Properties and Applications of PDMS for Biomedical Engineering: A Review
Abstract
:1. Introduction
2. PDMS Properties
3. PDMS Manufacturing Process
4. Methods to Characterize PDMS
- Gravimetry is a method based on gravitational techniques to quantify changes in PDMS sample weight. For example, this method is useful when it is needed to verify if there was or not degradation of the PDMS after chemical immersion [93];
- In order to obtain information on surface hydrophilicity, a goniometry test is performed. Micro water droplets are dropped on the PDMS surface and then the contact angle is measured. This technique allows for verification of if there was or not a change in the wettability of the PDMS after certain treatments [19,39,42,94];
- Nanoindentation offers the possibility of studying mechanical properties of the outermost layer of PDMS, which is susceptible to destruction due to different treatments, such as UV irradiation [95];
- X-ray photoelectron spectroscopy (XPS) is a technique based on the photoelectric effect, which allows identification of the elemental composition of the material. This method is useful when it is needed to verify if any changes in surface composition occurred after the PDMS received any treatment [38,39,98];
5. PDMS Microfabrication
- Microcontact printing: uses the relief pattern on the surface of a PDMS stamp to form patterns of self-assembled monolayers (SAMs) on the surfaces of substrates by contact;
- Replica moulding: replicates the relief pattern on the surface of a PDMS mould by using this structure as a mould for forming structures in a second UV-curable (or thermally curable) prepolymer;
- Micro-transfer moulding: a thin layer of liquid prepolymer is applied to the patterned surface of a PDMS mould. It is then placed in contact with the surface of a substrate and the liquid prepolymer is cured to a solid. After peeling off the mould, a patterned micro-structure is left on the surface of the substrate;
- Micro-moulding in capillaries: a PDMS mould is placed on the surface of a substrate to form a network of empty channels between them. The channels are filled with a low viscosity prepolymer, which is then cured to a solid. The mould is removed and a patterned micro-structure is left on the surface of the substrate;
- Solvent-assisted micro-moulding: a PDMS mould is wetted with a solvent, and it is placed in contact with a substrate (typically an organic polymer). The solvent starts to dissolve the substrate into a fluid or gel that is moulded against the relief structures in the mould. When the fluid solidifies, it forms a pattern relief structure complementary to that in the surface of the mould.
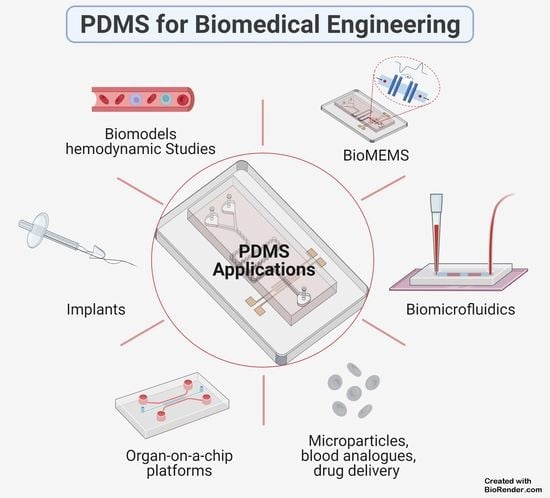
6. PDMS Applications
6.1. PDMS-Based Microchip
6.2. PDMS Biomodels for Hemodynamic Studies
6.3. PDMS-Based Blood Analogues
6.4. PDMS-Based Coatings for Medical Implants
7. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poll, M.; Zhou, F.; Ramstedt, M.; Hu, L.; Huck, W. A Self-assembly approach to chemical micropatterning of Poly(dimethylsiloxane). Angew. Chem. Int. Ed. 2007, 46, 6634–6637. [Google Scholar] [CrossRef] [PubMed]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in Poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Kuddannaya, S.; Bao, J.; Zhang, Y. Enhanced In Vitro biocompatibility of chemically modified Poly(dimethylsiloxane) surfaces for stable adhesion and long-term investigation of brain cerebral cortex cells. ACS Appl. Mater. Interfaces 2015, 7, 25529–25538. [Google Scholar] [CrossRef]
- Lee, S.; Shin, H.-J.; Yoon, S.-M.; Yi, D.K.; Choi, J.-Y.; Paik, U. Refractive index engineering of transparent ZrO2–polydimethylsiloxane nanocomposites. J. Mater. Chem. 2008, 18, 1751–1755. [Google Scholar] [CrossRef]
- Johnston, I.D.; Tracey, M.C.; Davis, J.B.; Tan, C.K.L. Micro throttle pump employing displacement amplification in an elastomeric substrate. J. Micromech. Microeng. 2005, 15, 1831–1839. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.A.; Santos, C.; Monge, N.; Santos, N.C.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Using plasma-mediated covalent functionalization of rhamnolipids on polydimethylsiloxane towards the antimicrobial improvement of catheter surfaces. Mater. Sci. Eng. C 2021, 112563. [Google Scholar] [CrossRef]
- Kumar, R.; Sahani, A. Role of superhydrophobic coatings in biomedical applications. Mater. Today 2021, 45, 5655–5659. [Google Scholar] [CrossRef]
- Wu, X.; Kim, S.-H.; Ji, C.-H.; Allen, M. A solid hydraulically amplified piezoelectric microvalve. J. Micromech. Microeng. 2011, 21, 95003–95011. [Google Scholar] [CrossRef] [Green Version]
- Bozukova, D.; Pagnoulle, C.; Jérôme, R.; Jérôme, C. Polymers in modern ophthalmic implants—Historical background and recent advances. Mater. Sci. Eng. R Rep. 2010, 69, 63–83. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Sinha, S.K.; Chau, F.S.; Wang, S. Lens integrated with self-aligned variable aperture using pneumatic actuation method. Sens. Actuators A Phys. 2010, 159, 105–110. [Google Scholar] [CrossRef]
- Doutel, E.; Viriato, N.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Geometrical effects in the hemodynamics of stenotic and non-stenotic left coronary arteries-numerical and in vitro approaches. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3207. [Google Scholar] [CrossRef] [PubMed]
- Usmani, A.; Muralidhar, K. Flow in an intracranial aneurysm model: Effect of parent artery orientation. J. Vis. 2018, 21, 795–818. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, D.-S.; Kim, I.-G.; Sohn, D.-W.; Park, J.-Y.; Choi, B.-K.; Kim, S.-W. Evaluation of the biocompatibility of a coating material for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Carta, R.; Jourand, P.; Hermans, B.; Thoné, J.; Brosteaux, D.; Vervust, T.; Bossuyt, F.; Axisa, F.; Vanfleteren, J.; Puers, R. Design and implementation of advanced systems in a flexible-stretchable technology for biomedical applications. Sens. Actuators A Phys. 2009, 156, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Chen, W.; Lam, R.H.W.; Fu, J. Photolithographic surface micromachining of polydimethylsiloxane (PDMS). Lab Chip 2012, 12, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Weibel, D.B.; DiLuzio, W.R.; Whitesides, G.M. Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007, 5, 209–218. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) based microfluidic devices for biomedical applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Schneider, F.; Fellner, T.; Wilde, J.; Wallrabe, U. Mechanical properties of silicones for MEMS. J. Micromech. Microeng. 2008, 18, 065008. [Google Scholar] [CrossRef]
- Bubendorfer, A.; Liu, X.; Ellis, A.V. Microfabrication of PDMS microchannels using SU-8/PMMA moldings and their sealing to polystyrene substrates. Smart Mater. Struct. 2007, 16, 367–371. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 Processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Jo, M.C.; Guldiken, R. Effects of polydimethylsiloxane (PDMS) microchannels on surface acoustic wave-based microfluidic devices. Microelectron. Eng. 2014, 113, 98–104. [Google Scholar] [CrossRef]
- Levitt, M.R.; Mandrycky, C.; Abel, A.; Kelly, C.M.; Levy, S.; Chivukula, V.K.; Zheng, Y.; Aliseda, A.; Kim, L.J. Genetic correlates of wall shear stress in a patient-specific 3D-printed cerebral aneurysm model. J. Neurointerv. Surg. 2019, 11, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Doutel, E.; Carneiro, J.; Oliveira, M.; Campos, J.B.L.M.; Miranda, J. Fabrication of 3d mili-scale channels for hemodynamic studies. J. Mech. Med. Biol. 2014, 5, 21. [Google Scholar] [CrossRef]
- Doutel, E.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Experimental and numerical methodology to analyze flows in a coronary bifurcation. Eur. J. Mech. B Fluids 2018, 67, 341–356. [Google Scholar] [CrossRef]
- Geoghegan, P.H.; Buchmann, N.A.; Spence, C.J.T.; Moore, S.; Jermy, M. Fabrication of rigid and flexible refractive-index-matched flow phantoms for flow visualisation and optical flow measurements. Exp. Fluids 2012, 52, 1331–1347. [Google Scholar] [CrossRef]
- Ford, M.D.; Nikolov, H.N.; Milner, J.S.; Lownie, S.P.; Demont, E.M.; Kalata, W.; Loth, F.; Holdsworth, D.W.; Steinman, D.A. PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models. J. Biomech. Eng. 2008, 130, 21015. [Google Scholar] [CrossRef] [Green Version]
- Brindise, M.C.; Rothenberger, S.; Dickerhoff, B.; Schnell, S.; Markl, M.; Saloner, D.; Rayz, V.L.; Vlachos, P.P. Multi-modality cerebral aneurysm haemodynamic analysis: In vivo 4D flow MRI, in vitro volumetric particle velocimetry and in silico computational fluid dynamics. J. R. Soc. Interface 2019, 16, 20190465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amili, O.; Golzarian, J.; Coletti, F. In Vitro Study of particle transport in successively bifurcating vessels. Ann. Biomed. Eng. 2019, 47, 2271–2283. [Google Scholar] [CrossRef]
- Li, Y.; Verrelli, D.I.; Yang, W.; Qian, Y.; Chong, W. A pilot validation of CFD model results against PIV observations of haemodynamics in intracranial aneurysms treated with flow-diverting stents. J. Biomech. 2020, 100, 109590. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, V.K.; Levitt, M.R.; Clark, A.; Barbour, M.C.; Sansom, K.; Johnson, L.; Kelly, C.M.; Geindreau, C.; Rolland du Roscoat, S.; Kim, L.J.; et al. Reconstructing patient-specific cerebral aneurysm vasculature for in vitro investigations and treatment efficacy assessments. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 61, 153–159. [Google Scholar] [CrossRef]
- Paliwal, N.; Damiano, R.J.; Varble, N.A.; Tutino, V.M.; Dou, Z.; Siddiqui, A.H.; Meng, H. Methodology for Computational Fluid Dynamic Validation for Medical Use: Application to Intracranial Aneurysm. J. Biomech. Eng. 2017, 139, 1210041–12100410. [Google Scholar] [CrossRef]
- Rossi de Aguiar, K.M.F.; Nascimento, M.V.; Faccioni, J.L.; Noeske, P.L.M.; Gätjen, L.; Rischka, K.; Rodrigues-Filho, U.P. Urethanes PDMS-based: Functional hybrid coatings for metallic dental implants. Appl. Surf. Sci. 2019, 484, 1128–1140. [Google Scholar] [CrossRef]
- Tran, P.A.; Fox, K.; Tran, N. Novel hierarchical tantalum oxide-PDMS hybrid coating for medical implants: One pot synthesis, characterization and modulation of fibroblast proliferation. J. Colloid Interface Sci. 2017, 485, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hijón, N.; Manzano, M.; Salinas, A.; Vallet-Regí, M. Bioactive CaO-SiO2-PDMS coatings on Ti6Al4V substrates. Chem. Mater. 2005, 17, 1591–1596. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, S.J.; Sohn, J.H.; Kim, I.G.; Kim, S.W.; Sohn, D.W.; Kim, J.H.; Choi, B. Biocompatibility of a pdms-coated micro-device: Bladder volume monitoring sensor. Chin. J. Polym. Sci. 2012, 30, 242–249. [Google Scholar] [CrossRef]
- Tavakoli, S.; Nemati, S.; Kharaziha, M.; Akbari-Alavijeh, S. Embedding CuO nanoparticles in PDMS-SiO2 coating to improve antibacterial characteristic and corrosion resistance. Colloids Interface Sci. Commun. 2019, 28, 20–28. [Google Scholar] [CrossRef]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef]
- Guo, R.; Liu, J. Implantable liquid metal-based flexible neural microelectrode array and its application in recovering animal locomotion functions. J. Micromech. Microeng. 2017, 27, 104002. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Zhao, J.; Sheadel, D.A.; Xue, W. Surface treatment of polymers for the fabrication of all-polymer MEMS devices. Sens. Actuators A Phys. 2012, 187, 43–49. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Victor, A.; Ribeiro, J.; Araújo, F.F. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Cardoso, C.; Fernandes, C.S.; Lima, R.; Ribeiro, J. Biomechanical analysis of PDMS channels using different hyperelastic numerical constitutive models. Mech. Res. Commun. 2018, 90, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.T.; Chen, Y.C.; Lin, P.-H.; Hsieh, C.C.; Hsu, F.T.; Lin, P.-H.; Chang, C.M.; Hsu, J.H.; Huang, J.C. Lens of controllable optical field with thin film metallic glasses for UV-LEDs. Opt. Express 2014, 22, 14411. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Zhang, B.; Han, Y.; Shen, C.; Lin, Q.; Chen, H. Development of antibacterial and high light transmittance bulk materials: Incorporation and sustained release of hydrophobic or hydrophilic antibiotics. Colloids Surf. B Biointerfaces 2016, 141, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.T.; Chen, Y.C.; Chen, Y.J.; Wang, W.C.; Yang, H.C.; Wu, H.C. Compound optical film using gray scale mask embedded with microvoids. Adv. Condens. Matter Phys. 2012, 2012, 942018. [Google Scholar] [CrossRef] [Green Version]
- Riehle, N.; Thude, S.; Götz, T.; Kandelbauer, A.; Thanos, S.; Tovar, G.; Lorenz, G. Influence of PDMS molecular weight on transparency and mechanical properties of soft polysiloxane-urea-elastomers for intraocular lens application. Eur. Polym. J. 2018, 101, 190–201. [Google Scholar] [CrossRef]
- Sales, F.; Souza, A.; Ariati, R.; Noronha, V.; Giovanetti, E.; Lima, R.; Ribeiro, J. Composite material of PDMS with interchangeable transmittance: Study of optical, mechanical properties and wettability. J. Compos. Sci. 2021, 5, 110. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- The Dow CompanyChemical. SYLGARDTM 184 Silicone Elastomer Technical Datasheet. Silicone Elastomer Technical Data Sheet 2017. Available online: https://consumer.dow.com/en-us/document-viewer.html?ramdomVar=3835418757322904567&docPath=/documents/en-us/productdatasheet/11/11-31/11-3184-sylgard-184-elastomer.pdf (accessed on 20 August 2021).
- Hong, J.; Lee, J.; Hong, C.; Shim, S. Effect of dispersion state of carbon nanotube on the thermal conductivity of poly(dimethyl siloxane) composites. Curr. Appl. Phys. 2010, 10, 359–363. [Google Scholar] [CrossRef]
- Armani, D.; Liu, C.; Aluru, N. Re-Configurable Fluid Circuits by PDMS Elastomer Micromachining. In Proceedings of the Technical Digest IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.99CH36291), Orlando, FL, USA, 21 January 1999; pp. 222–227. [Google Scholar]
- Müller, A.; Wapler, M.C.; Wallrabe, U. A quick and accurate method to determine the Poisson’s ratio and the coefficient of thermal expansion of PDMS. Soft Matter 2019, 15, 779–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Sun, Y.; Qian, B.; Gao, H.; Zuo, D. Experimental study on mechanical performance of polydimethylsiloxane (PDMS) at various temperatures. Polym. Test. 2020, 90, 106670. [Google Scholar] [CrossRef]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GRIFFITHS, E. international critical tables of numerical data, physics, chemistry and technology. Nature 1927, 119, 735–738. [Google Scholar] [CrossRef]
- Wu, M.H.; Urban, J.P.G.; Cui, Z.; Cui, Z.F. Development of PDMS microbioreactor with well-defined and homogenous culture environment for chondrocyte 3-D culture. Biomed. Microdevices 2006, 8, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Nguyen, N.T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 032204. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Kakinoki, S.; Iwasaki, Y. Long-lasting hydrophilic surface generated on poly(dimethyl siloxane) with photoreactive zwitterionic polymers. Colloids Surf. B Biointerfaces 2021, 205, 111900. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment-An SEM investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Makamba, H.; Kim, J.H.; Lim, K.; Park, N.; Hahn, J.H. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, S.; Cauich-Rodríguez, J.V.; Kreutzer, J.; Kallio, P. Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces. Appl. Surf. Sci. 2012, 258, 9864–9875. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of pdms for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Yang, Y.; Kulangara, K.; Lam, R.T.S.; Dharmawan, R.; Leong, K.W. Effects of Topographical and mechanical property alterations induced by oxygen plasma modification on stem cell behavior. ACS Nano 2012, 6, 8591–8598. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, Y.; Khandurina, J.; Guttman, A.; Lo, Y.-H. UV/ozone modification of poly(dimethylsiloxane) microfluidic channels. Sens. Actuators B Chem. 2004, 97, 402–408. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer 1998, 39, 1991–1998. [Google Scholar] [CrossRef]
- Makamba, H.; Hsieh, Y.Y.; Sung, W.C.; Chen, S.H. Stable permanently hydrophilic protein-resistant thin-film coatings on poly(dimethylsiloxane) substrates by electrostatic self-assembly and chemical cross-linking. Anal. Chem. 2005, 77, 3971–3978. [Google Scholar] [CrossRef]
- Boxshall, K.; Wu, M.-H.; Cui, Z.; Cui, Z.; Watts, J.F.; Baker, M.A. Simple surface treatments to modify protein adsorption and cell attachment properties within a poly(dimethylsiloxane) micro-bioreactor. Surf. Interface Anal. 2006, 38, 198–201. [Google Scholar] [CrossRef]
- Blättler, T.M.; Pasche, S.; Textor, M.; Griesser, H.J. High salt stability and protein resistance of poly(L-lysine)-g-poly(ethylene glycol) copolymers covalently immobilized via aldehyde plasma polymer interlayers on inorganic and polymeric substrates. Langmuir ACS J. Surf. Colloids 2006, 22, 5760–5769. [Google Scholar] [CrossRef]
- Xu, J.; Gleason, K.K. Conformal, amine-functionalized thin films by initiated chemical vapor deposition (iCVD) for hydrolytically stable microfluidic devices. Chem. Mater. 2010, 22, 1732–1738. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, X.; Xu, F.; Liu, X.; Liu, B.F. “Click” chemistry-based surface modification of poly(dimethylsiloxane) for protein separation in a microfluidic chip. Electrophoresis 2010, 31, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Surface-directed, graft polymerization within microfluidic channels. Anal. Chem. 2004, 76, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane composites characterization and its applications: A review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Gökaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 97377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.; Souza, M.S.; Pinho, D.; Agujetas, R.; Ferrera, C.; Lima, R.; Puga, H.; Ribeiro, J. 3D manufacturing of intracranial aneurysm biomodels for flow visualizations: Low cost fabrication processes. Mech. Res. Commun. 2020, 107, 103535. [Google Scholar] [CrossRef]
- Santiago-Alvarado, A.; Cruz-Félix, A.S.; González-García, J.; Sánchez-López, O.; Mendoza-Jasso, A.J.; Hernández-Castillo, I. Polynomial fitting techniques applied to opto-mechanical properties of PDMS Sylgard 184 for given curing parameters. Mater. Res. Express 2020, 7, 45301. [Google Scholar] [CrossRef]
- Khanafer, K.; Duprey, A.; Schlicht, M.; Berguer, R. Effects of strain rate, mixing ratio, and stress–strain definition on the mechanical behavior of the polydimethylsiloxane (PDMS) material as related to its biological applications. Biomed. Microdevices 2008, 11, 503. [Google Scholar] [CrossRef]
- Prajzler, V.; Nekvindova, P.; Spirkova, J.; Novotny, M. The evaluation of the refractive indices of bulk and thick polydimethylsiloxane and polydimethyl-diphenylsiloxane elastomers by the prism coupling technique. J. Mater. Sci. Mater. Electron. 2017, 28, 7951–7961. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Choi, J.S.; Piao, Y.; Seo, T.S. Fabrication of a circular PDMS microchannel for constructing a three-dimensional endothelial cell layer. Bioprocess. Biosyst. Eng. 2013, 36, 1871–1878. [Google Scholar] [CrossRef]
- Yoo, B.Y.; Kim, B.H.; Lee, J.S.; Shin, B.H.; Kwon, H.; Koh, W.G.; Heo, C.Y. Dual surface modification of PDMS-based silicone implants to suppress capsular contracture. Acta Biomater. 2018, 76, 56–70. [Google Scholar] [CrossRef]
- McMullan, D. Scanning electron microscopy 1928–1965. Scanning 1995, 17, 175–185. [Google Scholar] [CrossRef]
- Wong, B.; Zhang, Z.; Handa, Y.P. High-precision gravimetric technique for determining the solubility and diffusivity of gases in polymers. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, J.; Clark, C.; Deakes, J.; Burdon, D.; Lawrence, C. Clinical methods of goniometry: A comparative study. Disabil. Rehabil. 1992, 14, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.A. Nanoindentation studies of materials. Mater. Today 2006, 9, 32–40. [Google Scholar] [CrossRef]
- Chong, H.; Lou, J.; Bogie, K.M.; Zorman, C.A.; Majerus, S.J.A. Vascular pressure-flow measurement using CB-PDMS flexible strain sensor. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.C.; Arruda, E.M. An experimental and anaiytical investigation of the large strain compressive and tensile response of glassy polymers. Polym. Eng. Sci. 1990, 30, 1288–1298. [Google Scholar] [CrossRef]
- Fadley, C.S. X-ray photoelectron spectroscopy: Progress and perspectives. J. Electron. Spectrosc. Relat. Phenom. 2010, 178–179, 2–32. [Google Scholar] [CrossRef]
- Koenig, J.L. Fourier Transform Infrared Spectroscopy of Polymers BT—Spectroscopy: NMR, Fluorescence, FT-IR; Springer: Berlin/Heidelberg, Germany, 1984; pp. 87–154. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood cells separation and sorting techniques of passive microfluidic devices: From fabrication to applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.G.; Rehg, T.J. Spin Coating BT. In Liquid Film Coating: Scientific Principles and Their Technological Implications; Kistler, S.F., Schweizer, P.M., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 709–734. [Google Scholar]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface modification techniques for endothelial cell seeding in PDMS microfluidic devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Morra, M.; Occhiello, E.; Marola, R.; Garbassi, F.; Humphrey, P.; Johnson, D. On the aging of oxygen plasma-treated polydimethylsiloxane surfaces. J. Colloid Interface Sci. 1990, 137, 11–24. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, P.; Zhou, Q. Adhesion promotion between PDMS and glass by oxygen plasma pre-treatment. J. Adhes. Sci. Technol. 2014, 28, 1046–1054. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Shin, S.; Kim, N.; Hong, J.W. Comparison of surface modification techniques on polydimethylsiloxane to prevent protein adsorption. BioChip J. 2018, 12, 123–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, J.; Ge, Y.; Zhang, X.; Shi, H.; Yang, K.; Gao, X.; Shi, S.; Gong, Y. Fabrication of stable biomimetic coating on PDMS surface: Cooperativity of multivalent interactions. Appl. Surf. Sci. 2019, 469, 720–730. [Google Scholar] [CrossRef]
- Jinia, A.J.; Sunbul, N.B.; Meert, C.A.; Miller, C.A.; Clarke, S.D.; Kearfott, K.J.; Matuszak, M.M.; Pozzi, S.A. Review of sterilization techniques for medical and personal protective equipment contaminated with SARS-CoV-2. IEEE Access 2020, 8, 111347–111354. [Google Scholar] [CrossRef] [PubMed]
- Linke, B. Sterilization Methods and Impact on Electronics in Medical Devices. 2011. Available online: https://www.eetimes.com/document.asp?doc_id=1278906 (accessed on 20 September 2021).
- Harrington, R.E.; Guda, T.; Lambert, B.; Martin, J. Sterilization and Disinfection of Biomaterials for Medical Devices, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Rogers, W.J. Sterilisation Techniques for Polymers; Elsevier Masson SAS: Issy-les-Moulineaux, France, 2012. [Google Scholar]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Li, J.; Chen, Z.; Su, Z.; Liu, Z.; Yu, X. A glass/PDMS electrophoresis microchip embedded with molecular imprinting SPE monolith for contactless conductivity detection. Microchem. J. 2014, 114, 223–228. [Google Scholar] [CrossRef]
- Schöning, M.J.; Jacobs, M.; Muck, A.; Knobbe, D.T.; Wang, J.; Chatrathi, M.; Spillmann, S. Amperometric PDMS/glass capillary electrophoresis-based biosensor microchip for catechol and dopamine detection. Sens. Actuators B Chem. 2005, 108, 688–694. [Google Scholar] [CrossRef]
- Xu, K.; Clark, C.P.; Poe, B.L.; Lounsbury, J.A.; Nilsson, J.; Laurell, T.; Landers, J.P. Isolation of a low number of sperm cells from female DNA in a glass-PDMS-Glass microchip via bead-assisted acoustic differential extraction. Anal. Chem. 2019, 91, 2186–2191. [Google Scholar] [CrossRef]
- Hong, J.W.; Fujii, T.; Seki, M.; Yamamoto, T.; Endo, I. Integration of gene amplification and capillary gel electrophoresis on a polydimethylsiloxane-glass hybrid microchip. Electrophoresis 2001, 22, 328–333. [Google Scholar] [CrossRef]
- Xia, Y.-M.; Hua, Z.-S.; Srivannavit, O.; Ozel, A.; Gulari, E. Minimizing the surface effect of PDMS-glass microchip on polymerase chain reaction by dynamic polymer Passivation. J. Chem. Technol. Biotechnol. 2007, 82, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.Q.; Chen, W.Y.; Shao, S.Y.; Jia, X.Y.; Zhang, W.P. DNA amplification on a PDMS-glass hybrid microchip. J. Micromech. Microeng. 2006, 16, 425–433. [Google Scholar] [CrossRef]
- Nandi, P.; Desai, D.P.; Lunte, S.M. Development of a PDMS-based microchip electrophoresis device for continuous online in vivo monitoring of microdialysis samples. Electrophoresis 2010, 31, 1414–1422. [Google Scholar] [CrossRef]
- Ha, B.H.; Destgeer, G.; Park, J.; Jung, J.H.; Sung, H.J. Generation of complex, dynamic temperature gradients in a disposable microchip. Phys. Procedia 2015, 70, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Pinho, D.; Bento, D.; Lima, R.; Ribeiro, J. Wall expansion assessment of an intracranial aneurysm model by a 3D Digital Image Correlation System. Measurement 2016, 88, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Fiddes, L.K.; Raz, N.; Srigunapalan, S.; Tumarkan, E.; Simmons, C.A.; Wheeler, A.R.; Kumacheva, E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials 2010, 31, 3459–3464. [Google Scholar] [CrossRef]
- Siddique, A.; Pause, I.; Narayan, S.; Kruse, L.; Stark, R.W. Endothelialization of PDMS-based microfluidic devices under high shear stress conditions. Colloids Surf. B Biointerfaces 2021, 197, 111394. [Google Scholar] [CrossRef]
- Lima, R.; Wada, S.; Tanaka, S.; Takeda, M.; Ishikawa, T.; Tsubota, K.I.; Imai, Y.; Yamaguchi, T. In vitro blood flow in a rectangular PDMS microchannel: Experimental observations using a confocal micro-PIV system. Biomed. Microdevices 2008, 10, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.; Oliveira, M.S.N.; Ishikawa, T.; Kaji, H.; Tanaka, S.; Nishizawa, M.; Yamaguchi, T. Axisymmetric polydimethysiloxane microchannels for in vitro hemodynamic studies. Biofabrication 2009, 1, 35005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.Q.; Park, W.-T. Fabrication method of multi-depth circular microchannels for investigating arterial thrombosis-on-a-chip. Sens. Actuators B Chem. 2020, 321, 128590. [Google Scholar] [CrossRef]
- Agrawal, S.; Paknikar, K.; Bodas, D. Development of immunosensor using magnetic nanoparticles and circular microchannels in PDMS. Microelectron. Eng. 2014, 115, 66–69. [Google Scholar] [CrossRef]
- Morarka, A.; Agrawal, S.; Kale, S.; Kale, A.; Ogale, S.; Paknikar, K.; Bodas, D. Quantum dot based immunosensor using 3D circular microchannels fabricated in PDMS. Biosens. Bioelectron. 2011, 26, 3050–3053. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Ishikawa, T.; Lima, R.; Matsuki, N.; Imai, Y.; Kaji, H.; Nishizawa, M.; Yamaguchi, T. Red blood cell motions in high-hematocrit blood flowing through a stenosed microchannel. J. Biomech. 2009, 42, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, N.F.; Ristenpart, W.D. Mechanical response of red blood cells entering a constriction. Biomicrofluidics 2014, 8, 64123. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.; Pinho, D.; Lima, R.; Minas, G. A Passive microfluidic device based on crossflow filtration for cell separation measurements: A spectrophotometric characterization. Biosensors 2018, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Antaki, J.F.; Naik, T.; Bachman, T.N.; Kameneva, M.V.; Wu, Z.J. Microscopic investigation of erythrocyte deformation dynamics. Biorheology 2006, 43, 747–765. [Google Scholar]
- Lee, S.S.; Yim, Y.; Ahn, K.H.; Lee, S.J. Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed. Microdevices 2009, 11, 1021–1027. [Google Scholar] [CrossRef]
- Pinho, D.; Yaginuma, T.; Lima, R. A microfluidic device for partial cell separation and deformability assessment. BioChip J. 2013, 7, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Sousa, L.; Yaginuma, T.; Garcia, V.; Lima, R.; Miranda, J.M. Microbubble moving in blood flow in microchannels: Effect on the cell-free layer and cell local concentration. Biomed. Microdevices 2017, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Rodrigues, R.; Faustino, V.; Pinho, D.; Fernandes, C.; Pereira, A.; Garcia, V.; Miranda, J.; Lima, R. Deformation of red blood cells, air bubbles, and droplets in microfluidic devices: Flow visualizations and measurements. Micromachines 2018, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Shelby, J.P.; White, J.; Ganesan, K.; Rathod, P.K.; Chiu, D.T. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 14618–14622. [Google Scholar] [CrossRef] [Green Version]
- Boas, L.V.; Faustino, V.; Lima, R.; Miranda, J.M.; Minas, G.; Fernandes, C.S.V.; Catarino, S.O. Assessment of the deformability and velocity of healthy and artificially impaired red blood cells in narrow polydimethylsiloxane (PDMS) microchannels. Micromachines 2018, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.W.; Li, Q.S.; Lee, G.Y.H.; Kumar, A.P.; Ong, C.N.; Lim, C.T. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 2009, 11, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Pinho, D.; Yaginuma, T.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R. Extensional flow-based microfluidic device: Deformability assessment of red blood cells in contact with tumor cells. BioChip J. 2014, 8, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A microfluidic deformability assessment of pathological red blood cells flowing in a hyperbolic converging microchannel. Micromachines 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanopart. Res. 2016, 18, 194. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Fernandes, C.S.; Miranda, J.M.; Lima, R. In vitro blood flow visualizations and cell-free layer (CFL) measurements in a microchannel network. Exp. Therm. Fluid Sci. 2019, 109, 109847. [Google Scholar] [CrossRef]
- Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A rapid and low-cost nonlithographic method to fabricate biomedical microdevices for blood flow analysis. Micromachines 2015, 6, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Lopes, S.; Maia, I.; Lima, R.; Miranda, J.M. Bubbles moving in blood flow in a microchannel network: The effect on the local hematocrit. Micromachines 2020, 11, 344. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Pereira, A.I.; Lima, J.; Miranda, J.M.; Lima, R. Cell-free layer measurements of in vitro blood flow in a microfluidic network: An automatic and manual approach. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 629–637. [Google Scholar] [CrossRef]
- Leble, V.; Lima, R.; Dias, R.; Fernandes, C.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Asymmetry of red blood cell motions in a microchannel with a diverging and converging bifurcation. Biomicrofluidics 2011, 5, 044120. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Fujiwara, H.; Matsuki, N.; Yoshimoto, T.; Imai, Y.; Ueno, H.; Yamaguchi, T. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence. Biomed. Microdevices 2011, 13, 159–167. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadek, S.H.; Rubio, M.; Lima, R.; Vega, E.J. Blood Particulate Analogue Fluids: A Review. Materials 2021, 14, 2451. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Maia, I.; Souza, A.; Ribeiro, J.; Costa, P.; Puga, H.; Teixeira, S.; Lima, R.A. In vitro biomodels in stenotic arteries to perform blood analogues flow visualizations and measurements: A review. Open Biomed. Eng. J. 2021, 14, 87–102. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.M.; Souza, A.; Souza, M.S.; Bento, D.; Ribeiro, J.E.; Lima, R.; Pinho, D. Manual and automatic image analysis segmentation methods for blood flow studies in microchannels. Micromachines 2021, 12, 317. [Google Scholar] [CrossRef]
- Pinho, D.; Carvalho, V.; Gonçalves, I.M.; Teixeira, S.; Lima, R. Visualization and measurements of blood cells flowing in microfluidic systems and blood rheology: A personalized medicine perspective. J. Pers. Med. 2020, 10, 249. [Google Scholar] [CrossRef]
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.J.; Lima, R.; Campo-Deaño, L. Particulate blood analogues reproducing the erythrocytes cell-free layer in a microfluidic device containing a hyperbolic contraction. Micromachines 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Björnmalm, M.; Cui, J.; Wong, E.H.H.; Dai, Y.; Dai, Q.; Qiao, G.G.; Caruso, F. Structure governs the deformability of polymer particles in a microfluidic blood capillary model. ACS Macro Lett. 2015, 4, 1205–1209. [Google Scholar] [CrossRef]
- Maruyama, O.; Yamane, T.; Nishida, M.; Aouidef, A.; Tsutsui, T.; Jikuya, T.; Masuzawa, T. Fractural characteristic evaluation of a microcapsule suspension using a rotational shear stressor. ASAIO J. 2002, 48, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.A.M.; Rodrigues, A.R.O.; Faustino, V.; Pinho, D.; Castanheira, E.M.S.; Lima, R. Microfluidic deformability study of an innovative blood analogue fluid based on giant unilamellar vesicles. J. Funct. Biomater. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, R.; Vega, E.J.; Moita, A.S.; Miranda, J.M.; Pinho, D.; Moreira, A.L.N. Fast, flexible and low-cost multiphase blood analogue for biomedical and energy applications. Exp. Fluids 2020, 61, 231. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, N.; Rodríguez-Abreu, C.; Fernández-Nieves, A.; Solans, C. Fabrication of novel silicone capsules with tunable mechanical properties by microfluidic techniques. ACS Appl. Mater. Interfaces 2013, 5, 5247–5252. [Google Scholar] [CrossRef]
- Cui, J.; Björnmalm, M.; Liang, K.; Xu, C.; Best, J.P.; Zhang, X.; Caruso, F. Super-soft hydrogel particles with tunable elasticity in a microfluidic blood capillary model. Adv. Mater. 2014, 26, 7295–7299. [Google Scholar] [CrossRef]
- She, S.; Li, Q.; Shan, B.; Tong, W.; Gao, C. Fabrication of red-blood-cell-like polyelectrolyte microcapsules and their deformation and recovery behavior through a microcapillary. Adv. Mater. 2013, 25, 5814–5818. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Campo-Deaño, L.; Lima, R.; Pinho, F.T. In vitro particulate analogue fluids for experimental studies of rheological and hemorheological behavior of glucose-rich RBC suspensions. Biomicrofluidics 2017, 11, 54105. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Sánchez, B.N.; Silva, S.F.; Pinho, D.; Vega, E.J.; Lima, R. Generation of micro-sized PDMS particles by a flow focusing technique for biomicrofluidics applications. Biomicrofluidics 2016, 10, 014122. [Google Scholar] [CrossRef] [Green Version]
- Anes, C.F.; Pinho, D.; Muñoz-Sánchez, B.N.; Vega, E.J.; Lima, R. Shrinkage and colour in the production of micro-sized PDMS particles for microfluidic applications. J. Micromech. Microeng. 2018, 28, 75002. [Google Scholar] [CrossRef] [Green Version]
- Pinho, D.; Muñoz-Sánchez, B.N.; Anes, C.F.; Vega, E.J.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Chung, K.H.; Hong, H.B.; Lee, W.S. Production of PDMS microparticles by emulsification of two phases and their potential biological application. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 686–692. [Google Scholar] [CrossRef]
- López, M.; Rubio, M.; Sadek, S.H.; Vega, E.J. A simple emulsification technique for the production of micro-sized flexible powder of polydimethylsiloxane (PDMS). Powder Technol. 2020, 366, 610–616. [Google Scholar] [CrossRef]
- Carneiro, J.; Lima, R.; Campos, J.B.L.M.; Miranda, J.M. A microparticle blood analogue suspension matching blood rheology. Soft Matter 2021, 17, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Vega, E.J.; Cardoso, V.F.; Minas, G.; Montanero, J.M. Magnetic PDMS Microparticles for Biomedical and Energy Applications BT—VipIMAGE; Tavares, J.M.R.S., Natal Jorge, R.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 578–584. [Google Scholar]
- Barthes, J.; Lagarrigue, P.; Riabov, V.; Lutzweiller, G.; Kirsch, J.; Muller, C.; Courtial, E.-J.; Marquette, C.; Projetti, F.; Kzhyskowska, J.; et al. Biofunctionalization of 3D-printed silicone implants with immunomodulatory hydrogels for controlling the innate immune response: An in vivo model of tracheal defect repair. Biomaterials 2021, 268, 120549. [Google Scholar] [CrossRef]



| Property (Unity) | Result | References |
|---|---|---|
| Transmittance at range 390 nm to 780 nm (%) | 75–92 | [54,55] |
| Index of refraction | 1.4 | [56] |
| Thermal conductivity (W/m∙K) | 0.2–0.27 | [57,58] |
| Specific heat (kJ/kg∙K) | 1.46 | [56] |
| Dielectric strength (kV/mm) | 19 | [57] |
| Dielectric constant | 2.3–2.8 | [56] |
| Electrical conductivity (ohm∙m) | 4 × 1013 | [56] |
| Volume resistivity (ohm∙cm) | 2.9 × 1014 | [57] |
| Young’s modulus [kPa] | 360–870 | [59] |
| Poisson ratio | 0.5 | [60] |
| Tensile strength (MPa) | 2.24–6.7 | [56,57] |
| Hardness [Shore A] | 41–43 | [55,61] |
| Viscosity (Pa∙s) | 3.5 | [57] |
| Hydrophobicity—contact angle (°) | ~108° ± 7° | [62] |
| Melting Point (°C) | −49.9 to −40 | [63] |
| Temperature (°C) | Time |
|---|---|
| 25 | 48 h |
| 100 | 35 min |
| 125 | 20 min |
| 150 | 10 min |
| Application | PDMS Preparation | Motivations for Using PDMS | Reference |
|---|---|---|---|
| On-line sample pre-treatment and contactless conductivity detection | Mixing ratio—10:1, w/w Degassing time—20 min Curing temperature—80 °C Curing time—30 min Oxygen plasma treatment for 1–2 min | Low-cost, easy manufacture, suitability for mass production, transparency and elasticity. | [115] |
| Genetic analysis by functional integration of polymerase chain reaction (PCR) and capillary gel electrophoresis (CGE) | Mixing ratio—10:1, w/w Degassing time—15 min Curing temperature—65 °C Curing time—1 h Post-curing temperature—135 °C Post-curing time—15 min Hydrophilic treatment with HCl solution at 25 °C for 4 h | Low-cost, suitability for microscale moulding, high reproducibility on a micrometre scale, high gas permeability, low thermal conductivity and transparency. | [118] |
| Polymerase chain reaction (PCR) | Mixing ratio—10:1, w/w Curing temperature—95 °C Curing time—30 min | Low thermal conductivity, simple fabrication, low-cost, disposability, biocompatibility, irreversible bonding with glass and transparency. | [120] |
| Electrophoresis device for continuous on-line in vivo monitoring of micro dialysis samples | Mixing ratio—10.5:1.5, w/w 5 mm-thick layer curing temperature—90 °C 5 mm-thick layer curing time—25–30 min 1 mm-thick layer curing temperature–90 °C 1 mm-thick layer curing time—15–18 min Post-curing temperature—85 °C Post-curing time—overnight | Easy manufacture, good reproductivity and transparency. | [121] |
| Generation of temperature gradient | Mixing ratio—10:1, w/w | Low-cost, transparency, easy manufacture and low thermal conductivity | [122] |
| Geometry | Method | Advantages | Limitations | Application | Reference |
|---|---|---|---|---|---|
| Rectangular | Soft lithography | Generation of precise, reproducible and versatile microchannels; Precise control of experimental parameters and accurate measurements; Inexpensive, simple and rapid method. | Different geometry from in vivo microvessels; Difficulties in achieving stable cell seeding at the corners of the channel. | Integration of confocal micro-PIV with a PDMS microchannel to obtain blood velocity profiles | [126] |
| Circular | Wire casting technique | Simple and inexpensive method; Possibility of fabricating microchannels with different diameters; No need for a clean room or specialized equipment. | It is not possible to generate well-defined complex structures, such as bifurcations. | In vitro hemodynamic studies | [127] |
| Partially cured PDMS combined with thermal air expansion molding | Inexpensive and simple method; Possibility of fabricating multiple diameters of circular channel from 100 µm to 500 µm and different cross-sections. | It can be hard to fabricate a perfect circular channel. | Evaluate the clotting events in pathological vessels and testing device for antiplatelet and anticoagulant therapeutics | [128] | |
| Combination of soft lithography with the reflow phenomenon of a positive photoresist | Simple and efficient method; Possibility of fabricating microchannels with multiple diameters (from 100 µm to 400 µm) and various channel designs. | It can be hard to control the thickness of the photoresist, leading to a difficulty in generate perfect circular channels; Bonding the two semi-circular channels perfectly can be challenging. | This method allows endothelial cells culture, making this project suitable for drug screening and chemical/biological diagnostics | [90] | |
| Reshaping rectangular microchannels through polymerization of the liquid silicone oligomer around a gas steam | Ability of controlling the diameter from 40 µm to 100 µm; Possibility of fabricating constrictions. | Relatively complex and expensive method; Difficulty in controlling the exact diameter of the channel. | Mimic in vivo systems for cell flow studies | [124] |
| Application | PDMS Preparation | Motivation for Using PDMS | Reference |
|---|---|---|---|
| Urethanes PDMS-based hybrid coating for metallic dental implants | Hybrid urethanesil (PDMSUr) synthesized by ring opening polymerization of a bis(cyclic carbonate) derived from PDMS. Curing temperature—60 °C Curing time—24 h | Create hydrophobic and smooth surfaces, with less adhesion of bacteria, capable of adhering to tissue cells such as fibroblasts and osteoblasts. | [38] |
| Tantalum oxide-PDMS hybrid coating for medical implants | Modified sol-gel synthesis method, Tantalum oxide-PDMS solutions (10%, v/v). Curing temperature—room temperature Curing time—15 min | Medical grade PDMS has functional groups to bind to reactive surfaces such as activated metals or polymers. Ability to create micrometer-thick coatings. | [39] |
| Bioactive CaO-SiO2-PDMS coatings | Sol-gel dip-coating method. The produced coatings were kept at room temperature for 24 h for gelation. Curing temperature—150 °C Curing time—24 h | Mechanical properties and elasticity of PDMS | [40] |
| PDMS-based coating for a bladder volume monitoring sensor | Mixing ratio—10:2 (w/w) Curing temperature—80 °C Curing time—2 h | Biocompatibility, 10:2 ratio to increase tensile strength and improve Young’s modulus | [41] |
| CuO-PDMS-SiO2 coatings | Mixing ratio—10:1 (w/w) Curing temperature—150 °C Curing time—90 min | Improved biocompatibility, corrosion resistance and antibacterial property | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. https://doi.org/10.3390/jfb13010002
Miranda I, Souza A, Sousa P, Ribeiro J, Castanheira EMS, Lima R, Minas G. Properties and Applications of PDMS for Biomedical Engineering: A Review. Journal of Functional Biomaterials. 2022; 13(1):2. https://doi.org/10.3390/jfb13010002
Chicago/Turabian StyleMiranda, Inês, Andrews Souza, Paulo Sousa, João Ribeiro, Elisabete M. S. Castanheira, Rui Lima, and Graça Minas. 2022. "Properties and Applications of PDMS for Biomedical Engineering: A Review" Journal of Functional Biomaterials 13, no. 1: 2. https://doi.org/10.3390/jfb13010002