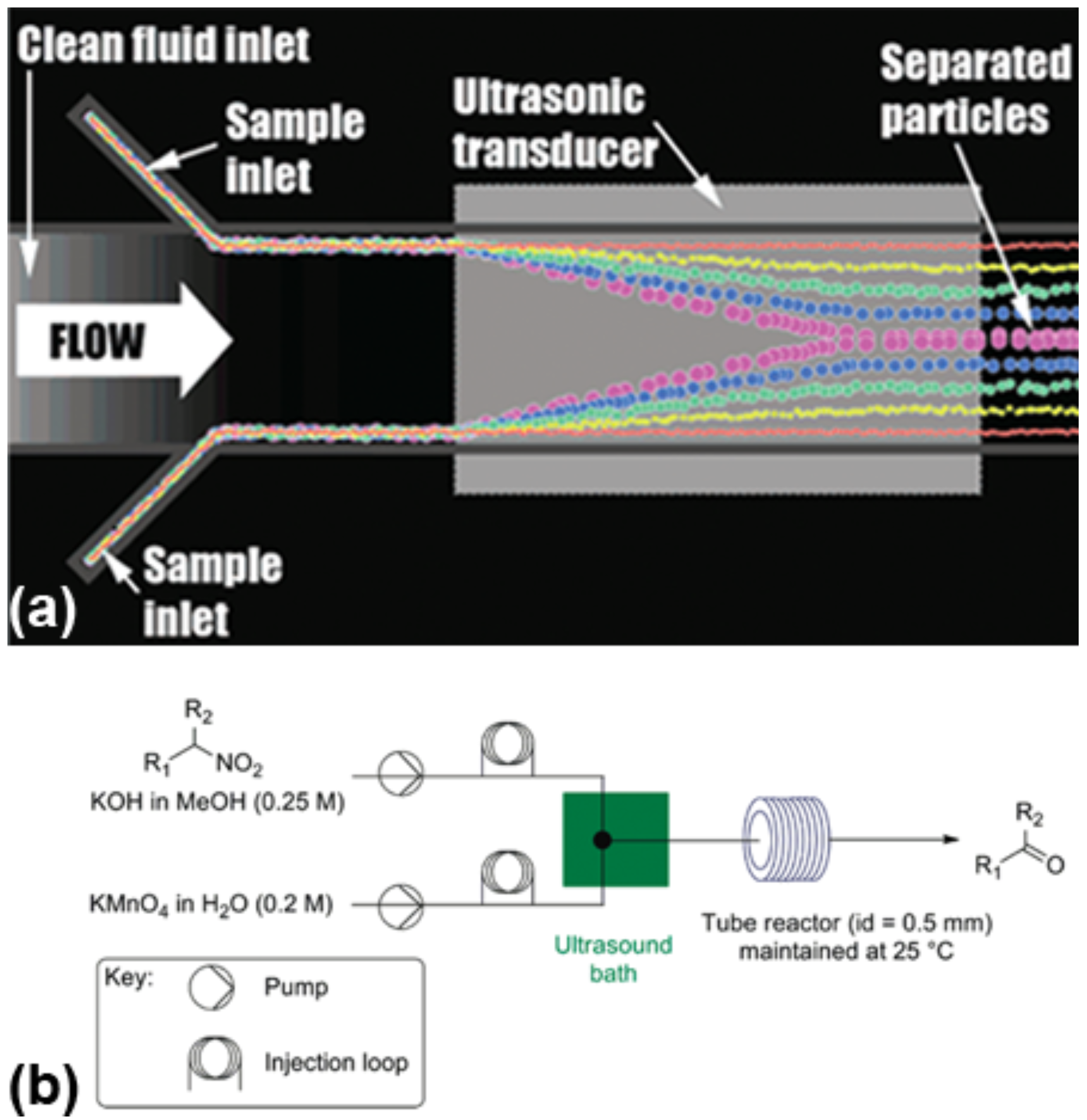
5. Outlook and Challenges
References
- Ottino, J.M.; Wiggins, S. Introduction: Mixing in microfluidics. Philos. Trans. R. Soc. A 2004, 362, 923–935. [Google Scholar] [CrossRef]
- Pamme, N. Magnetism and microfluidics. Lab Chip 2006, 6, 24–38. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Ann. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar]
- Hartman, R.L.; Jensen, K.F. Microchemical systems for continuous-flow synthesis. Lab Chip 2009, 9, 2495–2507. [Google Scholar] [CrossRef]
- Jensen, K.F. Microreaction engineering—Is small better? Chem. Eng. Sci. 2001, 56, 293–303. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef]
- Krishnadasan, S.; Brown, R.J.C.; Demello, A.J.; Demello, J.C. Intelligent routes to the controlled synthesis of nanoparticles. Lab Chip 2007, 7, 1434–1441. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When microfluidic devices go bad—How does fouling occur in microfluidic devices, and what can be done about it? Anal. Chem. 2005, 77, 429–432. [Google Scholar]
- Horie, T.; Sumino, M.; Tanaka, T.; Matsushita, Y.; Ichimura, T.; Yoshida, J. Photodimerization of maleic anhydride in a microreactor without clogging. Org. Proc. Res. Dev. 2010, 14, 405–410. [Google Scholar] [CrossRef]
- Kuhn, S.; Noel, T.; Gu, L.; Heider, P.L.; Jensen, K.F. A Teflon microreactor with integrated piezoelectric actuator to handle solid forming reactions. Lab Chip 2011, 11, 2488–2492. [Google Scholar]
- Nagasawa, H.; Mae, K. Development of a new microreactor based on annular microsegments for fine particle production. Ind. Eng. Chem. Res. 2006, 45, 2179–2186. [Google Scholar] [CrossRef]
- Noel, T.; Naber, J.R.; Hartman, R.L.; McMullen, J.P.; Jensen, K.F.; Buchwald, S.L. Palladium-catalyzed amination reactions in flow: Overcoming the challenges of clogging via acoustic irradiation. Chem. Sci. 2011, 2, 287–290. [Google Scholar]
- Poe, S.L.; Cummings, M.A.; Haaf, M.R.; McQuade, D.T. Solving the clogging problem: Precipitate-forming reactions in flow. Angew. Chem. Int. Ed. 2006, 45, 1544–1548. [Google Scholar]
- Pribyl, M.; Snita, D.; Marek, M. Nonlinear phenomena and qualitative evaluation of risk of clogging in a capillary microreactor under, imposed electric field. Chem. Eng. J. 2005, 105, 99–109. [Google Scholar] [CrossRef]
- Bengtsson, M.; Laurell, T. Ultrasonic agitation in microchannels. Anal. Bioanal. Chem. 2004, 378, 1716–1721. [Google Scholar] [CrossRef]
- Evander, M.; Johansson, L.; Lilliehorn, T.; Piskur, J.; Lindvall, M.; Johansson, S.; Almqvist, M.; Laurell, T.; Nilsson, J. Noninvasive acoustic cell trapping in a microfluidic perfusion system for online bioassays. Anal. Chem. 2007, 79, 2984–2991. [Google Scholar]
- Hawkes, J.J.; Barber, R.W.; Emerson, D.R.; Coakley, W.T. Continuous cell washing and mixing driven by an ultrasound standing wave within a microfluidic channel. Lab Chip 2004, 4, 446–452. [Google Scholar] [CrossRef]
- Hawkes, J.J.; Coakley, W.T. Force field particle filter, combining ultrasound standing waves and laminar flow. Sens. Act. B Chem. 2001, 75, 213–222. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar]
- Nilsson, A.; Petersson, F.; Jonsson, H.; Laurell, T. Acoustic control of suspended particles in micro fluidic chips. Lab Chip 2004, 4, 131–135. [Google Scholar] [CrossRef]
- Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 2007, 7, 1644–1659. [Google Scholar] [CrossRef]
- Petersson, F.; Aberg, L.; Sward-Nilsson, A.M.; Laurell, T. Free flow acoustophoresis: Microfluidic-based mode of particle and cell separation. Anal. Chem. 2007, 79, 5117–5123. [Google Scholar] [CrossRef]
- Petersson, F.; Nilsson, A.; Holm, C.; Jonsson, H.; Laurell, T. Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces. Lab Chip 2005, 5, 20–22. [Google Scholar] [CrossRef]
- Petersson, F.; Nilsson, A.; Jonsson, H.; Laurell, T. Carrier medium exchange through ultrasonic particle switching in microfluidic channels. Anal. Chem. 2005, 77, 1216–1221. [Google Scholar]
- Dittrich, P.S.; Tachikawa, K.; Manz, A. Micro total analysis systems. Latest advancements and trends. Anal. Chem. 2006, 78, 3887–3907. [Google Scholar]
- Pamme, N.; Koyama, R.; Manz, A. Counting and sizing of particles and particle agglomerates in a microfluidic device using laser light scattering: Application to a particle-enhanced immunoassay. Lab Chip 2003, 3, 187–192. [Google Scholar] [CrossRef]
- Pamme, N.; Manz, A. On-chip free-flow magnetophoresis: Continuous flow separation of magnetic particles and agglomerates. Anal. Chem. 2004, 76, 7250–7256. [Google Scholar] [CrossRef]
- Vilkner, T.; Shivji, A.; Manz, A. Dry powder injection on chip. Lab Chip 2005, 5, 140–145. [Google Scholar] [CrossRef]
- Pamme, N.; Wilhelm, C. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip 2006, 6, 974–980. [Google Scholar] [CrossRef]
- Rodriguez-Villarreal, A.I.; Tarn, M.D.; Madden, L.A.; Lutz, J.B.; Greenman, J.; Samitier, J.; Pamme, N. Flow focussing of particles and cells based on their intrinsic properties using a simple diamagnetic repulsion setup. Lab Chip 2011, 11, 1240–1248. [Google Scholar]
- Hartman, R.L. Managing solids in microreactors for the upstream continuous processing of fine chemicals. Org. Proc. Res. Dev. 2012. [Google Scholar]
- Hartman, R.L.; Naber, J.R.; Zaborenko, N.; Buchwald, S.L.; Jensen, K.F. Overcoming the challenges of solid bridging and constriction during Pd-catalyzed C-N bond formation in microreactors. Org. Proc. Res. Dev. 2010, 14, 1347–1357. [Google Scholar] [CrossRef]
- Kuhn, S.; Hartman, R.L.; Sultana, M.; Nagy, K.D.; Marre, S.; Jensen, K.F. Teflon-coated silicon microreactors: Impact on segmented liquid-liquid multiphase flows. Langmuir 2011, 27, 6519–6527. [Google Scholar]
- Honda, T.; Miyazaki, M.; Nakamura, H.; Maeda, H. Controllable polymerization of N-carboxy anhydrides in a microreaction system. Lab Chip 2005, 5, 812–818. [Google Scholar] [CrossRef]
- Li, W.; Pharn, H.H.; Nie, Z.; MacDonald, B.; Guenther, A.; Kumacheva, E. Multi-step microfluidic polymerization reactions conducted in droplets: The internal trigger approach. J. Am. Chem. Soc. 2008, 130, 9935–9941. [Google Scholar]
- Marcati, A.; Serra, C.; Bouquey, M.; Prat, L. Handling of polymer particles in microchannels. Chem. Eng. Tech. 2010, 33, 1779–1787. [Google Scholar] [CrossRef]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef]
- Kralj, J.G.; Lis, M.T.W.; Schmidt, M.A.; Jensen, K.F. Continuous dielectrophoretic size-based particle sorting. Anal. Chem. 2006, 78, 5019–5025. [Google Scholar]
- Hessel, V. Novel process windows—Gate to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 2009, 32, 1655–1681. [Google Scholar] [CrossRef]
- Kockmann, N.; Gottsponer, M.; Roberge, D.M. Scale-up concept of single-channel microreactors from process development to industrial production. Chem. Eng. J. 2011, 167, 718–726. [Google Scholar] [CrossRef]
- Roberge, D.M.; Zimmermann, B.; Rainone, F.; Gottsponer, M.; Eyholzer, M.; Kockmann, N. Microreactor technology and continuous processes in the fine chemical and pharmaceutical industry: Is the revolution underway? Org. Proc. Res. Dev. 2008, 12, 905–910. [Google Scholar] [CrossRef]
- Adamo, A.; Jensen, K.F. Microfluidic based single cell microinjection. Lab Chip 2008, 8, 1258–1261. [Google Scholar] [CrossRef]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005, 26, R73–R98. [Google Scholar] [CrossRef]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef]
- Salieb-Beugelaar, G.B.; Simone, G.; Arora, A.; Philippi, A.; Manz, A. Latest developments in microfluidic cell biology and analysis systems. Anal. Chem. 2010, 82, 4848–4864. [Google Scholar] [CrossRef]
- Bruzewicz, D.A.; McGuigan, A.P.; Whitesides, G.M. Fabrication of a modular tissue construct in a microfluidic chip. Lab Chip 2008, 8, 663–671. [Google Scholar] [CrossRef]
- Marcy, Y.; Ishoey, T.; Lasken, R.S.; Stockwell, T.B.; Walenz, B.P.; Halpern, A.L.; Beeson, K.Y.; Goldberg, S.M.D.; Quake, S.R. Nanoliter reactors improve multiple displacement amplification of genomes from single cells. PLoS Genet. 2007, 3, 1702–1708. [Google Scholar]
- Kortmann, H.; Blank, L.M.; Schmid, A. Single Cell Analytics: An Overview. In High Resolution Microbial Single Cell Analytics; Muller, S., Bley, T., Eds.; Springer-Verlag Berlin: Berlin, Germany, 2011; Volume 124, pp. 99–122. [Google Scholar]
- Schmid, A.; Kortmann, H.; Dittrich, P.S.; Blank, L.M. Chemical and biological single cell analysis. Curr. Opin. Biotechnol. 2010, 21, 12–20. [Google Scholar] [CrossRef]
- Jaggi, R.D.; Sandoz, R.; Effenhauser, C.S. Microfluidic depletion of red blood cells from whole blood in high-aspect-ratio microchannels. Microfluid. Nanofluid. 2007, 3, 47–53. [Google Scholar]
- Pommer, M.S.; Zhang, Y.T.; Keerthi, N.; Chen, D.; Thomson, J.A.; Meinhart, C.D.; Soh, H.T. Dielectrophoretic separation of platelets from diluted whole blood in microfluidic channels. Electrophoresis 2008, 29, 1213–1218. [Google Scholar] [CrossRef]
- Chabert, M.; Viovy, J.L. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3191–3196. [Google Scholar] [CrossRef]
- Dorvee, J.R.; Sailor, M.J.; Miskelly, G.M. Digital microfluidics and delivery of molecular payloads with magnetic porous silicon chaperones. Dalton Trans. 2008, 721–730. [Google Scholar]
- Hung, L.H.; Choi, K.M.; Tseng, W.Y.; Tan, Y.C.; Shea, K.J.; Lee, A.P. Alternating droplet generation and controlled dynamic droplet fusion in microfluidic device for CdS nanoparticle synthesis. Lab Chip 2006, 6, 174–178. [Google Scholar] [CrossRef]
- Nakamura, H.; Yamaguchi, Y.; Miyazaki, M.; Maeda, H.; Uehara, M.; Mulvaney, P. Preparation of CdSe nanocrystals in a micro-flow-reactor. Chem. Commun. 2002, 2844–2845. [Google Scholar]
- Edel, J.B.; Fortt, R.; de Mello, J.C.; de Mello, A.J. Microfluidic routes to the controlled production of nanoparticles. Chem. Commun. 2002, 1136–1137. [Google Scholar]
- Khan, S.A.; Jensen, K.F. Microfluidic synthesis of titania shells on colloidal silica. Adv. Mat. 2007, 19, 2556–2560. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Gunther, A.; Schmidt, M.A.; Jensen, K.F.; Bawendi, M.G. A microfabricated gas-liquid segmented flow reactor for high-temperature synthesis: The case of CdSe quantum dots. Angew. Chem. Int. Ed. 2005, 44, 5447–5451. [Google Scholar] [CrossRef]
- Hartman, R.L. Managing solids in microreactors for the upstream continuous processing of fine chemicals. Org. Proc. Res. Dev. 2012. [Google Scholar]
- Roberge, D.M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Microreactor technology: A revolution for the fine chemical and pharmaceutical industries? Chem. Eng. Technol. 2005, 28, 318–323. [Google Scholar] [CrossRef]
- Noel, T.; Buchwald, S.L. Cross-coupling in flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361. [Google Scholar]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Chen, D.L.; Gerdts, C.J.; Ismagilov, R.F. Using microfluidics to observe the effect of mixing on nucleation of protein crystals. J. Am. Chem. Soc. 2005, 127, 9672–9673. [Google Scholar]
- Koc, Y.; de Mello, A.J.; McHale, G.; Newton, M.I.; Roach, P.; Shirtcliffe, N.J. Nano-scale superhydrophobicity: Suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip 2008, 8, 582–586. [Google Scholar] [CrossRef]
- Marre, S.; Park, J.; Rempel, J.; Guan, J.; Bawendi, M.G.; Jensen, K.F. Supercritical continuous-microflow synthesis of narrow size distribution quantum dots. Adv. Mater. 2008, 20, 4830–4834. [Google Scholar] [CrossRef]
- Kockmann, N.; Kastner, J.; Woias, P. Reactive particle precipitation in liquid microchannel flow. Chem. Eng. J. 2008, 135, S110–S116. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 3rd ed; Butterworth Heinemann: Oxford, UK, 1997. [Google Scholar]
- Slaughter, D.W.; Doherty, M.F. Calculation of solid-liquid equilibrium and crystallization paths for melt crystallization processes. Chem. Eng. Sci. 1995, 50, 1679–1694. [Google Scholar] [CrossRef]
- Winn, D.; Doherty, M.F. Modeling crystal shapes of organic materials grown from solution. Aiche J. 2000, 46, 1348–1367. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Sizemore, J.P.; Doherty, M.F. Shape evolution of 3-dimensional faceted crystals. Aiche J. 2006, 52, 1906–1915. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Ramachandran, V.; Fogler, H.S. Plugging by hydrodynamic bridging during flow of stable colloidal particles within cylindrical pores. J. Fluid Mech. 1999, 385, 129–156. [Google Scholar] [CrossRef]
- Schenkel, J.H.; Kitchener, J.A. A test of the Derjaguin-Verwey-Overbeek theory with a colloidal suspension. Trans. Faraday Soc. 1960, 56, 161–173. [Google Scholar] [CrossRef]
- Feke, D.L.; Prabhu, N.D.; Mann, J.A.; Mann, J.A. A formulation of the short-range repulsion between spherical colloidal particles. J. Phys. Chem. 1984, 88, 5735–5739. [Google Scholar] [CrossRef]
- Bowen, R.W.; Sharif, A.O. Adaptive finite-element solution of the nonlinear Poisson-Boltzmann equation: A charged spherical particle at various distances from a charged cylindrical pore in a charged planar surface. J. Colloid Interface Sci. 1997, 187, 363–374. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Sharma, A. Lifshitz-van der waals energy of spherical-particles in cylindrical pores. J. Colloid Interface Sci. 1995, 171, 288–296. [Google Scholar] [CrossRef]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; Stone, H.A.; Weitz, D.A. Mechanism for clogging of microchannels. Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 2006, 74. [Google Scholar]
- Ramachandran, V.; Fogler, H.S. Multilayer deposition of stable colloidal particles during flow within cylindrical pores. Langmuir 1998, 14, 4435–4444. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannel. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar]
- Gunther, A.; Jensen, K.F. Multiphase microfluidics: From flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef]
- Gunther, A.; Jhunjhunwala, M.; Thalmann, M.; Schmidt, M.A.; Jensen, K.F. Micromixing of miscible liquids in segmented gas-liquid flow. Langmuir 2005, 21, 1547–1555. [Google Scholar] [CrossRef]
- Gunther, A.; Khan, S.A.; Thalmann, M.; Trachsel, F.; Jensen, K.F. Transport and reaction in microscale segmented gas-liquid flow. Lab Chip 2004, 4, 278–286. [Google Scholar] [CrossRef]
- Wang, F.K.; Chon, C.H.; Li, D.Q. Particle separation by a moving air-liquid interface in a microchannel. J. Colloid Interface Sci. 2010, 352, 580–584. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Abkarian, M.; Mahadevan, L.; Stone, H.A. Mechanics of interfacial composite materials. Langmuir 2006, 22, 10204–10208. [Google Scholar] [CrossRef]
- Subramanian, R.S.; Larsen, R.J.; Stone, H.A. Stability of a flat gas-liquid interface containing nonidentical spheres to gas transport: Toward an explanation of particle stabilization of gas bubbles. Langmuir 2005, 21, 4526–4531. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Abkarian, M.; Stone, H.A. Controlled assembly of jammed colloidal shells on fluid droplets. Nat. Mater. 2005, 4, 553–556. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Mejean, C.; Abkarian, M.; Stone, H.A. Microstructure, morphology, and lifetime of armored bubbles exposed to surfactants. Langmuir 2006, 22, 5986–5990. [Google Scholar] [CrossRef]
- Lautz, J.; Sankin, G.; Yuan, F.; Zhong, P. Displacement of particles in microfluidics by laser-generated tandem bubbles. Appl. Phys. Lett. 2010, 97, 183701–183701-3. [Google Scholar]
- Wang, M.M.; Tu, E.; Raymond, D.E.; Yang, J.M.; Zhang, H.C.; Hagen, N.; Dees, B.; Mercer, E.M.; Forster, A.H.; Kariv, I.; et al. Microfluidic sorting of mammalian cells by optical force switching. Nat. Biotechnol. 2005, 23, 83–87. [Google Scholar]
- Durr, M.; Kentsch, J.; Muller, T.; Schnelle, T.; Stelzle, M. Microdevices for manipulation and accumulation of micro- and nanoparticles by dielectrophoresis. Electrophoresis 2003, 24, 722–731. [Google Scholar] [CrossRef]
- Gijs, M.A.M. Magnetic bead handling on-chip: New opportunities for analytical applications. Microfluid. Nanofluid. 2004, 1, 22–40. [Google Scholar]
- Lu, L.H.; Ryu, K.S.; Liu, C. A magnetic microstirrer and array for microfluidic mixing. J. Microelectromech. Syst. 2002, 11, 462–469. [Google Scholar] [CrossRef]
- Challis, R.E.; Povey, M.J.W.; Mather, M.L.; Holmes, A.K. Ultrasound techniques for characterizing colloidal dispersions. Rep. Prog. Phys. 2005, 68, 1541–1637. [Google Scholar] [CrossRef]
- Lilliehorn, T.; Simu, U.; Nilsson, M.; Almqvist, M.; Stepinski, T.; Laurell, T.; Nilsson, J.; Johansson, S. Trapping of microparticles in the near field of an ultrasonic transducer. Ultrasonics 2005, 43, 293–303. [Google Scholar] [CrossRef]
- Mason, W.P. Physical Acoustics; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Poesio, P.; Ooms, G. Formation and ultrasonic removal of fouling particle structures in a natural porous material. J. Pet. Sci. Eng. 2004, 45, 159–178. [Google Scholar] [CrossRef]
- Spengler, J.; Jekel, M. Ultrasound conditioning of suspensions—Studies of streaming influence on particle aggregation on a lab- and pilot-plant scale. Ultrasonics 2000, 38, 624–628. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar]
- Sedelmeier, J.; Ley, S.V.; Baxendale, I.R.; Baumann, M. KMnO(4)-mediated oxidation as a continuous flow process. Org. Lett. 2010, 12, 3618–3621. [Google Scholar]
- Browne, D.L.; Deadman, B.J.; Ashe, R.; Baxendale, I.R.; Ley, S.V. Continuous flow processing of slurries: Evaluation of an agitated cell reactor. Org. Proc. Res. Dev. 2011, 15, 693–697. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Flowers, B.S.; Hartman, R.L. Particle Handling Techniques in Microchemical Processes. Challenges 2012, 3, 194-211. https://doi.org/10.3390/challe3020194
Flowers BS, Hartman RL. Particle Handling Techniques in Microchemical Processes. Challenges. 2012; 3(2):194-211. https://doi.org/10.3390/challe3020194
Chicago/Turabian StyleFlowers, Brian S., and Ryan L. Hartman. 2012. "Particle Handling Techniques in Microchemical Processes" Challenges 3, no. 2: 194-211. https://doi.org/10.3390/challe3020194



