1. Introduction
Coffee cultivation and first transformation represent an important source of income for many developing countries in the tropical regions of Middle and South America, Africa and Asia. Coffee crops are represented by three botanical species, and, among these,
Coffea arabica(Arabica) makes up approximately 70% of the world’s coffee production [
1]. The other species are
C. canephora, mostly represented by the variety Robusta, and
C. liberica. Of all of the
Coffea species, only
C. arabica is self-fertile, and therefore can be self-pollinated. The most cultivated varieties of
C. arabica in the various regions are Typica and Bourbon, with the derived cultivars Paca, Pache, Caturra, Pacamara, Java, Ruiru, Geisha, Catuaí, Icatu, Blue Mountain and Castillo. Of these, Castillo, grown in Colombia, is highly resistant to coffee leaf rust fungi called “roya” [
2]. The
C. arabica species also typically has lower caffeine contents than
C. canephora. Robusta, for its high caffeine content, presents high resistance to pests and insects and can be grown at lower altitudes and higher temperatures [
3].
Coffee growing is highly affected by climate change and the adaptation of agronomic practices, tailored for the local contexts, are critical for the future of coffee production. Ramirez-Villegas [
4] prospected that in Colombia, an increase in rainfall could impact 80% of crops and 60% of cultivated land.
In Ethiopia, the rapidly increasing temperatures kill the coffee plants at an alarming rate [
5].
Modelling of the influence of climate change on Arabica coffee led to the prediction that there will be a 65%–100% decrease in Arabica production by the year 2080 due to the reduction of suitable cultivation localities [
1]. The decreases in climatic suitability was predicted to be more intense at lower altitudes and high latitudes with a consequent production shift among the major regions of Arabica coffee cultivation [
6].
Arabica coffee quality is strongly affected by temperature increases since, for optimum growth and taste, a temperature of about 18–21°C is required, while the exposure to temperatures of 23 °C or higher can in most cases accelerate ripening of fruits and negatively affect the quality of the product [
1].
Another threat linked to the increased temperatures and rainfall is the rise of the coffee leaf rust, that has become prevalent at higher altitudes than before and has attacked Arabica coffee throughout South America and Africa. As a remedy against the disease, the Colombia Cenicafe research center, has created two strands of coffee rust-resistant cultivars, Colombia and Castillo [
7].
In addition, the populations of the coffee berry borer,
Hypothenemus hampei, the most dangerous pest for coffee plants, increased at the higher temperatures. Moreover, a further increases of over 2 °C could force
H. hampei to migrate to higher altitudes [
8]. In order to limit the damaging effects of the
H. hampei, farmers need to implement early-warning systems [
4].
It is generally agreed that the best way to preserve Arabica coffee is through the use of shade trees that, planted near coffee plants, block the impact of sun with a temperature reduction of up to 4 °C [
4,
8,
9]. According to the predictions, the implementation of shade trees in Colombia and Ethiopia would lower, by about 34%, the increase in population of
H. hampei [
9].
The selection of new Arabica plant varieties is one of the lines of defence from the damages of temperature and rainfall increase.
Ways to modify the taste of Robusta coffee, more tolerant to heat and not depending much on rainfall, were also pursued but have failed so far [
7].
Beyond harvesting high quality coffee cherries, the coffee industry and local producers rely on procedures for coffee bean processing, that may involve controlled fermentation, to obtain the desired characteristics, in terms of flavours, proteolysis and lipolysis, synthesis of volatiles such as aldehydes and chetones, free fatty acids and acidity (sour/citric) in the product. Control on these processes is required to avoid spoilage by bacteria and undesired fungi, such as ochratoxin producing
Aspergillus spp. [
10,
11].
This review illustrates the present and future challenges affecting the coffee production industry, especially the recently established specialty coffee section, and the technological approaches that coffee producers can put into practice in order to assure a high quality product and specialty grade coffees every year, including improvements in moisture avoidance, optimization of storage conditions and packaging.
2. Harvesting of Ripe Coffee Cherries
In coffee cherry harvesting a crucial step is separating fruits at different maturation stages. Under- or overripe cherries can seriously impact on the taste of the final product. In recent years, mechanical harvesters have been developed to pick mainly mature fruits more loosely attached to the coffee plant, by setting vibration and velocity parameters. Similarly, growers may choose to harvest one side of the coffee row at a time, based on sun exposure and quicker fruit maturation. New generation colour sorters, separating ripe yellow and red cherries from the unripe green ones, are available, as well as harvesting machines that screen red ripe cherries by colour, making it easier to produce a high quality harvest. Where the wet-processing methods are used, water provides a mean for density separation of cherries and the pulping machines can be set to pulp only ripe ones.
After the cherries have been harvested, they are processed to separate skin, pulp and mucilage (
Figure 1) either by sun-drying or by wet mechanical processing.
3. Processing of Coffee Cherries
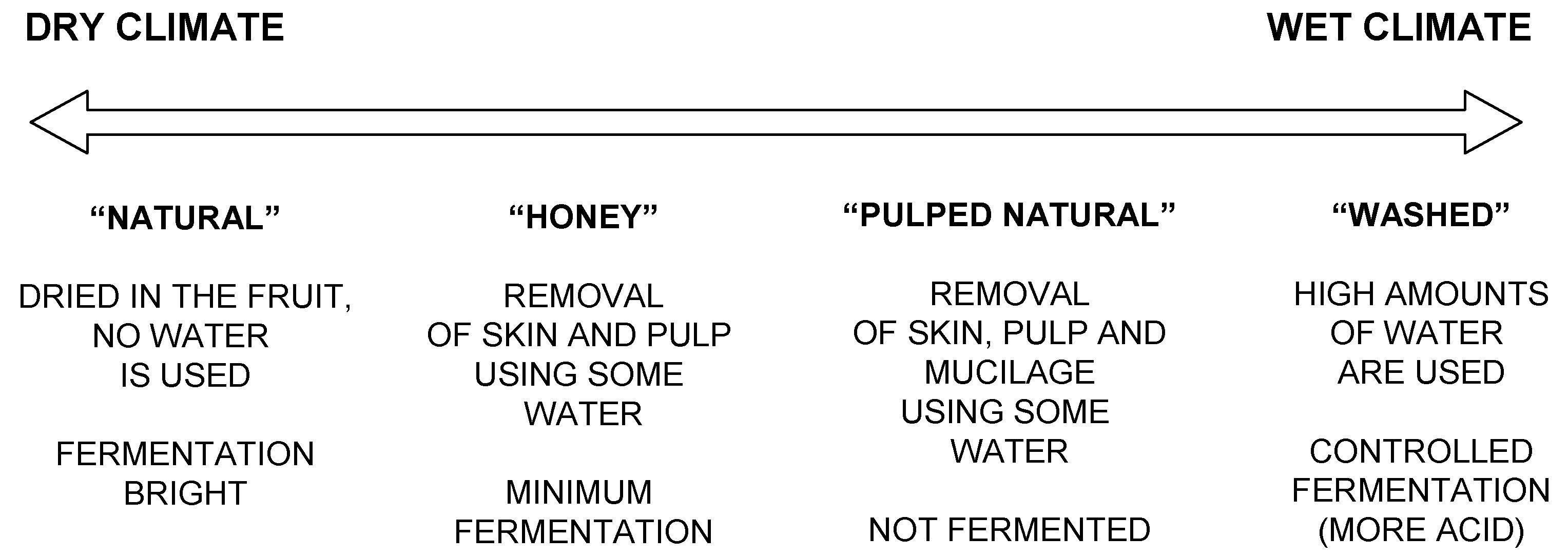
According to the method used for processing the well ripened coffee cherries, the world coffee bean production is classified as “dry natural”, “pulped natural”, “wet hulled” or “fully washed”. These processes are applied to remove the mucilage and to reduce the moisture content of the bean.
A tonne of ripe arabica cherry yields about 120 kg of mucilage in which sugars represent about 9 kg of the dry mass. Mucilage sugars constitute the fermentable carbohydrate for coffee fermentation. Pectic substances amount to about 35% of the dry mass of the mucilage, including polygalacturonic acid chains linked by glycosidic bonds that need to be released by enzymes. Minerals, such as Ca, K and P are also present [
12,
13,
14,
15,
16,
17].
In most farms, coffee is dried until approximately 13% water content, but this value is dependent on the variety and the farmer’s preference. The different methods adopted vary according to the climate characteristics of the production regions as shown in
Figure 2.
The process applied to remove the fruit outer layers influences the development of flavours. The “pulped natural” process is the one that relies least on fermentation, followed by the “honey” process, with minimum fermentation and acidity; by the “washed” process, with controlled fermentation and more acidity; and finally by the “natural” (dried in the fruit) process, with the longest fermentation times. Mechanically washed coffee (not based on fermentation) can be of comparable quality with the fermented product.
There’s still a large area for discoveries on the effects of the processing method and on the opportunity to adopt natural methods or wet ones. “Natural” coffees (naturals) tend to have a more complex presence of flavour-active compounds (discussed in paragraph 4 and
Table 1), that provide the high “body” (silky mouth feel), less acidity, and a wider spectrum of fruity notes compared to washed coffees. On the other hand, naturals tend to score lower in terms of the cleanliness, i.e., absence of sensory defects, of the cup. They are also more vulnerable to damage, given the longer periods of exposure to external agents; this is why they are rarer to find among specialty coffees. Nevertheless, specialty-level naturals can have an excellent combination of clear flavours, that join nuance and complexity with great intensity and a full body, making them able to satisfy the most demanding palates.
3.1. Natural or Sun-Dried in the Fruit—Dry Process
In the “natural” (sun-dried in the fruit) dry process, no layer is removed from the coffee cherries and the final product develops characteristic fruity and cherry flavours. During drying, fermentation occurs.
Coffee processed by natural sun-drying represents more than 80% of Yemen Arabica and 60% of Brazilian and Ethiopian Arabica, and almost all Robusta varieties. Recently, a higher level of care has been put into the process, from ripening to drying, to achieve high quality productions and Robusta coffee has also made its way into the specialty coffee market.
Natural (or dry) processing is the easiest way to transform coffee cherries into green beans, yet it is also one of the most difficult ways to produce high quality coffee [
13,
14]. On the other hand, it is a cheap and simple processing method that relies exclusively on sunlight and use of flat surfaces where the cherries are layered to dry. There are different techniques for revolving the cherries, and different drying supports are used: some farmers use raised beds, others dry the coffee on patio pavements, and others combine these methods with mechanical dryers.
3.2. Water-Linked Processing Methods: Pulped Natural, Honey, Fully Washed
Water-linked processing methods, such as pulped natural, honey and fully washed methods, have become common in several countries and are applied to meet market requirements for the aroma of coffee, and in the production of specialty coffees. However, the washed methods do not necessarily increase the coffee aroma. By removing the outer layers of the cherry and leaving only a small portion of the mucilage, these methods speed up the drying process and reduce the risk of excessive fermentation or mould growth. Immersion in water allows the separation of damaged beans, and removal of floating lighter cherries. Though the presence of mucilage is considered by some scientist to allow the transfer of fruity aromas from the cherry to the beans, this is actually a contentious point, and there have been no conclusive biophysics studies to demonstrate the importance of fruit compounds to contribute to flavouring. The majority of scientists believe that the mucilage acts as a greenhouse, permitting the internal bean decomposition through enzymatic and kinetic processes and acting as a protective outer layer to isolate the bean and preserve it from environmental factors.
On the other hand, de-pulping allows for a more standardized coffee bean production process and the availability of a mechanical depulper, which is a huge investment for the farmer, permits the obtainment of higher quality coffee beans [
11,
13,
14].
The discussion on how washing vs. fermentation can affect quality is controversial. Enzymes are involved, but they are produced mostly by bacteria. Some of these bacteria are inside the berry itself (endophytic) and therefore even when the fruit layers are removed, fermentation can still occur. As pectins are fermented to simple sugars, yeasts begin to take over and if this goes too far then the “fermented” taste problems arise. In addition, the purely functional definition of the fermentation stage as the separation of mucilage is incomplete. Most scientists agree that by-products (both enzymatic and biological) are created during this stage that can add flavours and complexity to coffee. If allowed to go too far, these by-products can damage the quality of coffee. On the other hand, the process is complex and is not dependent only on mucilage removal [
11,
14].
3.3. Pulped Natural Coffee
In the Pulped Natural method, it is possible to remove the pulp by using high-tech pressure washing machines. After the coffee has been picked and sorted, it is sent to a processing station where it is pulped, i.e., the coffee cherry is cut with a plastic knife and the coffee bean is squeezed out of its shell in a centrifuge. Sugars in the mucilage are removed by fermentation and mechanical scrubbing. Fermentation of the mucilage occurs by stacking the beans outdoors and rinsing them in water. Several washes are performed and, finally, centrifuge pumps remove the excess water. The coffee is packed in sacks and allowed to dry to 40% moisture content. Further drying of a pulped natural coffee is a highly sensitive process and must be carried out under constant supervision. The beans are turned several times a day to get an even drying and prevent excessive fermentation or rot. During the drying process the remaining layer of the mucilage dries around the bean and affects the coffee flavour profile. A well dried pulped natural coffee has an intense sweetness, great mouth feel and rounded acidity. After the coffee has reached a humidity of 13%, it is packed in Grain-Pro bags and then left to rest for a couple of weeks. Pulped natural is a very common coffee processing method, particularly in large areas of Brazil [
11,
14].
3.4. Semi-Dry Processing-Honey Coffee
This process makes use of depulpers and robots to remove skin and pulp and leaves varying amounts of mucilage, from 20% to 80%. Then beans are dried quickly in the sun, or slowly in the shade, thus obtaining yellow, red and black honey coffee, respectively (
Figure 3). These quality coffee beans are also described in terms of percentage of honey level.
Farmers often separate their crop into different categories. Some beans have less mucilage, and therefore dry quicker [
11]. Other types of varieties have a thicker layer of mucilage, and need a longer drying time. A yellow honey (approx. 25% mucilage) typically has the least cloud/shade cover during drying in order to speed up the drying time to about 8 days, and will gain a yellow colour. Red honey (approx. 50% mucilage) takes about 12 days to dry and is typically developed with cloud cover or shading. Black honey is usually 100% covered by mucilage so as to prolong the drying period to about 30 days. In each area, the weather conditions and the initial moisture level of coffee cherries contribute to the duration of the period required for the drying process.
3.5. Wet Hulled/Semi-Washed Process
In Indonesia the farmers make use of de-pulping machines that take out the outer skin, and de-hulling machines that free the beans from the dried hull, called “luwak”. Mucilage remains coated on the beans, that are stored for up to a day to dry, then the mucilage is washed off in a semi-wet process and the coffee is partially dried to 30% to 35% moisture.
3.6. Washed/Fully Washed-Wet Process
In this process, skin, pulp and mucilage are removed using water and fermentation. This is the conventional form of Arabica coffee processing in most regions of the world. The wet process is also used for a small percentage of Robustas, although the trend to use the wet process for Robustas is increasing [
11,
14]. In this method a naturally occurring honey-like layer outside each bean is fermented and washed under controlled steps. Fermentation makes the honey layer viscous in water so it can be washed off after 12–48 h.
After drying, coffee is stored in a warehouse for a minimum of one month, so that moisture levels in each bean distribute evenly [
11,
12,
13].
4. Factors that Affect the Chemical Composition of the Grains and Flavours
Coffee flavour is the result of a complex chain of chemical transformations. The green bean has only a faint odour that is not at all reminiscent of the coffee aroma. It contains, however, all of the necessary precursors to generate the coffee flavour during roasting. The levels and biochemical status of these precursors may vary in relation to genetic traits, environmental factors, maturation level, postharvest treatment, and storage. Maturation favours the development of high-quality flavour in the coffee brew. Green coffee beans, however, may generate high-quality coffee flavour also when they are not completely ripe. Biochemical aspects were studied in immature and mature green coffee suspensions incubated under air or argon. Aerobic incubation triggers the fragmentation or digestion of the 11S seed storage protein and the release of free amino acids [
17,
18,
19,
20,
21]. On the analytical side, a specific pool of flavour precursors was monitored: chlorogenic acids, green coffee proteins, and free amino acids. A link between maturation, the redox behaviour of green coffee suspensions, and their sensory scores was identified. Beans were shown to be sensitive to oxidation of chlorogenic acids.
Desirable flavours have been described [
22], as bright or dry—highly acidic leaving a dry aftertaste; caramel or syrupy; chocolaty—aftertaste similar to chocolate or vanilla; earthy—a soil-like quality; fragrant—an aroma ranging from floral to nutty to spicy, etc.; fruity—having a berry scent; mellow—a smooth taste lacking acidity but not flat; nutty; sweet—a lack of harshness; wild–a flavour considered favourable; winy-aftertaste—resembling a mature wine.
Several chemicals derive from the plant metabolites, and others are products of chemical reactions. Phenolic compounds have been described as contributors to the quality of coffee flavour with a smoky-like odour [
22]. Guaiacol is formed by a thermal degradation of ferulic acid, and the level increases with roasting. Pyrazines are formed by complex interactions between α-amino acids and carbohydrates. Alkyl pyrazines, such as 2-ethyl 5-methylpyrazine, have a key role, with nutty and coffee-like odours. Pyrroles, originating from the degradation of amino acids and Amadori intermediates, have floreal, rose-like, smoky flavours.
Farah [
21] described the major coffee chemical compounds contributing to flavour and sensory aspects. Flament [
22] analysed coffee bean flavour-active compounds, with attention to volatiles, aldehydes, esters, alcohols, and others, contributing to the smell of green coffee and the flavour of roasted coffee. Mastronardi studied the effect of origin and process treatment on the roasting and development of aromas and sensorial evaluation [
23]. Montavon [
24] studied green coffee protein profiles during maturation and with a relationship to coffee cup quality. Czerny and Grosch [
25] identified some odorants of raw Arabica coffee and their changes during roasting. Aroma extract dilution analysis of raw Arabica coffee revealed 3-isobutyl-2-methoxypyrazine, contributing to a peas-like odour with a note of raw coffee; with 2-methoxy-3,5-dimethylpyrazine (II), ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, and 3-isopropyl-2-methoxypyrazine as potent odorants. Twelve odorants occurring in raw coffee and (E)-β-damascenone were also quantified after roasting. Methional, 3-hydroxy-4, 5-dimethyl-2(5H)-furanone, vanillin, (E)-β-damascenone, and 4-vinyl- and 4-ethylguaiacol (with spicy, smoky and sweet flavour) increased strongly during the roasting process. Among chetones, (E)-β-damascenone has a honey-like, fruity, sweet, woody, tea-like flavour.
Semmelroch and Grosch [
26] mentioned that 22 compounds are responsible for the aroma of coffee amongst the hundreds of compounds identified. Studying the ratio of concentration to odor threshold) they found 2-furfurylthiol, 3-mercapto-3-methylbutyl formate, methanethiol, α-damascenone, methylpropanal, and 3-methylbutanal as the most potent odorants. However, the rankings of the odour activity values (OAV) were different in the two coffee brews: Robusta and Arabica. The extraction yields obtained during the preparation of Robusta and Arabica brews were determined for 17 odorants. Polar compounds (e.g., guaiacol, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 3-hydroxy-4,5-dimethyl-2(5H)-furanone, 2,3-butanedione) were extracted with higher yields (75%–100%); non-polar compounds (e.g., α-damascenone, 2-isobutyl-3-methoxypyrazine) gave yields of only 10%–25%.
Kitzberger [
27] evaluated the content in bioactive compounds in roasted coffees, finding 5-caffeoylquinic acid (5-CQA) (from 936 to 1695 mg 100 g
−1), and the diterpenescafestol (from 414 to 742 mg 100 g
−1), and kahweol (from 439 to 1068 mg 100 g
−1)
Kouadio [
28] described that storage of the cherries before sun-drying led to the acidification of the cherries from pH 5.27 to 3.6 and to the degradation of chlorogenic acids, while the caffeine content remained stable. Mussatto [
29] described the chemical components of different coffee varieties. Fisk [
30] studied and identified several flavour chemicals discriminating roast and ground coffee aroma. Using methods of olfactometric analysis, such as Aroma Extraction Dilution Analysis (AEDA) and Combined Hedonic Aroma Response Method (CHARM), Mayer found the olfactory importance of tens of volatile compounds present in the aroma of coffee [
31]. Potent odorants in a sample of medium-roasted Arabica coffee and in the corresponding brew were quantified. Large amounts (>75%) of acetaldehyde, 2,3-butanedione, 2,3-pentanedione, vanillin and some furanones were extracted from the coffee brew, whereas the yields of the more nonpolar compounds, such as 3-isobutyl-2-methoxypyrazine, (
E)-α-damascenone and the unstable 2-furfurylthiol were low (<25%). The aroma of the brew was mainly attributable to some alkylpyrazines, furanones and phenols, and by 2-furfurylthiol, methional and 3-mercapto-3-methylbutyl formate. The higher impact of both methional and the formate on the aroma of the brew and the lower aroma activity of 4-vinylguaiacol were in contrast to results obtained in a previous study for ground coffee of the same provenance and roast degree.
Dirinck [
32] established a study to classify Arabica and Robusta according to the flavour-developing compounds in brewed coffee. Selmar [
33] found that, during post-harvest treatment, coffee beans start to germinate, with an increase in enzymes of the tricarboxylic cycle, such as isocitrate lyase, and that wet and dry processing differently affect the changes in metabolites. Bytof [
34] deepened the findings on seed germination and how they affect the flavour in the post-harvest phase of coffee processing. Selmar [
35] studied the storage of green coffee after wet processing, as parchment coffee or hulled beans, concluding that Maillard reactions occurring during storage are the cause of the decrease in potential aroma precursors such as amino acids and sugars.
Schedig [
36] studied the development of odours during the storage of raw coffee beans (green coffee). These may influence the aroma of the coffee beverage. To gain insight into the aroma compounds responsible for such odour changes, a comparative aroma extract dilution analysis was applied on not stored, raw Arabica coffee beans from Colombia with a water content of 11.75% and on the same beans with a water content of 13.5%, which were stored for 9 months at 40 °C. In combination with flavour dilution factors, the results showed strong increases in (E)-β-damascenone (cooked apple-like), 2-methoxy-4-vinylphenol (clove-like), whereas others, such as the earthy smelling 3-isopropyl-2-methoxypyrazine as well as 2-phenylethanol and 3-methoxyphenol, remained unchanged during storage. In addition, 2-methoxy-5-vinylphenol (intense smoky odour) increased significantly during storage. Quantitative measurements performed on raw coffee samples stored at various temperatures, water contents, and oxygen availabilities indicated methyl esters of 2- and 3-methylbutanoic acid as responsible for the pronounced and fruity odour perceived in the stored green coffee, whereas 2-methoxy-4-vinylphenol and 2-methoxy-5-vinylphenol led to clove-like odour. On the basis of the results obtained, in particular the reduction of the water content in combination with lower temperatures, the aroma in raw coffee beans may be preserved during storage.
5. Coffee Bean Microbiota in Fermentation Processes
The fermentation of coffee is a highly complex process that is influenced by climatic conditions, particularly temperature and humidity. If the fermentation is not carefully monitored, the coffee can acquire undesirable, sour flavours.
Hull, or dried pulp and parchment, is what remains after mucilage has been removed or degraded by enzymes. Hulling of dried parchment leads to green coffee.
A new trend is to study assisted fermentation by using microorganisms as starter strains, or enzymes degrading the mucilage into free sugars. There are three classes of pectinolytic enzymes. Those produced by plants and fungi are pectin esterases which remove methoxy groups of the uronic acids exposing carboxylic groups through which Ca2+ coordinates the chains. Certain fungi also can produce the pectin lyase, an enzyme that attacks the 1,4-glycosidic links of fully esterified (methoxylated) chains of pectin. Lastly, polygalacturonase is produced by certain bacteria and it also attacks the glycosidic links but only of partially de-esterified chains or segments. The oxidative yeast Cryptococcus is common in fruits and it is pectinolytic, but numerous isolates from coffee have been checked and did not show this activity. A few Candida species are also reported to liquefy Ca2+-pectate.
The action of the microflora, in concert with chemical factors (lime, hydroxy carbonates that precipitate pectates) contribute to make the mucilage less sticky, freeing the bean with their parchment to be washed easily by water treatment, making possible the subsequent drying of the beans.
A master thesis has been recently published on the application of enzymes as a processing support to produce coffee beans with good quality [
37].
Independent variables that affect the quality of the resulting beans and therefore the coffee drink, are (a) the composition of the enzymes mix; (b) physico-chemical parameters (pH, temperature); (c) the sequence of different enzymes used and exposure time of the beans; (d) the inoculation with fermentation bacteria; (e) the time of exposure to fermentation and fermentation conditions (pH, temperature).
Finally, environmental conditions influence the types of microorganisms present at different stages of fermentation, thereby affecting the quality of the final product. Six parameters are particularly important in this respect: namely sugar concentration (measured in Brix degrees), water activity (Aw), availability of oxygen, temperature, acidity and time.
The relationship between coffee fermentation and coffee aroma is intricate and delicate [
19]. The coffee aroma profile is determined by the fermentation process during coffee processing. The fermentation process in coffee processing is conducted mainly for mucilage removal but induces the modulation of the coffee aroma.
A control over the fermentation process imparts desirable attributes and prevents undesirable fermentation which generates off-flavours [
22], whereas critical factors such as elevated moisture levels and temperatures in green coffee may generate an over-fermented flavour defect during shipping or storage. Specific ethyl esters of short chain fatty acids have been linked to actual or potential key aroma compounds responsible for the over-fermented flavour defect, with individual contribution as a function of the actual concentration in a given sample.
Moreover, undesired microbial species can be responsible for flavour defects such as the highly acidifying ones, those producing acetic acid and moulds, that impart musty aromas and support the growth of ochratoxin-forming fungi, discussed in the last part of the review. There is a need to accurately control the growth of the organisms consuming the polysaccharides, and the formation of aromatic volatiles, such as phenolics, aldehydes and chetones.
Several methods have attempted to develop novel niche products, such as elephant digested berries collected from dung, marketed under the Trademark “Black Ivory” coffee sold by Elephant Polo store [
38] in Comfort, Texas, and Kopi Luwak coffee; known as the most expensive coffee in the world, obtained by digestion and gut fermentation by asian palm civets. In the “Kopi Luwak beans” coffee, drupes are consumed by wild animals or fed to animals (asian palm civets/cat civets). Beans are recovered after passage into the digestive tract by manual selection. The digested beans present a specific flavour and characteristics. Novel production methods require new approaches to exploit the coffee microbiome and novel associations of gut-microbiome strains in the development of desired flavours [
39].
5.1. Bacteria and Yeasts Associated to Coffee Fermentation Processes
Different types of microorganisms interact with natural coffee mucilage in very diverse ways, some simultaneously and others in succession [
39]. The main aim is to control the process in order to avoid over-fermentation linked flavours [
40,
41].
Masoud studied the yeasts involved in fermentation of
Coffea Arabica in East Africa. In fermenting
Coffea arabica beans, yeasts were dominant [
41]. The predominant yeasts were
Pichia kluyveri and
Pichia anomala.
Hanseniaspora uvarum was predominant during fermentation but decreased during drying.
Kluyveromyces marxianus,
Candida pseudointermedia,
Issatchenkia orientalis,
Pichia ohmeri and
Torulaspora delbrueckii occurred at lower concentrations.
Saccharomyces cerevisiae and
Candida xestobii were not isolated by culture dependent methods. Avallone [
42] studied pectolyticmicrorganisms during coffee fermentation, identifying the positive action of
Lactobacillus brevis, showing good pectinolyitic activity. Other studies regarded the fermentation of sugars and mucilage by various naturally occurring microrganisms [
43,
44,
45].
De Melo Pereira [
46] evaluated yeasts in coffee fermentation by the wet process.
Pichia fermentans, and
Pichia kluyveri were the most frequent isolates, followed by the
Candida species.
Candida glabrata, C. quercitrusa, Saccharomyces sp., Pichia guilliermondii, Pichia caribbica, Hanseniaspora opuntiae and
P. fermentans were found with lower frequency.
The microflora of coffee before and under fermentation has been studied for the role of enzymes of lactic acid bacteria (
Lactobacillus brevis, Leuconostoc mesenteroides) [
42] and
Bacillus subtilis, such as pectinases, xylanases and enzymes depolymerising polysaccharides, simultaneously or preceding yeasts growth.
In a study by Silva [
47,
48,
49] on natural coffee fermentation, among Gram-positive bacteria, representing 85.5% of bacteria isolated,
Bacillus was the predominant genus (51%). Gram-negative species of the genera
Serratia,
Enterobacter and
Acinetobacter were also found. A total of 22% of microorganisms isolated were mycetes.
Debaryomyces (27%),
Pichia (18.9%) and
Candida (8.0%) were the most common genera present, while
Aspergillus was the most abundant.
Previously, Silva [
48], found
Aeromonas,
Pseudomonas,
Enterobacter and
Serratia among the bacterial species. Among Gram-positive bacteria, 23 were spore-forming and included six
Bacillus species, and 118 were non-spore-formers such as
Cellulomonas,
Arthrobacter,
Microbacterium,
Brochothrix,
Dermabacter,
Lactobacillus. As for yeast isolates, such as
Pichia,
Candida,
Arxula,
Saccharomycopsis, 24 different species were identified, with almost all of them being fermentative. There were many rarely described yeasts such as
Pichia lynferdii,
Arxula adeninivorans. Among fungal isolates, 52 were identified to species level, such as
Cladosporium, Fusarium and
Penicillium spp. [
49].
Vilela [
50] analysed the molecular ecology and polyphasic characterization of the microbiota associated with coffee (
Coffea arabica L.) fermentation in a semi-dry process. The bacterial and fungal isolates were phenotypically characterised.
Bacillus subtilis,
Escherichia coli,
Enterobacter agglomerans,
Bacillus cereus and
Klebsiella pneumoniae were the predominant bacteria detected during the processing of the coffee, and
Pichia anomala,
Torulaspora delbrueckii and
Rhodotorula mucilaginosa were the dominant yeasts, while
Aspergillus was the most common fungus. All of the yeast and bacterial species were isolated using culture-dependent methods. Therefore, different methods of microbiological analysis brought different results. Presently, metagenomic studies may allow the standardisation of the analyses and the achievement of comparable results among different groups.
Evangelista [
51] tested starter cultures in a semi-dry coffee (
Coffea arabica) fermentation process. The authors used
Saccharomyces cerevisiae UFLA YCN727,
S. cerevisiae UFLA YCN724,
Candida parapsilosis UFLA YCN448,
Pichia guilliermondii UFLA YCN731;
Debaryomyces hansenii,
Cystofilobasidium ferigula and
Trichosporon cavernicola. In the inoculated samples they found a mixed bacterial population, composed of the genera
Weissella,
Leuconostoc,
Gluconobacter,
Pseudomonas, Pantoea,
Erwinia and
Klebsiella.Silva [
52] evaluated a potential starter culture to enhance the quality of coffee fermentation.
Bacillus cereus,
Bacillus megaterium,
Bacillus subtilis,
Candida parapsilosis,
Pichia caribbica,
Pichia guilliermondii and
Saccharomyces cerevisiae were tested. Among the species used, they found some strains producing 2-propanediol, hexanoic acid, decanoic acid, nonanoic acid and ethyl acetate. The UFLA CN448 and UFLA CN724 strains were characterized by guaiacol, butyric acid and citronellol.
S. cerevisiae UFLACN727,
P. guilliermondii UFLACN731 and
C. parapsilosis UFLACN448 isolates were identified as promising candidates for addition as starter cultures to coffee fermentation.
Lin [
53] studied the depulping of coffee fruits and green coffee bean fermentation using enzymes,
Leuconostoc mesenteroides,
Kluyveromyces marxianus,
Kluveromyces lactis,
Rhizopus oryzae, and
Aspergillus niger. The fermentation time required using
Aspergillus niger and enzymes was within 24 h, with high reduced sugar (8–15° brix) content, weak acidity, pH in the range 3–4.7), and cupping determined by a caramel flavour.
5.2. Role of Microorganisms in Taste Defects
The role of microorganisms in taste defects of wet processed coffee is a matter of debate [
40]. Of the numerous defects often attributed to problems during fermentation, the three most serious are “fermented taste”, “sour” and “stinkers”. Because fermentation can occur in the intact fruit, especially if harvested and not processed rapidly, the same defects can arise in natural coffee and, by extension, in wet processed coffee even if the fault was actually related to the fermentation step.
A fermented taste has fruity aldehyde tones; “sour” is likened to onion; “stinker” is a powerful foul taste, and a single stinker bean can affect several kilograms of product. Stinker beans have been attributed to the dominant growth of
Bacillus brevis or too high levels of lactic acid bacteria, possibly associated with derivatives of methyl-butanoic acid, cyclohexanoic acid esters and S-containing organic compounds. Some compounds that can be traced to a defect also indicate the source where few organisms produce the compound. Earthy and mouldy odours [
54] can be attributed principally to 2-methyl-isoborneol and geosmin, respectively. These compounds are produced notably by species of
Eurotium, other moulds and some
Actinomycetes.
In some processing chains, whether or not fermentation has been conducted, a soaking step is sometimes applied. This is sometimes called secondary fermentation where a fermentation step is included in the process or fermentation where mechanical mucilage removal has been used. After mucilage removal, the parchments are held under water for a period from overnight up to, rarely, 48 h. The principle effect is to cause the beans to become more uniformly dark blue-green, a desirable physical characteristic that, in itself, has no taste implication. The colour is the same as that generated by the hydrated bean in response to physical injury, or damaged by coffee berry borers or by cutting with a scalpel. The colour is likely to be a hydrolysis reaction akin to the chlorogenic acid reaction (
Table 1).
Some authorities claim that soaking removes or reduces any harsh “edge” the cup may have. It is unclear whether this is due to leaching, or to seeds’ metabolism under anoxic conditions [
12,
13,
53]. For coffee without this “edge” there is no change in cup quality for up to 7 days. The harshness is usually attributed to phenolic compounds, thus the implication would be that, through one mechanism or another, certain phenolic compounds are removed or altered.
After washing of the parchments, considerable yeast and bacteria remain on the surface and even in the bean tissue. However, there is very little substrate for microbial growth therefore the bean is metabolically quiescent, during this period under water. Short periods of soaking do not seem to be associated with flavour defects, unless tainted water is used for the soaking.
5.3. Ochratoxin A (OTA) Producing Fungi in Coffee Production and Storage
The European Union or individual countries have imposed tight limits of ochratoxin levels in coffee beans that are in the range of 5–10 ppb, or 10–20 ng/gr. The European Mycotoxin Awareness Network (EMAN) groups include several food safety institutes, one of which is the Institute of Food Productions of the National Research Council of Italy (CNR-ISPA, Italy) [
55]. In this context, several EU projects have been carried out to improve the analysis and detection of mycotoxins producing strains on foods and on the environment [
56].
Coffee cherry drying, was identified as one of the steps during which ochratoxin A (OTA) formation can take place, particularly under humid tropical conditions. In particular, in coffee, ochratoxigenic fungi may develop on ripe and unripe cherries [
57], on beans, during fermentation [
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70], during storage, packaging and during roasting and brewing [
71,
72,
73,
74].
Recently, a protocol set up by the Food and Agriculture Organization (FAO) has been published to optimize the conditions of storage and packaging to protect the beans from ochratoxin producing fungi [
12].
Kouadio [
69] analysed the mixing of coffee cherries dried on aerated beds and the frequency of mixing on the fungal growth and production of OTA and defined the temperature and water activity conditions for the production of ochratoxin A by fungi grown on a coffee-based culture medium. Subsequently, they showed the development of toxigenic fungi after cherry harvest before the start of sun-drying, in Robusta varieties [
28].
To avoid toxigenic fungi growth, recommendations about the correct drying process have been established but also strategies to stimulate plant defence responses to toxigenic fungi should be pursued [
75]. The coffee beans need to be harvested intact, and kept on aerated beds, eventually covered with polyethylene films to protect them from moisture and rain, and stored in well aerated deposits far from the walls. A ripe coffee cherry on a healthy plant needs to be cared for properly and picked at the peak of ripeness, preserving it from contacts with overripe, broken or rotten berries. The coffee cherry undergoing initial processing has to be dried uniformly avoiding rewetting. The coffee must rest before undergoing the last stages of raw processing and preparation for shipping. At this time, relative humidity, temperature and storage containers and conditions all become critical. The natural occurrence of OTA in green coffee beans has been reported by several authors in concentrations from 0.2 to 360 μg∙kg
−1. In coffee beans and in roasted coffee, brewed with different methods, the possibility of incurring the imposed limits (in the range of 5–10 ppb, or 10–20 ng/gr) of the EU or an individual country is probable.
The roasting process decreases the OTA content by 50%. Brewing with a French press, or by dripping, as in American coffee making, enables a transfer of up to 50% of the toxin in the final drink [
74]. Paterson [
76] pointed to the need to pay attention also to aflatoxins and eventually to fumonisins.
The possibility for heavy coffee drinkers to accumulate ochratoxin at levels within the detection limit of modern HPLC methods supports the need for end users (brewers, roasters and coffee drinkers) to occasionally check their blood to control ochratoxin A and its derivatives in serum [
77], for safety purposes, since the toxin is linked to kidney cancer.
Tests have been made to evaluate the ability of growth of ochratoxigenic fungi on a coffee bean medium [
76,
77], and on the growth in presence or absence of antagonistic yeasts and microorganisms [
77,
78,
79].
Several critical points arise in all these steps, and small mistakes in screening or larger mistakes in the selection of packaging or the storage conditions prior to shipping can cause contamination by ochratoxigenic fungi and, occasionally, their transfer to a whole coffee shipment. In
Figure 4 various stages of coffee harvesting and processing steps that are at risk of toxigenic fungi development are described.
The berries put out for sun drying on the day of harvest were devoid of fungi. The 3% of A. niger isolates found on coffee were able to produce OTA, while 77% of A. carbonarius isolates and 75% of A. ochraceus isolates produced this mycotoxin. However, A. niger was isolated more frequently than A. carbonarius and A. ochraceus.
The presence of skins with the parchment is unlikely to affect the fermentation course with respect to OTA production. The presence of dry cherries, which go through the pulper due to their small size, present a different scenario. If we assume that a greater development of OTA-producers could have occurred under the extended period of oxidative and mesohydric conditions of this material, the fermentation would not protect and significant OTA production could occur, if not during fermentation, later during drying.
The conditions of low oxygen tension dictates that the oxidative, mesophilic species of Aspergillus capable of OTA production will not thrive during fermentation. In laboratory studies, large numbers of spores, introduced into the fermentation mass at the beginning of fermentation did not result in any OTA appearing in the beans and the organism (A. ochraceus) could not be recovered from the beans after drying. However, this test is only valid for models where the spores of the OTA producer from the fruit skins or soil are the source of contamination. In fact, a proportion of beans, infected before harvest, harbour these fungi and there is some evidence to suggest that fermentation can kill them in the beans. However, it is clear that fermentation does not always do so. In other tests where pulping was delayed for up to six days after harvest, the protective effect of fermentation against OTA accumulation was not observed. This could be interpreted as there being some threshold biomass above which the mesophilic fungi can survive the fermentation.
Iamanaka [
80] studied the mycobiota of coffee beans and its influence on the coffee beverage. There is an input of microbial contamination during the processing of the beans, especially just after harvesting and drying. Coffee beans were collected from different stages of the coffee production chain and data were correlated with the sensory characteristics of the final beverage. Samples were collected from the tree (mature cherries), from the ground, from the patio (mature, immature and dried floaters or overripe cherries from the tree) and from storage facilities. In general, coffee samples from Brazil showed fungal infection and contamination. The most common fungi were
Penicillium brevicompactum,
Aspergillus section
nigri,
Penicillium sp. nov. (related to
Penicillium crustosum) and
Fusarium sp. Both
P. brevicompactum and
Penicillium sp. nov. were found at all processing stages, also in the cherries.
The use of patios with earth floors should be avoided to produce the highest quality coffee [
62,
67,
68]. Wet processing is applied to Arabica coffee beans and the mycobiota was highly influenced by (a) fermentation (water quality and equipment used) and (b) drying (time period, cleanliness of equipment and environment).
Palacios-Cabrera [
81] investigated OTA production during transportation and verified that the temperature inside coffee containers changed drastically during the travel from a coffee exporting country such as Brazil in summer to an importing country such as Italy in winter. Condensation can occur during raw coffee transportation to the consuming countries and lead to mould growth. Storage and transportation trials have shown that condensation and wetting of coffee occur mainly during transport overland to the harbour for shipping and/or upon arrival at the destination.
Velmourougane [
82,
83] studied mold incidence, ochratoxin A contamination, and cup quality during preparation of arabica and robusta cherries in farms, and the containment of
Aspergillus ochraceus and Ochratoxin-A production in coffee during processing through commercial yeast inoculation, using
S. cerevisiae as an antagonistic yeast, with positive results. In addition, wireless sensors and RFID technology have been proposed to control coffee processing in tanks [
84].
6. Specialty Coffees Classification Criteria
While espresso is the main driver of coffee consumption in Italy, with studies on espresso coffee quality determinants [
85], in Northern Europe brewed coffee and American style coffees are widely used. Recently, a niche market has been established for high quality coffees with unique flavours. The term “specialty coffee” was first coined by Erna Knutsen, of Knutsen Coffee Ltd., in a speech to the delegates of an international coffee conference in Montreuil, France, in 1978. Special geographic microclimates produce beans with unique flavour profiles, which she referred to as “specialty coffees”. Underlying this idea of coffee appellations was the fundamental premise that specialty coffee beans would always be well prepared, freshly roasted, and properly brewed. This was the craft of the specialty coffee industry that had been slowly evolving during the last 10 years. The Specialty Coffee Association of America (SCAA) continues to define specialty in this context.
In this respect, Golden Mountain coffee growers in Thailand, must be mentioned as an example, since they put a premium on taking extra care, both before and after processing, to inspect beans and ensure the export of only the finest choice of high-quality products. Both electronic light sensors and individuals pick out imperfections, ensuring that when the beans reach roasters and customers they reflect all the hard work involved in growing and processing them. They use special food-grade packaging to seal in freshness and ensure that excellent cupping scores are maintained from origin to roastery. The staff constantly visit partner producers and oversee drying, hulling, storage, exports, and imports, maintaining quality along the entire supply chain.
Until the moment that the roasted coffee is brewed and transformed into a beverage, the concept of specialty coffee is locked up as a possibility, just a potentially wonderful gustatory experience. Plant husbandry is essential to the preservation of the highest quality of coffee berries.
A ripe coffee cherry on a healthy plant of suitable ancestry planted in the right soil, blessed with appropriate climatic conditions and cared for properly, must be picked at the peak of ripeness in order to preserve the potential for greatness. The coffee cherry must undergo some initial processing at this point. For the majority of specialty coffee this begins with the delivery of the ripe cherry to a wet mill. The time that elapses between harvest and the beginning of processing can have a dramatic impact on the final results for the coffee, therefore specialty coffee is dependent on a quick delivery from the tree to the mill.
Whether the coffee is mechanically pulped and then fully washed, or if it is processed in a demucilaging machine, the initial processing stage must be carefully managed so that the coffee is not harmed. After removal of the skin and pulp, the coffee must be dried, and this is another critical activity. Dried too quickly or too slowly, dried unevenly, dried and then rewetted, not dried sufficiently: all these factors can be disastrous to the final quality of the coffee. From here the coffee must rest before undergoing the last stages of raw processing and preparation for shipping. At this time, relative humidity, temperature and storage containers and conditions all become critical. Finally, the coffee must be hulled, separated by size and packaged for shipping. More critical points arise here, and small mistakes in screening or larger mistakes in the selection of packaging or the storage conditions prior to shipping can drain the coffee of its potential.
The Specialty Coffee Association of America (SCAA) defines specialty coffee in its green stage as coffee that is free of primary defects, is properly sized and dried, presents no faults or taints in the cup and has distinctive attributes. In practical terms, this means that the coffee must be able to pass aspect grading and cupping tests. After roasting and before brewing, the coffee must be ground. Grinding is best done as close in time to brewing as possible, as many delicate aromatic compounds are fully released upon grinding. The increase in surface area, necessary for a good brewing and cupping experience, leads to the rapid oxidation of the coffee and staling. The size of the ground particles is also important, depending on the method of brewing to be used. Whether the coffee is to be prepared as an espresso, as a drip coffee or in a steeping method like a French press, the application of standards of water quality, brewing temperature, coffee to water ratio and extraction must be applied, to produce a specialty coffee beverage [
86].
The cup of coffee is then graded according to the classification of Specialty Grade, or Below Specialty Grade. The Technical Standards Committee (TSC) of the SCAA recommended certain standards for cupping coffee, such as sample preparation to evaluation the quality., i.e., sensory testing, flavour description, scoring. A sensory lexicon has been established, for the tasting and cupping experience, applying sensory science to name coffee’s primary sensory attributes and pave the way for a replicable measuring of those qualities. The Coffee Quality Institute (CQI) protocols introduced a Cupping Form, with sensory descriptive analysis, to show the quantifiable difference in sensory characteristics of two quality coffees. Based on the protocol, it was evaluated that a good Castillo is fruity but not citric, with notes of dark chocolate and roasted nuts, while a good Caturra is floral with cocoa and caramel notes.
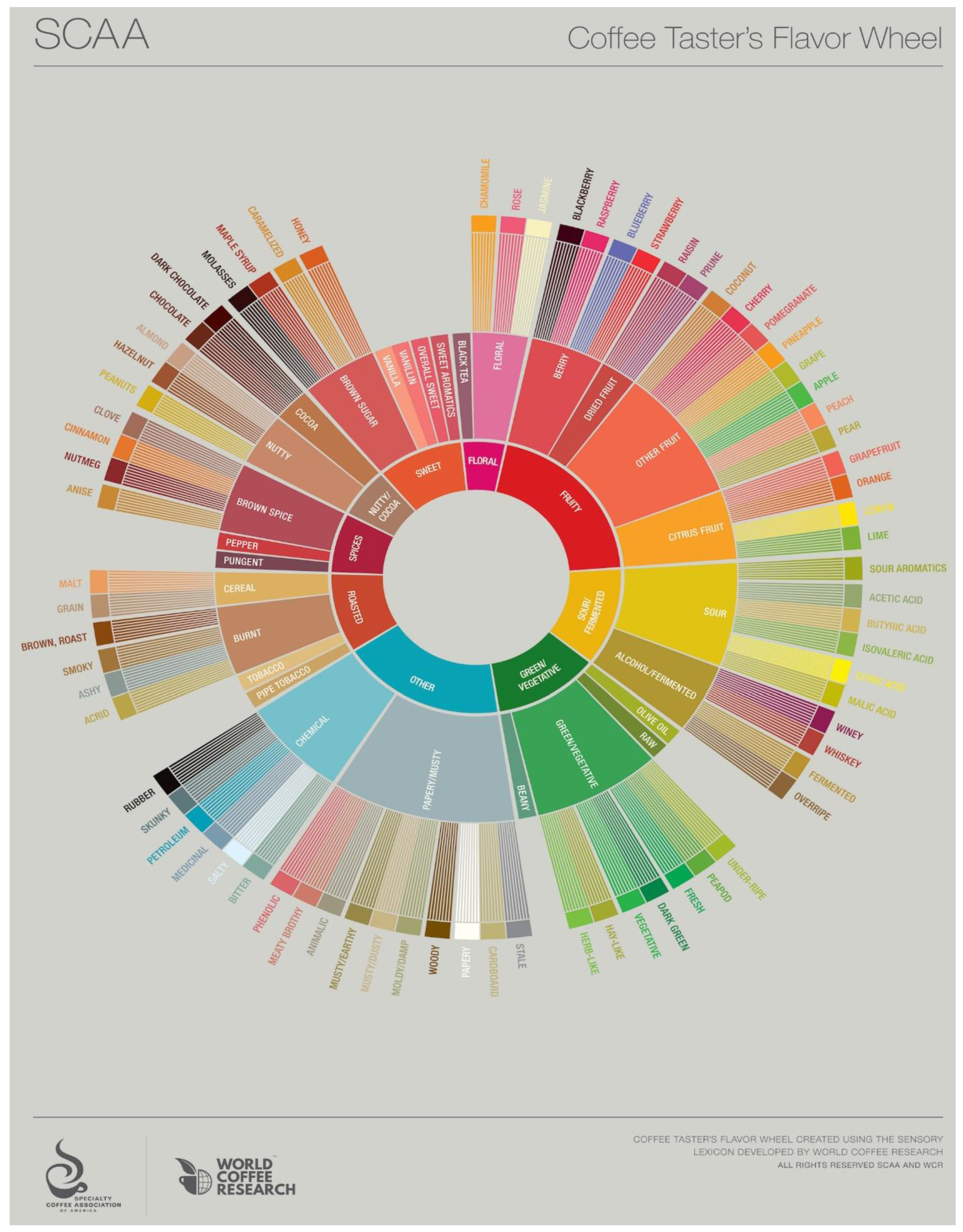
In
Figure 5 the Coffee Taster’s Flavour Wheel (©2016), by SCAA and World Coffee Research (WCR), licensed under a Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License (CC BY-NC-ND 4.0), is reproduced, by courtesy of Emma Sage of SCAA [
87]. The work is based on the World Coffee Research Sensory Lexicon, with the collaboration of professional sensory panelists, scientists, coffee buyers, and roasting companies collaborating via WCR and SCAA.
The SCAA Cupping Form provides a means of recording important flavour attributes for coffee: fragrance/aroma, flavour, aftertaste, acidity, body, balance, uniformity, clean cup, sweetness, defects, and “overall” attributes. The overall score is based on the flavour experience of the individual “cupper” as a personal appraisal. The specific flavour attributes are positive scores of quality reflecting a judgment rating by the cupper. Defects are negative scores denoting unpleasant flavour sensations that detract from the quality of the coffee. These are classified in two ways: a taint is an off-flavour that is noticeable, but not overwhelming, usually found in the aromatic aspects. A “taint” is given a “2” rating in intensity. A “fault” is an off-flavour, usually found in the taste aspects, that is either overwhelming or renders the sample unpalatable and is given an intensity rating of “4”. The defect must first be classified as a taint or a fault, then described (“sour,” “rubbery,” “ferment,” “phenolic”) and the description written down. The number of cups in which the defect was found is then noted, and the intensity of the defect is recorded as either a 2 or 4. The defect score is multiplied and subtracted from the total score according to directions on the cupping form. The defects contribute to assignment of the final scoring to “Below Specialty quality”, and identification of the product as non-specialty.
Through the evolution of coffee production methods, fermentation processes and high standards of quality, the producers have been able to increase their income and to produce specialty coffees with high value for the coffee experience sector.
Piccino [
88] showed the major chemicals influencing the aroma of three specialty quality coffees (“Gran Cru”, “Sublime” and “Authentique”). They found 2,4-nonadienal, 2,4-heptadienal, 2-phenylacetaldehyde, 2-methyl-5-propylpyrazine as markers of the gradation of quality. They studied the “Bourbon Pointu” coffee,
Coffea Arabica var.
laurina, originated from a spontaneous mutation of the Bourbon variety in Reunion Island, as “specialty coffee”. Solid phase extraction (SPE) was used to extract the volatiles. After identification by gas chromatography–mass spectrometry, the odorants were determined by the odour activity value (OAV). This allowed the differentiation of coffee brews from the three trade classifications. Aromatic composition of “Bourbon Pointu” coffee was characterised by a predominance of aldehydes with high OAV (e.g., (
E,
E)-nona-2,4-dienal: 13,600) for coffee brews belonging to “Grand Cru” classification, of 2-phenylacetaldehyde (270) for coffee brews of “Sublime” classification and of a group of pyrazine compounds (e.g., 2-methyl-5-propylpyrazine: 16,750) for coffee brews of “Authentique” classification.
7. Freshness Preservation of Roasted Coffee
Roasted coffee must be packed as soon as possible to prevent the loss of VOCs and oxidation. Therefore, semi-rigid gas-impervious containers, capable of counteracting the pressure generated by the CO
2 release from the coffee beans, are used. To avoid package swelling, leaking or bursting, coffees are partially degassed by treatments that minimize aroma loss, and packaged in active packaging systems that are equipped with a vent valve to allow the release of CO
2 during storage [
89]. During the roasting process, volatile and non-volatile compounds that form the typical flavour of coffee are generated. The packaging material is equally important for the roasted and roasted and ground (R&G) product from precursors present in the green bean. At the same time, the formation of CO
2 and other gases takes place as a consequence of Maillard, Strecker, and pyrolysis reactions, with CO
2 accounting for more than 80% of the gases formed. A part of the CO
2 remains entrapped in the beans and is released during storage [
90].
The containers are resealable and allow the preservation of the sensorial quality of the product after the first opening. Freshness is a critical factor in the quality rating of coffees and even more so in the specialty coffee sector; it is influenced by the roasting and degassing treatments applied to coffee beans before packaging and by the packaging conditions, which must minimize the loss of volatile compounds (VOCs).
A recent study by Glöss [
89] evaluated the effect of different packaging materials on freshness preservation for freshly roasted
Coffea arabica from Guatemala, Antigua (La Ceiba). Both whole roasted beans in 250 g pack sizes and R&G coffee packed as individual single serve capsules were analysed after storage in four different packaging materials, ranging from a simple paper pack to a plastic composite film with a thick aluminium layer. At the time of analysis, ground coffee was filled in a headspace vial under nitrogen atmosphere and analysed by GC-MS.
The proposed indicators of freshness, i.e., ratios 2-butanone/2-methylfuran and 2,3-butanedione/2-methylfuran for whole beans, and dimethyl disulfide/methanethiol and 2-butanone/methanethiol for roasted and grinded coffee, highlighted the role of the aluminium layer in preserving freshness. The ratio 2-butanone/2-methylfuran, probably the best known ratio for analyzing the shelf-life of coffee, is related with coffee staling since it increases with the loss of the highly volatile compound 2-methylfuran. This ratio is influenced by the permeability of the packaging material and packaging with an aluminium layer showed only very small changes of it over time.
The ratio 2,3-butanedione/2-methylfuran increased for coffees packed in paper and plastic composite films, while, in different studies, it was found to slightly decrease for packages with an aluminium layer. This was attributed to the high reactivity of 2,3-butanedione.
Freshness indices dimethyldisulfide/methanethiol and 2-butanone/methanethiol are influenced by the reactivity of methanethiol by oxidation and dimerization to dimethyl disulfide and therefore are influenced by the decreased concentration of methanethiol. A capsule with a 100% aluminium body and aluminium cover did not show any evolution of both freshness indices over the 46 weeks of storage and exhibited a low capsule-to-capsule variability.
A further advancement and future challenge in coffee process control is the application of selected microbiota and enzymes to accelerate the coffee transformation processes for the development of desirable flavours [
17,
91,
92].
Recently, special attention has been paid to the chemical analysis and evaluation of flavour components such as roasty smelling 2-furfurylthiol and di- or trihydroxybenzenes [
93]. Mozambioside is an Arabica-specific bitter-tasting furokauraneglucoside [
94]. In contrast to Catuai and Tipica coffees [
95], the application of chromatographical and statistical techniques; the application of advanced technologies, such as Raman spectroscopy in the determination of robusta in a coffee blend [
96]; and proton-transfer reaction mass spectrometry (PTR-MS), coupled with GC/MS in the identification of volatile organic compounds [
97]. Proton Transfer Reaction—Mass Spectrometry (PTR-MS) has proven to ba applicable as an instrumental approach to predict the sensory profile of espresso coffee [
98,
99].
8. Conclusions
The coffee industry relies on microbiology and fermentation processes to allow coffee beans to develop specific characteristics linked to the coffee variety, the process used, and the conditions in drying and storage. All these aspects must be tackled to improve the quality of the coffees and try to assure the availability of high quality coffee beans. The avoidance of the growth of toxigenic fungi and the limitation of ochratoxin A synthesis by the use of antagonistic strains may help in a sustainable production of coffees within the high standards set up by the EU countries. In addition, studies have shown the feasibility to apply enzymatic hydrolysis to dissolve the mucilage and to add starter strains to speed up the wet coffee processing. Furthermore, innovative technologies may be applied to protect the stored coffee beans from fungal contamination, such as a new packaging material surface coated with antifungal volatiles. Lastly, specialty coffees may take the lead and we need to assure the availability of coffees with a high cupping score, to sustain consumer demand.