Negative Correlation between Serum Cytokine Levels and Cognitive Abilities in Children with Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment of Cognitive Functioning
2.3. Cytokine Measurements
2.4. Statistical Analysis
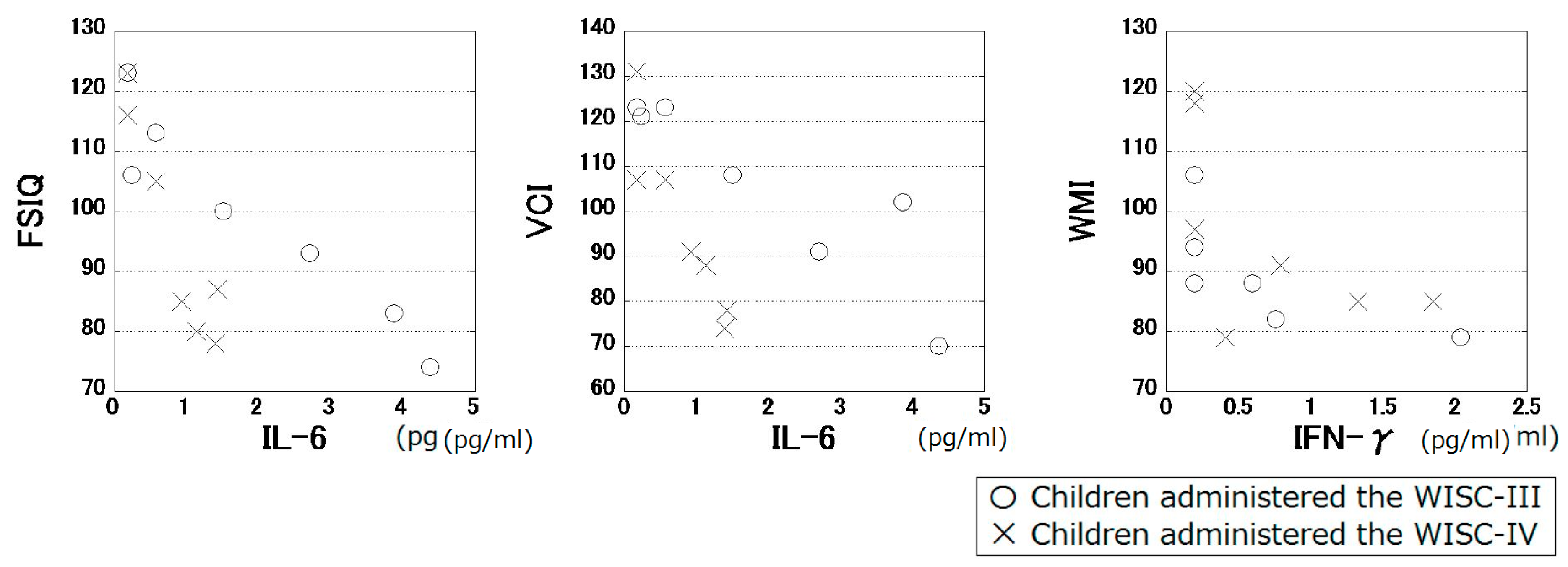
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gilmore, J.H.; Fredrik Jarskog, L.; Vadlamudi, S.; Lauder, J.M. Prenatal infection and risk for schizophrenia: IL-1β, IL-6, and TNFα inhibit cortical neuron dendrite development. Neuropsychopharmacology 2004, 29, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Mehler, M.F.; Kessler, J.A. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997, 20, 357–365. [Google Scholar] [CrossRef]
- Marsland, A.L.; Petersen, K.L.; Sathanoori, R.; Muldoon, M.F.; Neumann, S.A.; Ryan, C.; Flory, J.D.; Manuck, S.B. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom. Med. 2006, 68, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Lekander, M.; von Essen, J.; Schultzberg, M.; Andreasson, A.N.; Garlind, A.; Hansson, L.O.; Nilsson, L.G. Cytokines and memory across the mature life span of women. Scand. J. Psychol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Guthikonda, P. Circulating cytokines in alzheimer’s disease. J. Psychiatr. Res. 1997, 31, 657–660. [Google Scholar] [CrossRef]
- Sasayama, D.; Hori, H.; Teraishi, T.; Hattori, K.; Ota, M.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Higuchi, T.; Amano, N.; et al. Association of interleukin-1β genetic polymorphisms with cognitive performance in elderly females without dementia. J. Hum. Genet. 2011, 56, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, D.; Hori, H.; Teraishi, T.; Hattori, K.; Ota, M.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Amano, N.; Kunugi, H. Association of cognitive performance with interleukin-6 receptor Asp358Ala polymorphism in healthy adults. J. Neural Transm. 2011, 119, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Sacerdote, P.; Panerai, A.E.; Bianchi, M.; Aloisi, A.M.; Iosue, S.; Sala, M. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav. Brain Res. 2004, 153, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Heyser, C.J.; Masliah, E.; Samimi, A.; Campbell, I.L.; Gold, L.H. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA 1997, 94, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, D.; Hattori, K.; Wakabayashi, C.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013, 47, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, D.; Wakabayashi, C.; Hori, H.; Teraishi, T.; Hattori, K.; Ota, M.; Ishikawa, M.; Arima, K.; Higuchi, T.; Amano, N.; et al. Association of plasma IL-6 and soluble IL-6 receptor levels with the Asp358Ala polymorphism of the IL-6 receptor gene in schizophrenic patients. J. Psychiatr. Res. 2011, 45, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J. Neuroimmunol. 1996, 66, 143–145. [Google Scholar] [CrossRef]
- Aureli, A.; Sebastiani, P.; Del Beato, T.; Marimpietri, A.E.; Graziani, A.; Sechi, E.; Di Loreto, S. Involvement of IL-6 and IL-1 receptor antagonist on intellectual disability. Immunol. Lett. 2014, 162, 124–131. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Joseph, R.M.; Kuban, K.C.; Allred, E.N.; Ware, J.; Coster, T.; Fichorova, R.N.; Dammann, O.; Leviton, A. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 months in extremely preterm infants. Pediatr. Res. 2014, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Shimizu, Y.; Rutter, M. No effect of MMR withdrawal on the incidence of autism: A total population study. J. Child. Psychol. Psychiatry 2005, 46, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Schmidt, M.J.; Mirnics, K. Immune system gene dysregulation in autism and schizophrenia. Dev. Neurobiol. 2012, 72, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Guest, P.C.; Rahmoune, H.; Wang, L.; Levin, Y.; Ingudomnukul, E.; Ruta, L.; Kent, L.; Spain, M.; Baron-Cohen, S.; et al. Sex-specific serum biomarker patterns in adults with asperger’s syndrome. Mol. Psychiatry 2011, 16, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Munson, J.; Dawson, G.; Sterling, L.; Beauchaine, T.; Zhou, A.; Elizabeth, K.; Lord, C.; Rogers, S.; Sigman, M.; Estes, A.; et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am. J. Ment. Retard. 2008, 113, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Black, D.O.; Wallace, G.L.; Sokoloff, J.L.; Kenworthy, L. Brief report: IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. J. Autism. Dev. Disord. 2009, 39, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Japanese WISC-III Publication Committee. Japanese Wechsler Intelligence Scale for Children, 3rd ed.; Nihon Bunka Kagakusha: Tokyo, Japan, 1998. [Google Scholar]
- Japanese WISC-IV Publication Committee. Japanese Wechsler Intelligent Scale for Children, 4th ed.; Nihon Bunka Kagakusha: Tokyo, Japan, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Van Damme, J.; Opdenakker, G.; Simpson, R.J.; Rubira, M.R.; Cayphas, S.; Vink, A.; Billiau, A.; Van Snick, J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J. Exp. Med. 1987, 165, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Ponath, G.; Rothermundt, M.; Riess, O.; Funke, H.; Berger, K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology 2008, 33, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Hong, C.J.; Liu, M.E.; Hou, S.J.; Yen, F.C.; Hsieh, C.H.; Liou, Y.J. Interleukin-1 beta (C-511T) genetic polymorphism is associated with cognitive performance in elderly males without dementia. Neurobiol. Aging 2010, 31, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Crimmins, E.M.; Karlamangla, A.; Hu, P.; Seeman, T.E. Inflammation and rate of cognitive change in high-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewski, J.M.; Song, Y.; Fung, C.H.; Rodriguez, J.C.; Jouldjian, S.; Alessi, C.A.; Breen, E.C.; Irwin, M.R.; Martin, J.L. Self-reported sleep duration mitigates the association between inflammation and cognitive functioning in hospitalized older men. Front. Psychol. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.D.; Hong, S.; Crescini, M.; Ellis, R.; Perez-Santiago, J.; Spina, C.; Letendre, S. Cerebrospinal fluid (CSF) CD8+ t-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS ONE 2015, 10, e0116526. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Croen, L.A.; Yoshida, C.K.; Heuer, L.; Hansen, R.; Zerbo, O.; DeLorenze, G.N.; Kharrazi, M.; Yolken, R.; Ashwood, P.; et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatry 2017, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrenstein, O.S.; Neta, G.I.; Andrews, W.; Goldenberg, R.; Goepfert, A.; Zhang, J. Child intellectual development in relation to cytokine levels in umbilical cord blood. Am. J. Epidemiol. 2012, 175, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Vaz, G.N.; Saito, V.M.; Teixeira, A.L. Further evidence for the role of interferon-gamma on anxiety- and depressive-like behaviors: Involvement of hippocampal neurogenesis and NGF production. Neurosci. Lett. 2014, 578, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.A.; Sudol, K.L.; Narrow, W.C.; Bowers, W.J. Interferon-γ differentially affects alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009, 175, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Subjects Administered the WISC-III | Subjects Administered the WISC-IV | Total |
|---|---|---|---|
| N (boys/girls) | 7 (5/2) | 7 (4/3) | 14 (9/5) |
| Age (years) | 11.7 ± 1.7 | 11.6 ± 2.6 | 11.6 ± 2.1 |
| Body Weight (kg) | 45.6 ± 8.8 | 54.4 ± 26.2 | 50.0 ± 19.3 |
| Medication status | |||
| On any mediation | 4 | 3 | 7 |
| Stimulant | 2 | 2 | 4 |
| Atomoxetine | 1 | 0 | 1 |
| Guanfacine | 0 | 1 | 1 |
| Atypical antipsychotics | 1 | 0 | 1 |
| WISC scores (standard scores) | |||
| FSIQ | 98.9 ± 17.0 | 96.3 ± 18.2 | 97.6 ± 17.0 |
| VCI | 105.4 ± 19.8 | 96.6 ± 19.8 | 101.0 ± 19.6 |
| POI (WISC-III) or PRI (WISC-IV) | 96.7 ± 13.5 | 96.0 ± 20.1 | 96.4 ± 16.4 |
| FDI (WISC-III) or WMI (WISC-IV) | 89.3 ± 8.8 | 96.4 ± 16.4 | 92.9 ± 13.2 |
| PSI | 94.6 ± 19.8 | 96.9 ± 13.3 | 95.7 ± 16.2 |
| Time of blood collection | 10.34 h ± 158 min | 13.11 h ± 169 min | 11.53 h ± 177 min |
| IFN-γ (pg/mL) | 0.6 ± 0.7 | 0.7 ± 0.7 | 0.7 ± 0.6 |
| IL-1β (pg/mL) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.3 |
| IL-2 (pg/mL) | 5.4 ± 8.7 | 2.0 ± 1.7 | 3.7 ± 6.3 |
| IL-4 (pg/mL) | 2.2 ± 2.8 | 2.1 ± 1.7 | 2.1 ± 2.2 |
| IL-6 (pg/mL) | 1.9 ± 1.7 | 0.8 ± 0.5 | 1.4 ± 1.4 |
| IL-8 (pg/mL) | 10.2 ± 10.3 | 7.8 ± 6.4 | 9.0 ± 8.3 |
| IL-10 (pg/mL) | 1.4 ± 0.9 | 1.8 ± 1.5 | 1.6 ± 1.2 |
| TNF-α (pg/mL) | 0.6 ± 0.6 | 1.1 ± 0.8 | 0.8 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasayama, D.; Kurahashi, K.; Oda, K.; Yasaki, T.; Yamada, Y.; Sugiyama, N.; Inaba, Y.; Harada, Y.; Washizuka, S.; Honda, H. Negative Correlation between Serum Cytokine Levels and Cognitive Abilities in Children with Autism Spectrum Disorder. J. Intell. 2017, 5, 19. https://doi.org/10.3390/jintelligence5020019
Sasayama D, Kurahashi K, Oda K, Yasaki T, Yamada Y, Sugiyama N, Inaba Y, Harada Y, Washizuka S, Honda H. Negative Correlation between Serum Cytokine Levels and Cognitive Abilities in Children with Autism Spectrum Disorder. Journal of Intelligence. 2017; 5(2):19. https://doi.org/10.3390/jintelligence5020019
Chicago/Turabian StyleSasayama, Daimei, Kana Kurahashi, Kayoko Oda, Takehiko Yasaki, Yoshiyuki Yamada, Nobuhiro Sugiyama, Yuji Inaba, Yuzuru Harada, Shinsuke Washizuka, and Hideo Honda. 2017. "Negative Correlation between Serum Cytokine Levels and Cognitive Abilities in Children with Autism Spectrum Disorder" Journal of Intelligence 5, no. 2: 19. https://doi.org/10.3390/jintelligence5020019






