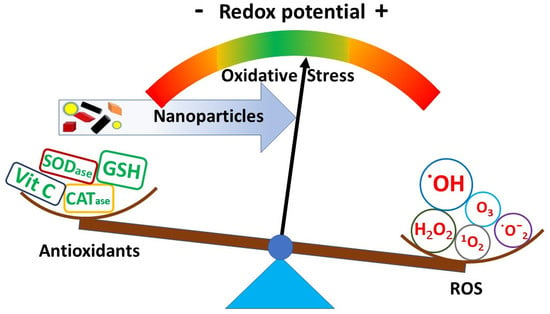
Nanomaterials and Oxidative Stress
Abstract
:Acknowledgments
Declaration
Author Contributions
Conflicts of Interest
References
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, M.; Sil, P.C. Nanotoxicity: Oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Hall, N.D.; Bacon, P.A.; Dieppe, P.A.; Halliwell, B.; Gutteridge, J.M.C. The importance of iron in rheumatoid disease. Lancet 1981, 2, 1142–1144. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Richmond, R.; Halliwell, B. Oxygen free-radicals and lipid-peroxidation—Inhibition by the protein ceruloplasmin. FEBS Lett. 1980, 112, 269–272. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Burello, E.; Worth, A.P. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology 2011, 5, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.X.; Liu, R.; George, S.; Xiong, S.J.; Wang, X.; Zhang, H.Y.; et al. High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Plumlee, G.S.; Morman, S.A.; Ziegler, T.L. The toxicological geochemistry of earth materials: An overview of processes and the interdisciplinary methods used to understand them. Rev. Mineral. Geochem. 2006, 64, 5–57. [Google Scholar] [CrossRef]
- Lujan, H.; Sayes, C.M. Cytotoxicological pathways induced after nanoparticle exposure: Studies of oxidative stress at the ‘nano-bio’ interface. Toxicol. Res. 2017, 6, 580–594. [Google Scholar] [CrossRef]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Bustos, A.R.M.; Reipa, V.; Riley, K.R.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Tu, X.; Dizdaroglu, M.; Zheng, M.; Nelson, B.C. Protective roles of single-wall carbon nanotubes in ultrasonication-induced DNA base damage. Small 2013, 9, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Wages, P.A.; Cheng, W.Y.; Gibbs-Flournoy, E.; Samet, J.M. Live-cell imaging approaches for the investigation of xenobiotic-induced oxidant stress. BBA Gen. Subj. 2016, 1860, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Rhieu, S.Y.; Urbas, A.A.; Bearden, D.W.; Marino, J.P.; Lippa, K.A.; Reipa, V. Probing the intracellular glutathione redox potential by in-cell NMR spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Mercatelli, E.; Barbieri, L.; Luchinat, E.; Banci, L. Direct structural evidence of protein redox regulation obtained by in-cell NMR. BBA Mol. Cell Res. 2016, 1863, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Papayan, G.; Petrishchev, N.; Galagudza, M. Autofluorescence spectroscopy for nadh and flavoproteins redox state monitoring in the isolated rat heart subjected to ischemia-reperfusion. Photodiagn. Photodyn. Ther. 2014, 11, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Reipa, V.; Watson, S.S.; Stanley, D.L.; Rabb, S.A.; Nelson, B.C. DNA damaging potential of photoactivated p25 titanium dioxide nanoparticles. Chem. Res. Toxicol. 2014, 27, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Hollingsworth, J.A.; Hu, B.; Steinbruck, A.; Stark, P.C.; Rios Valdez, C.; Vuyisich, M.; Stewart, M.H.; Atha, D.H.; Nelson, B.C.; et al. Functionalization-dependent induction of cellular survival pathways by cdse quantum dots in primary normal human bronchial epithelial cells. ACS Nano 2013, 7, 8397–8411. [Google Scholar] [CrossRef] [PubMed]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Tournebize, J.; Sapin-Minet, A.; Bartosz, G.; Leroy, P.; Boudier, A. Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 2013, 116, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Von Dem Bussche, A.; Buechner, M.; Yan, A.H.; Kane, A.B.; Hurt, R.H. Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small 2008, 4, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Guadagnini, R.; Kenzaoui, B.H.; Walker, L.; Pojana, G.; Magdolenova, Z.; Bilanicova, D.; Saunders, M.; Juillerat-Jeanneret, L.; Marcomini, A.; Huk, A.; et al. Toxicity screenings of nanomaterials: Challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology 2015, 9, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.R.; Dusinska, M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagen. 2015, 56, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, K.H.; Ji, L.F.; Tang, B. Effect of gold nanoparticles on glutathione depletion-induced hydrogen peroxide generation and apoptosis in hl7702 cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tournebize, J.; Boudier, A.; Joubert, O.; Eidi, H.; Bartosz, G.; Maincent, P.; Leroy, P.; Sapin-Minet, A. Impact of gold nanoparticle coating on redox homeostasis. Int. J. Pharm. 2012, 438, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Li, C.C.; Yao, C.J.; Ding, L.; Lei, Z.D.; Wu, M.H. Techniques for investigating molecular toxicology of nanomaterials. J. Biomed. Nanotechnol. 2016, 12, 1115–1135. [Google Scholar] [CrossRef] [PubMed]
- Atha, D.H.; Nagy, A.; Steinbruck, A.; Dennis, A.M.; Hollingsworth, J.A.; Dua, V.; Iyer, R.; Nelson, B.C. Quantifying engineered nanomaterial toxicity: Comparison of common cytotoxicity and gene expression measurements. J. Nanobiotechnol. 2017, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Reipa, V. Analytical measurements of nanoparticles in challenging and complex environments. In Metrology and Standatdization for Nanotechnology; Mansfield, E., Ed.; Wiley-VCH: Weinheim, Germany, 2017; pp. 175–196. [Google Scholar]
- Elliott, J.T.; Rosslein, M.; Song, N.W.; Toman, B.; Kinsner-Ovaskainen, A.; Maniratanachote, R.; Salit, M.L.; Petersen, E.J.; Sequeira, F.; Romsos, E.L.; et al. Toward achieving harmonization in a nanocytotoxicity assay measurement through an interlaboratory comparison study. ALTEX Altern. Anim. Exp. 2017, 34, 201–218. [Google Scholar]
- Material Measurement Laboratory. Available online: https://www.nist.gov/mml/nano-measurement-protocols (accessed on 27 February 2018).
- Johnston, H.J.; Verdon, R.; Gillies, S.; Brown, D.M.; Fernandes, T.F.; Henry, T.B.; Rossi, A.G.; Tran, L.; Tucker, C.; Tyler, C.R.; et al. Adoption of in vitro systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanomaterials. Crit. Rev. Toxicol. 2018, 48, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.G.; Serchi, T.; Hoffmann, L.; Blomeke, B.; Gutleb, A.C. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part. Fibre Toxicol. 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.A.; Mombelli, E.; Pietroiusti, A.; Tran, L.; Worth, A.; Fadeel, B.; McCall, M.J. Applying quantitative structure-activity relationship approaches to nanotoxicology: Current status and future potential. Toxicology 2013, 313, 15–23. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reipa, V.; Atha, D.H. Nanomaterials and Oxidative Stress. Challenges 2018, 9, 17. https://doi.org/10.3390/challe9010017
Reipa V, Atha DH. Nanomaterials and Oxidative Stress. Challenges. 2018; 9(1):17. https://doi.org/10.3390/challe9010017
Chicago/Turabian StyleReipa, Vytas, and Donald H. Atha. 2018. "Nanomaterials and Oxidative Stress" Challenges 9, no. 1: 17. https://doi.org/10.3390/challe9010017