3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. ACP and CSH Preparation
2.2. Printing Ink Preparation
2.3. Scaffold Fabrication
2.4. Structural and Morphological Characterization
2.5. Surface Roughness
2.6. Mechanical Characterization
2.7. Swelling Property
2.8. Degradation Rate
2.9. Cell Culture and Cell Seeding
2.10. Attachment, Viability, and Proliferation of Cells on the Scaffolds
2.11. Statistical Analysis
3. Results
3.1. Scaffold Characterization
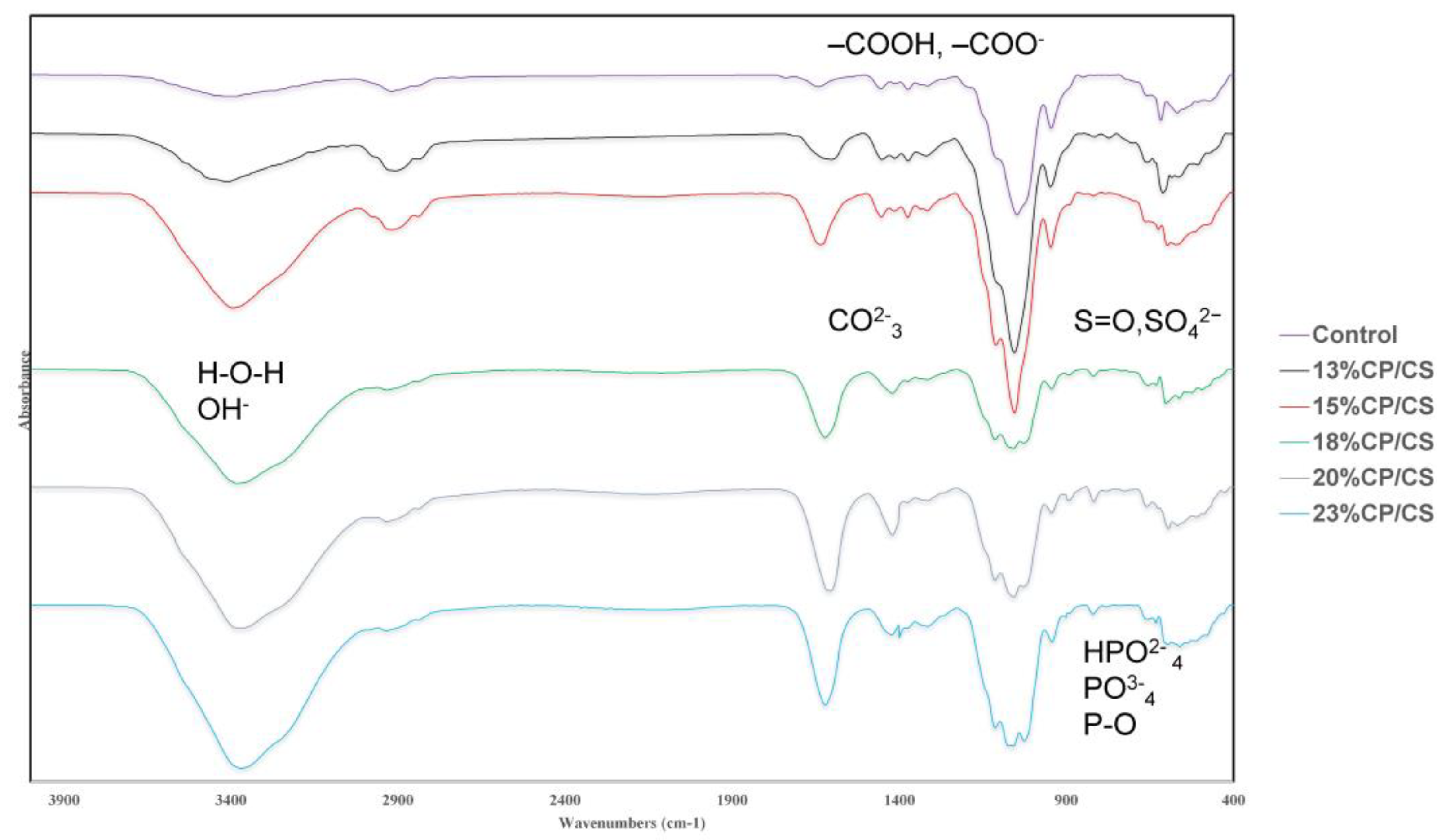
3.2. FTIR
3.3. Surface Roughness
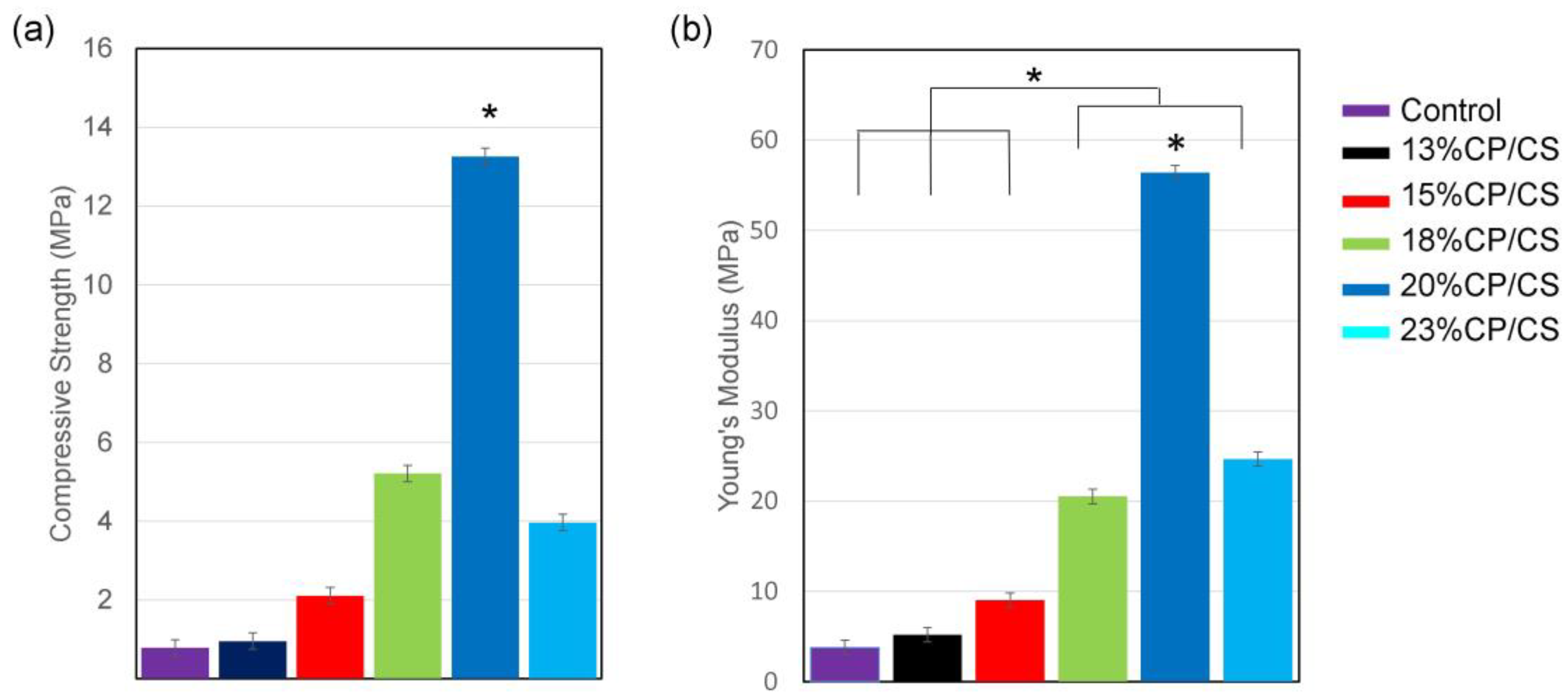
3.4. Mechanical Characterization
3.5. Swelling Property
3.6. Degradation Rate
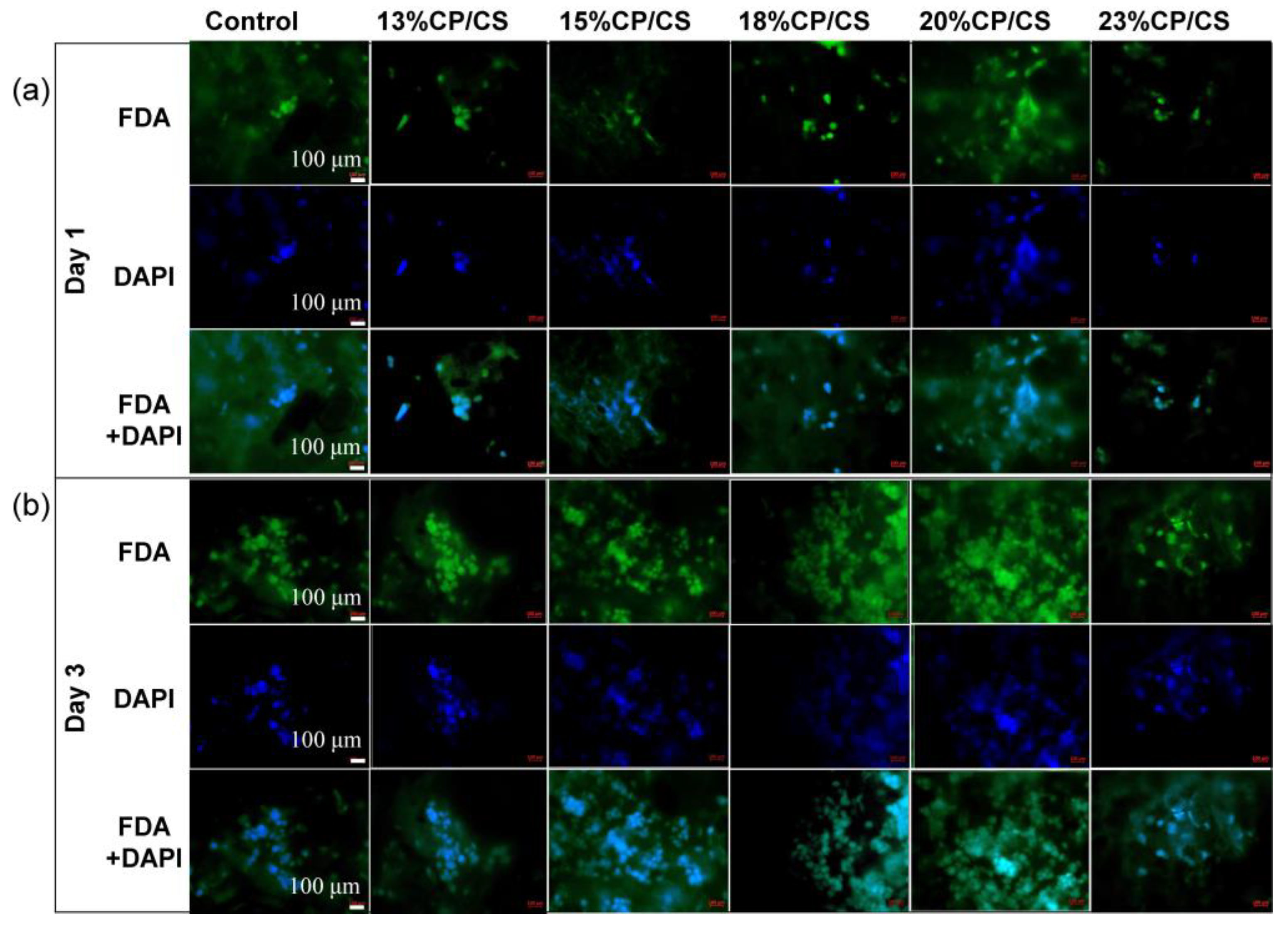
3.7. Cell Attachment and Spread
3.8. Cell Viability
3.9. Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tingli, L.; Zhao, W.; Hui, Q.; Kong, D.; Chen, T. Research Progress of Scaffold Materials in Bone Tissue Engineering. Mater. Rev. 2011, 25, 125–131. [Google Scholar]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.; Chua, C.; Leong, K.; Cheah, C.; Cheang, P.; Bakar, M.A.; Cha, S. Scaffold development using selective laser sintering of polyetheretherketone–hydroxyapatite biocomposite blends. Biomaterials 2003, 24, 3115–3123. [Google Scholar] [CrossRef]
- Wang, L.; Gramlich, W.M.; Gardner, D.J. Improving the impact strength of Poly (lactic acid) (PLA) in fused layer modeling (FLM). Polymer 2017, 114, 242–248. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of additive manufactured tissue engineering scaffolds: Relationship between geometry and performance. Burn. Trauma 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The impact of bioceramic scaffolds on bone regeneration in preclinical in vivo studies: A systematic review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.H.; Takagi, S.; Sun, L.; Hussain, L.; Chow, L.C.; Guthrie, W.F.; Yen, J.H. Development of a nonrigid, durable calcium phosphate cement for use in periodontal bone repair. J. Am. Dent. Assoc. 2006, 137, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.-M.; Chen, Z.; Liu, X.; Liu, H.; Lian, X.; Wang, X.; Cui, F.-Z. Mechanical properties and in vitro bioactivity of injectable and self-setting calcium sulfate/nano-HA/collagen bone graft substitute. J. Mech. Behav. Biomed. Mater. 2012, 12, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bioinspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, F.; Weng, W. Preparation and characterization of novel biphasic calcium phosphate powders (alpha-TCP/HA) derived from carbonated amorphous calcium phosphates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 508–517. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous Calcium Phosphate-Based Bioactive Polymeric Composites for Mineralized Tissue Regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; Díaz de León, A.; Alvarez, A.J.; Ortega-Lara, W. Alginate/Gelatin Hydrogels Reinforced with TiO2 and β-TCP Fabricated by Microextrusion-based Printing for Tissue Regeneration. Polymers 2019, 11, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, R.M.; Turner, T.M.; Hall, D.J.; Inoue, N.; Gitelis, S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin. Orthop. Relat. Res. 2007, 459, 110–117. [Google Scholar] [CrossRef]
- Nilsson, M.; Wang, J.-S.; Wielanek, L.; Tanner, K.; Lidgren, L. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J. Bone Jt. Surg. 2004, 86, 120–125. [Google Scholar] [CrossRef]
- Bahn, S.L. Plaster: A bone substitute. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 672–681. [Google Scholar] [CrossRef]
- Peltier, L.F.; Jones, R. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J. Bone Jt. Surg. Am. Vol. 1978, 60, 820–822. [Google Scholar] [CrossRef]
- Sidqui, M.; Collin, P.; Vitte, C.; Forest, N. Osteoblast adherence and resorption activity of isolated osteoclasts on calcium sulphate hemihydrate. Biomaterials 1995, 16, 1327–1332. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Garg, A. Review of bone-grafting materials. In Bone Biology, Harvesting, Grafting for Dental Implants: Rationale and Clinical Applications, 1st ed.; Quintessence Publishing: Berlin, Germany, 2004; pp. 21–56. [Google Scholar]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Chapter Four—Role of Alginate in Bone Tissue Engineering. Adv. Food Nutr. Res. 2014, 73, 45–57. [Google Scholar] [PubMed]
- Pei, P.; Wei, D.; Zhu, M.; Du, X.; Zhu, Y. The effect of calcium sulfate incorporation on physiochemical and biological properties of 3D-printed mesoporous calcium silicate cement scaffolds. Microporous Mesoporous Mater. 2017, 241, 11–20. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Wahab, I.F.; Abd Razak, S.I. Polysaccharides as composite biomaterials. In Composites from Renewable and Sustainable Materials; Intech Publisher: London, UK, 2016; pp. 65–84. [Google Scholar]
- Fernández-Cervantes, I.; Morales, M.; Agustín-Serrano, R.; Cardenas-García, M.; Pérez-Luna, P.; Arroyo-Reyes, B.; Maldonado-García, A. Polylactic acid/sodium alginate/hydroxyapatite composite scaffolds with trabecular tissue morphology designed by a bone remodeling model using 3D printing. J. Mater. Sci. 2019, 54, 9478–9496. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Placht, A.M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2017, 11, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes—In vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef]
- Iqbal, H.; Ali, M.; Zeeshan, R.; Mutahir, Z.; Iqbal, F.; Nawaz, M.A.H.; Shahzadi, L.; Chaudhry, A.A.; Yar, M.; Luan, S. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf. B Biointerfaces 2017, 160, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Cherng, A.; Takagi, S.; Chow, L. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J. Biomed. Mater. Res. 1997, 35, 273–277. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.C.; Sun, J.Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-D.; Lee, S.-Y.; Poma, M.; Wu, J.-Y.; Wang, D.-C.; Yang, J.-C. A novel resorbable α-calcium sulfate hemihydrate/amorphous calcium phosphate bone substitute for dental implantation surgery. Mater. Sci. Eng. C 2012, 32, 440–446. [Google Scholar] [CrossRef]
- Li, T.-T.; Ebert, K.; Vogel, J.; Groth, T. Comparative studies on osteogenic potential of micro-and nanofibre scaffolds prepared by electrospinning of poly (ε-caprolactone). Prog. Biomater. 2013, 2, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, S.; Guessasma, S. Compression performance of hollow structures: From topology optimisation to design 3D printing. Int. J. Mech. Sci. 2017, 133, 728–739. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Saed, A.; Behravesh, A.H.; Hasannia, S.; Akhoundi, B.; Hedayati, S.K.; Gashtasbi, F. An in vitro study on the key features of Poly L-lactic acid/biphasic calcium phosphate scaffolds fabricated via DLP 3D printing for bone grafting. Eur. Polym. J. 2020, 141, 110057. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecturein vivo. Biomed. Mater. 2011, 6, 015007. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, G.; Kim, J.Y.; Cho, D.W. Evaluating cell proliferation based on internal pore size and 3D scaffold architecture fabricated using solid freeform fabrication technology. J. Mater. Sci. Mater. Med. 2010, 21, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Rotbaum, Y.; Puiu, C.; Rittel, D.; Domingos, M. Quasi-static and dynamic in vitro mechanical response of 3D printed scaffolds with tailored pore size and architectures. Mater. Sci. Eng. C 2019, 96, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redey, S.A.; Nardin, M.; Bernache-Assolant, D.; Rey, C.; Delannoy, P.; Sedel, L.; Marie, P.J. Behavior of human osteoblastic cells on stoichiometric hydroxyapatite and type A carbonate apatite: Role of surface energy. J. Biomed. Mater. Res. 2000, 50, 353–364. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar] [CrossRef] [Green Version]
- Zan, X.; Sitasuwan, P.; Feng, S.; Wang, Q. Effect of Roughness on in Situ Biomineralized CaP-Collagen Coating on the Osteogenesis of Mesenchymal Stem Cells. Langmuir 2016, 32, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Hartcher-O’Brien, J.; Evers, J.; Tempelman, E. Surface roughness of 3D printed materials: Comparing physical measurements and human perception. Mater. Today Commun. 2019, 19, 300–305. [Google Scholar] [CrossRef]
- Lee, B.-T. Fabrication of calcium phosphate–calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010, 21, 1867–1874. [Google Scholar]
- Sun, H.; Hu, C.; Zhou, C.; Wu, L.; Sun, J.; Zhou, X.; Xing, F.; Long, C.; Kong, Q.; Liang, J.; et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 108540. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Suvannapruk, W.; Wasoontararat, K. Low temperature preparation of calcium phosphate structure via phosphorization of 3D-printed calcium sulfate hemihydrate based material. J. Mater. Sci. Mater. Med. 2010, 21, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Musskaya, O.N.; Krut’ko, V.K.; Kulak, A.I.; Filatov, S.A.; Batyrev, E.V.; Safronova, T.V. Calcium Phosphate Compositions with Polyvinyl Alcohol for 3D Printing. Inorg. Mater. Appl. Res. 2020, 11, 192–197. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, M.; Zheng, X.; Liang, X.; Su, X.; Zhang, Y.; Lu, A.; Zhang, L. Effects of chitin whiskers on physical properties and osteoblast culture of alginate based nanocomposite hydrogels. Biomacromolecules 2015, 16, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Czaderna, A.; Kocemba, A.; Kozanecki, M.; Mucha, M.; Mróz, P. The influence of cellulose derivatives on water structure in gypsum. Constr. Build. Mater. 2018, 160, 628–638. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- He, W.; Wu, Z.; Wu, Y.; Zhong, Z.; Hong, Y. Construction of the Gypsum-Coated Scaffolds for In Situ Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 31527–31541. [Google Scholar] [CrossRef]
- Witek, L.; Shi, Y.; Smay, J. Controlling calcium and phosphate ion release of 3D printed bioactive ceramic scaffolds: An in vitro study. J. Adv. Ceram. 2017, 6, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.; Gupte, M.J.; Zhang, Z.; Swanson, W.B.; McCauley, L.K.; Ma, P.X. Three-Dimensional Electrodeposition of Calcium Phosphates on Porous Nanofibrous Scaffolds and Their Controlled Release of Calcium for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 32503–32513. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.-n.; Jiao, K.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.-l.; Mao, J.; Chen, J.-h.; Pashley, D.H.; Tay, F.R. A review of the bioactivity of hydraulic calcium silicate cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Solati-Hashjin, M.; Zabihi-Neyshabouri, E.; Rabiee, S.M. Novel calcium phosphate coated calcium silicate-based cement: In vitro evaluation. Biomed. Mater. 2020, 15, 035008. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nakamura, T.; Okada, Y.; Fukumoto, A.; Furukawa, T.; Kato, H.; Kokubo, T.; Kikutani, T. Bioactive bone cement: Comparison of apatite and wollastonite containing glass-ceramic, hydroxyapatite, and β-tricalcium phosphate fillers on bone-bonding strength. J. Biomed. Mater. Res. 1998, 42, 223–237. [Google Scholar] [CrossRef]
- Constantz, B.R.; Barr, B.M.; Ison, I.C.; Fulmer, M.T.; Baker, J.; McKinney, L.; Goodman, S.B.; Gunasekaren, S.; Delaney, D.C.; Ross, J. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. 1998, 43, 451–461. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]

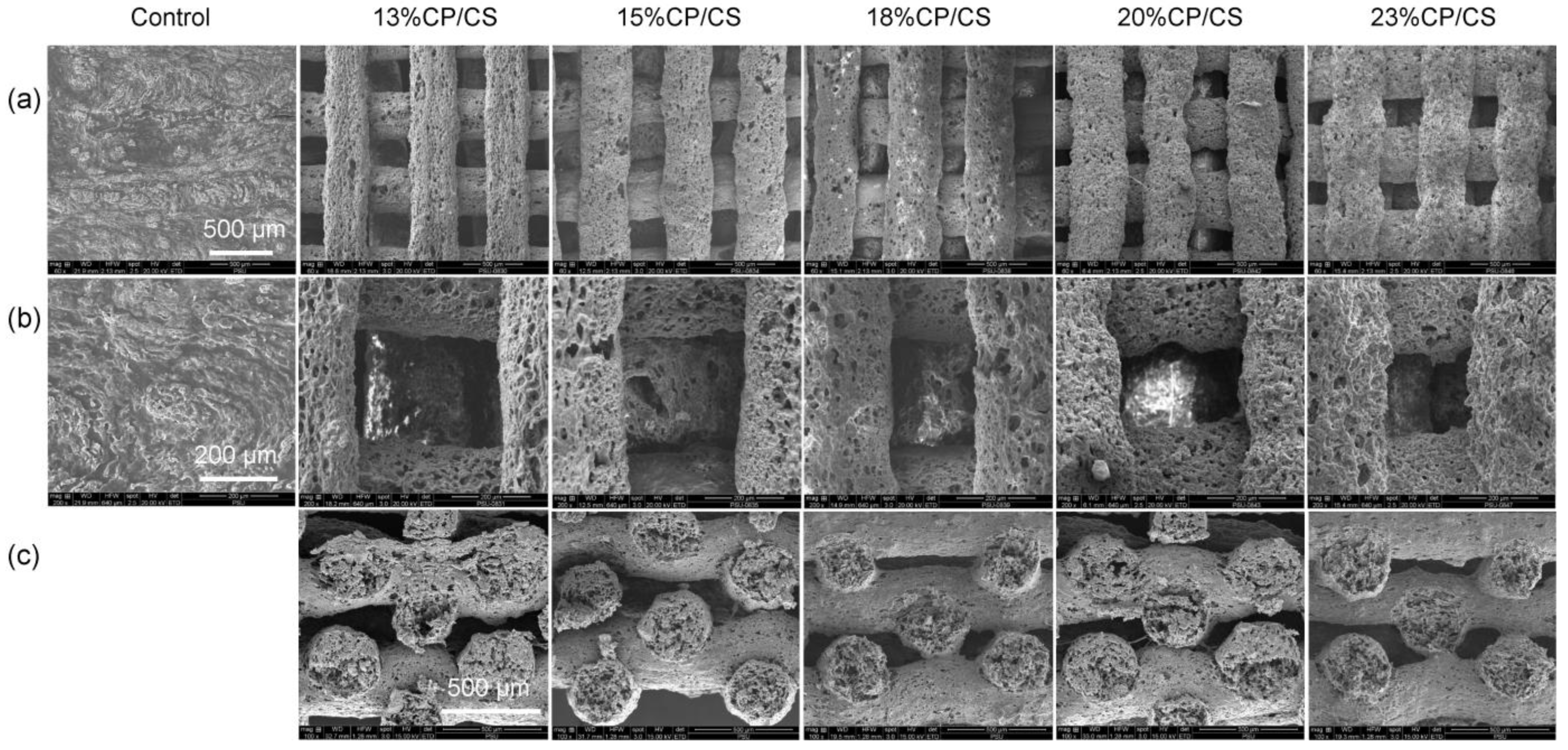




| Groups | Pore Size (μm), Surface | p-Value | Pore Size (Μm), Cross-Section | p-Value | Surface Roughness Average (nm) | p-Value |
|---|---|---|---|---|---|---|
| Control | 0.00 ± 0.00 | 0.000 | 0.00 ± 0.00 | 0.000 | 9.02 ± 4.49 | 0.007 |
| 13% CP/CS | 315.61 ± 42.31 | 334.00 ± 86.65 | 55.34 ± 4.66 | |||
| 15% CP/CS | 299.68 ± 48.92 | 377.09 ± 69.77 | 45.93 ± 7.46 | |||
| 18% CP/CS | 298.65 ± 55.09 | 323.96 ± 83.05 | 43.22 ± 4.89 | |||
| 20% CP/CS | 316.36 ± 52.95 | 316.60 ± 71.57 | 62.53 ± 9.43 | |||
| 23% CP/CS | 306.48 ± 48.14 | 327.99 ± 80.36 | 59.00 ± 5.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanaanek, N.; Suttapreyasri, S.; Samruajbenjakun, B. 3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization. J. Funct. Biomater. 2022, 13, 47. https://doi.org/10.3390/jfb13020047
Wattanaanek N, Suttapreyasri S, Samruajbenjakun B. 3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization. Journal of Functional Biomaterials. 2022; 13(2):47. https://doi.org/10.3390/jfb13020047
Chicago/Turabian StyleWattanaanek, Nattanan, Srisurang Suttapreyasri, and Bancha Samruajbenjakun. 2022. "3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization" Journal of Functional Biomaterials 13, no. 2: 47. https://doi.org/10.3390/jfb13020047
APA StyleWattanaanek, N., Suttapreyasri, S., & Samruajbenjakun, B. (2022). 3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization. Journal of Functional Biomaterials, 13(2), 47. https://doi.org/10.3390/jfb13020047






