Industrial Developmental Toxicants and Congenital Heart Disease in Urban and Rural Alberta, Canada
Abstract
:1. Introduction
2. Results
2.1. Principal Component Analysis (PCA)
2.2. Distribution of Emitting Facilities and Exposure on Alberta Postal Codes
2.3. Distribution of CHD in Urban and Rural Alberta
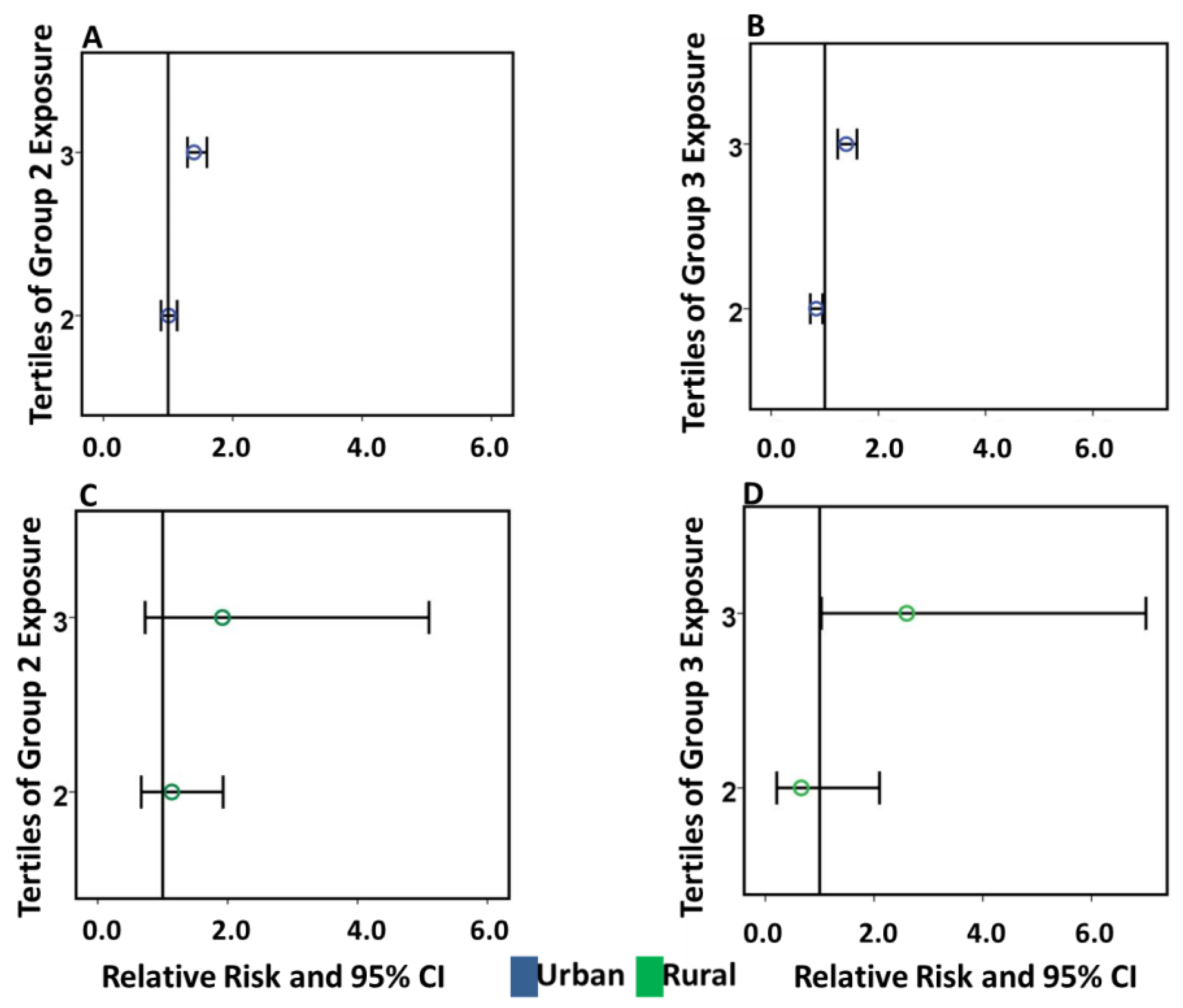
2.4. Adjusted Inverse Distance Weighted Exposure on CHD in Urban and Rural Postal Codes
2.4.1. Urban Postal Codes
2.4.2. Rural Postal Codes
2.4.3. Geographic Distribution of Urban and Rural Postal Codes with the Highest Exposure to Emissions
3. Discussion
3.1. Multipollutant Exposures and CHD
3.2. Negative Associations of Multipollutant Exposures and CHD in Urban Postal Codes
3.3. Rural Higher Average of CHD by Postal Code
3.4. Plausible Pathogenetic Mechanisms for Industrial Multipollutants and CHD
4. Materials and Methods
4.1. Study Population
4.2. Pollution Data
4.3. Exposure Assessment
4.4. Statistical Analysis
5. Strengths and Limitations
6. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval
Declarations
Abbreviations
| CHD | Congenital Heart Disease |
| EF | Emitting Facility |
| IDW | Inverse Distance Weight |
| NPRI | National Pollutant Release Inventory |
| PCA | Principal Component Analysis |
| RR | Relative Risk |
| SES | Socio-Economic Status |
References
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Chiappa, E.; Gargani, L.; Picano, E. Potential effects of environmental chemical contamination in congenital heart disease. Pediatr. Cardiol. 2014, 35, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Joseph, K.S.; Lisonkova, S.; Rouleau, J.; Van den Hof, M.; Sauve, R.; Kramer, M.S.; Canadian Perinatal Surveillance System (Public Health Agency of Canada). Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation 2013, 128, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.B.; Witt, K.L. Genetic toxicities of human teratogens. Mutat. Res. 1997, 396, 9–43. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Kartiko, S.; Finnell, R.H. Importance of gene-environment interactions in the etiology of selected birth defects. Clin. Genet. 2009, 75, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Pulignani, S.; Andreassi, M.G. Genetic and Epigenetic Mechanisms Linking Air Pollution and Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Martinez, D.; Manzanares, S.; Dadvand, P.; Schembari, A.; Rankin, J.; Nieuwenhuijsen, M. Ambient air pollution and risk of congenital anomalies: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2011, 119, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.K.; Zmirou-Navier, D.; Padilla, C.; Deguen, S. Effects of Air Pollution on the Risk of Congenital Anomalies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2014, 11, 7642–7668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, L.M.; Guo, R.; Witter, R.Z.; Savitz, D.A.; Newman, L.S.; Adgate, J.L. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ. Health Perspect. 2014, 122, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.H.; Brender, J.D.; Suarez, L.; Zhan, F.B.; Mistry, J.H.; Scheuerle, A.; Moody, K. Maternal residential proximity to waste sites and industrial facilities and conotruncal heart defects in offspring. Paediatr. Perinat. Epidemiol. 2009, 23, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Yauck, J.S.; Malloy, M.E.; Blair, K.; Simpson, P.M.; McCarver, D.G. Proximity of residence to trichloroethylene-emitting sites and increased risk of offspring congenital heart defects among older women. Birth Defects Res. (Part A) Clin. Mol. Teratol. 2004, 70, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health Part B 2008, 11, 373–517. [Google Scholar] [CrossRef] [PubMed]
- Stingone, J.A.; Luben, T.J.; Daniels, J.L.; Fuentes, M.; Richardson, D.B.; Aylsworth, A.S.; Herring, A.H.; Anderka, M.; Botto, L.; Correa, A.; et al. Maternal Exposure to Criteria Air Pollutants and Congenital Heart Defects in Offspring: Results from the National Birth Defects Prevention Study. Environ. Health Perspect. 2014, 122, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, E.M.; Smits, J.E.G.; St Clair, C.C. Evidence of low toxicity of oil sands process affected water to birds invites re-evaluation of avian protection strategies. Conserv. Physiol. 2015, 3, cov038. [Google Scholar] [CrossRef] [PubMed]
- Canada Public Health Agency. Congenital Anomalies in Canada: A Perinatal Health Surveillance Report; Canada Public Health Agency: Ottawa, ON, Canada, 2013.
- Mauderly, J.L.; Burnett, R.T.; Castillejos, M.; Ozkaynak, H.; Samet, J.M.; Stieb, D.M.; Vedal, S.; Wyzga, R.E. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal. Toxicol. 2010, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, S.M.; Desrosiers, T.A.; Lawson, C.; Lupo, P.J.; Riehle-Colarusso, T.J.; Stewart, P.A.; van Wijngaarden, E.; Waters, M.A.; Correa, A.; National Birth Defects Prevention Study. Association between maternal occupational exposure to organic solvents and congenital heart defects, National Birth Defects Prevention Study, 1997–2002. Occup. Environ. Med. 2012, 69, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianicolo, E.A.; Mangia, C.; Cervino, M.; Bruni, A.; Andreassi, M.G.; Latini, G. Congenital anomalies among live births in a high environmental risk area—A case-control study in Brindisi (southern Italy). Environ. Res. 2014, 128, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.B.; Montgomery, T.L.; Leavitt, J.M.; Colbaugh, W.C.; Thomas, F.W. Principal Plume Dispersion Models: TVA Power Plants. J. Air Pollut. Control Assoc. 1971, 21, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.W.; Carpenter, S.B.; Gartrell, F.E. Stacks-How High? J. Air Pollut. Control Assoc. 1963, 13, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R. Theory and Objectives of Air Dispersion Modelling. Available online: http://www.hazmatoklahoma.com/documents/Theory_and_Objectives_of_Air_Dispersion_Modelling.pdf (accessed on 28 June 2018).
- Lateb, M.; Meroney, R.N.; Yataghene, M.; Fellouah, H.; Saleh, F.; Boufadel, M.C. On the use of numerical modelling for near-field pollutant dispersion in urban environments—A review. Environ. Pollut. 2016, 208, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Korek, M.; Johansson, C.; Svensson, N.; Lind, T.; Beelen, R.; Hoek, G.; Pershagen, G.; Bellander, T. Can dispersion modeling of air pollution be improved by land-use regression? An example from Stockholm, Sweden. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.-Y.; Li, Z.; Tao, W.-Q. Buoyancy flows and pollutant dispersion through different scale urban areas: CFD simulations and wind-tunnel measurements. Build. Environ. 2016, 104, 76–91. [Google Scholar] [CrossRef]
- Hansen, J.M. Oxidative stress as a mechanism of teratogenesis. Birth Defects Res. (Part C) Embryo Today 2006, 78, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D.; Shinde, M.U.; Zhan, F.B.; Gong, X.; Langlois, P.H. Maternal residential proximity to chlorinated solvent emissions and birth defects in offspring: A case-control study. Environ. Health 2014, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Ronan, G.; Chevrier, C.; Cordier, S.; Celebi, C.; Cordier, S. Combined effect of prenatal solvent exposure and GSTT1 or GSTM1 polymorphisms in the risk of birth defects. Birth Defects Res. (Part A) Clin. Mol. Teratol. 2012, 94, 481–485. [Google Scholar]
- Bitto, A.; Pizzino, G.; Irrera, N.; Galfo, F.; Squadrito, F. Epigenetic Modifications Due to Heavy Metals Exposure in Children Living in Polluted Areas. Curr. Genom. 2014, 15, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Serra-Juhé, C.; Cuscó, I.; Homs, A.; Flores, R.; Torán, N.; Pérez-Jurado, L.A. DNA methylation abnormalities in congenital heart disease. Epigenetics 2015, 10, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenquist, T.H. Folate, homocysteine and the cardiac neural crest. Dev. Dyn. 2013, 242, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Erickson, S.W.; MacLeod, S.L.; Cleves, M.A.; Hu, P.; Karim, M.A.; Hobbs, C.A. Maternal Genome-Wide DNA Methylation Patterns and Congenital Heart Defects. PLoS ONE 2011, 6, e16506. [Google Scholar] [CrossRef] [PubMed]
- Anatomy of Canadian Postal Codes. Available online: http://aix1.uottawa.ca/~rroberge/postal%20codes.htm (accessed on 5 February 2018).
- Office of Environmental Health Hazard Assessment Proposition 65 California: State of California Environmental Protection Agency. 2014. Available online: http://www.oehha.org/prop65/prop65_list/Newlist.html (accessed on 5 February 2018).
- Legendre, P.; Legendre, L. Ordination in reduced space. In Numerical Ecology, 3rd ed.; Legendre, P., Legendre, L., Eds.; Elsevier: New York, NY, USA, 2012; pp. 425–520. [Google Scholar]
- Statistics Canada North American Industrial Classification System. Available online: http://www.statcan.gc.ca/subjects-sujets/standard-norme/naics-scian/2012/introduction-eng.htm (accessed on 5 February 2018).
- Alberta Health Services. Alberta Perinatal Health Program. Available online: http://aphp.dapasoft.com/Lists/HTMLPages/aboutUs.aspx (accessed on 5 February 2018).
- DMTI Spatial CanMap. 2013. Available online: https://www.dmtispatial.com/canmap/ (accessed on 5 February 2018).
- Chan, E.; Serrano, J.; Chen, L.; Stieb, D.M.; Jerrett, M.; Osornio-Vargas, A. Development of a Canadian socioeconomic status index for the study of health outcomes related to environmental pollution. BMC Public Health 2015, 15, 714. [Google Scholar] [CrossRef] [PubMed]
- Hystad, P.; Setton, E.; Cervantes, A.; Poplawski, K.; Deschenes, S.; Brauer, M.; van Donkelaar, A.; Lamsal, L.; Martin, R.; Jerrett, M.; et al. Creating National Air Pollution Models for Population Exposure Assessment in Canada. Environ. Health Perspect. 2011, 119, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colapinto, C.K.; O’Connor, D.L.; Tremblay, M.S. Folate status of the population in the Canadian Health Measures Survey. CMAJ 2011, 182, E100–E106. [Google Scholar] [CrossRef] [PubMed]
- Trines, J.; Fruitman, D.; Zuo, K.J.; Smallhorn, J.F.; Hornberger, L.K.; Mackie, A.S. Effectiveness of Prenatal Screening for Congenital Heart Disease: Assessment in a Jurisdiction with Universal Access to Health Care. Can. J. Cardiol. 2013, 29, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Siffel, C.; Correa, A. Residential mobility during pregnancy: Patterns and correlates. MCH 2010, 14, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Canfield, M.A.; Ramadhani, T.A.; Langlois, P.H.; Waller, D.K. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incardona, J.P.; Carls, M.G.; Holland, L.; Linbo, T.L.; Baldwin, D.H.; Myers, M.S.; Peck, K.A.; Tagal, M.; Rice, S.D.; Scholz, N.L. Very low embryonic crude oil exposures cause lasting cardiac defects in salmon and herring. Sci. Rep. 2015, 5, 13499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWitt, J.C.; Millsap, D.S.; Yeager, R.L.; Heise, S.S.; Sparks, D.W.; Henshel, D.S. External heart deformities in passerine birds exposed to environmental mixtures of polychlorinated biphenyls during development. Environ. Toxicol. Chem. 2006, 25, 541–551. [Google Scholar] [CrossRef] [PubMed]




| Industrial Emissions | Principal Components | ||
|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |
| Benzene | 0.98 | 0.11 | 0.01 |
| Carbon Disulfide | 0.95 | −0.09 | −0.04 |
| Carbon Monoxide | 0.95 | 0.21 | 0.12 |
| Sulphur Dioxide | 0.86 | −0.11 | 0.47 |
| Toluene | 0.86 | −0.04 | 0.04 |
| 1,3-Butadiene | 0.64 | 0.66 | 0.01 |
| Chloroform | 0.02 | 0.85 | 0.04 |
| Ethylene Oxide | 0.17 | 0.96 | 0.06 |
| Methanol | 0.03 | 0.86 | 0.06 |
| Methyl-isobutyl-ketone | 0.11 | 0.90 | 0.05 |
| Trichloroethylene | 0.11 | 0.79 | 0.05 |
| Arsenic | 0.16 | 0.11 | 0.95 |
| Cadmium | 0.36 | 0.06 | 0.60 |
| Hexachlorobenzene | −0.15 | −0.05 | 0.91 |
| Lead | 0.29 | 0.29 | 0.72 |
| Mercury | −0.52 | −0.08 | 0.97 |
| 2-Ethoxyethanol | −0.05 | −0.04 | −0.08 |
| N-Methyl-2-Pyrrolidone | −0.03 | 0.04 | 0.01 |
| Variable | Urban | Rural | * p Value |
|---|---|---|---|
| Total postal codes | 53,561 | 679 | |
| Number of postal codes with EF in a 10 km radius | 51,546 | 531 | |
| Number of postal codes without EF in a 10 km radius | 2015 | 148 | |
| Count of EF per Postal Code (Min) | 1 | 1 | |
| Count of EF per Postal Code (Max) | 252 | 183 | |
| Median number of EF impacting each postal code (IQR) | 60 (84) | 10 (15) | |
| Sum total IDW emissions in tonnes | 170,497 | 1632 | |
| Sum Group 1 IDW emissions in tonnes | 168,434 | 1608 | |
| Sum Group 2 IDW emissions in kg | 205,089,526 | 23,689 | |
| Sum Group 3 IDW emissions in kg | 9043 | 21 | |
| Median total emissions in tonnes (IQR) | 0.6 (2.4) | 0.07 (0.3) | <0.001 |
| Median Group 1 emission in tonnes (IQR) | 0.6 (2.3) | 0.07 (0.3) | <0.001 |
| Median Group 2 emissions in kg (IQR) | 6 (39) | 0.0000 (0.00) † | <0.001 |
| Median Group 3 emissions in kg (IQR) | 2.2 × 10−9 (1.5 × 10−7) | 0.0000 (0.00) † | <0.001 |
| Total CHD counts n = 2413 (%) | 1967 (81.5) | 446 (18.4) | |
| Poisson mean of CHD counts (95% CI) | 0.04 (0.04, 0.04) | 0.66 (0.59, 0.72) |
| Region | Exposure Category | Total Postal Codes Count | Postal Codes with CHD (%) | Number of CHD Cases | Min Cases | Max Cases | Min Exposure (tonnes) | Max Exposure (tonnes) |
|---|---|---|---|---|---|---|---|---|
| Urban | Group 1 in 10th Decile | 5410 | 293 (5) | 317 | 1 | 4 | 10 | 116 |
| Group 2 in 3rd Tertile | 18,062 | 730 (4) | 786 | 1 | 4 | 23 | 2461 ‡ | |
| Group 3 in 3rd Tertile | 18,068 | 741 (4) | 799 | 1 | 4 | 0.091 | 3.9 ‡ | |
| Rural | Group 1 in 10th Decile | 14 | 7 (50) | 20 | 1 | 9 | 11 | 500 |
| Group 2 in 3rd Tertile | 18 | 6(33) | 22 | 1 | 9 | 38 | 17,340 ‡ | |
| Group 3 in 3rd Tertile | 12 | 6 (50) | 22 | 1 | 9 | 0.1 | 12 ‡ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwezi, D.P.; Hornberger, L.K.; Serrano-Lomelin, J.; Nielsen, C.C.; Fruitman, D.; Osornio-Vargas, A. Industrial Developmental Toxicants and Congenital Heart Disease in Urban and Rural Alberta, Canada. Challenges 2018, 9, 26. https://doi.org/10.3390/challe9020026
Ngwezi DP, Hornberger LK, Serrano-Lomelin J, Nielsen CC, Fruitman D, Osornio-Vargas A. Industrial Developmental Toxicants and Congenital Heart Disease in Urban and Rural Alberta, Canada. Challenges. 2018; 9(2):26. https://doi.org/10.3390/challe9020026
Chicago/Turabian StyleNgwezi, Deliwe P., Lisa K. Hornberger, Jesus Serrano-Lomelin, Charlene C. Nielsen, Deborah Fruitman, and Alvaro Osornio-Vargas. 2018. "Industrial Developmental Toxicants and Congenital Heart Disease in Urban and Rural Alberta, Canada" Challenges 9, no. 2: 26. https://doi.org/10.3390/challe9020026





