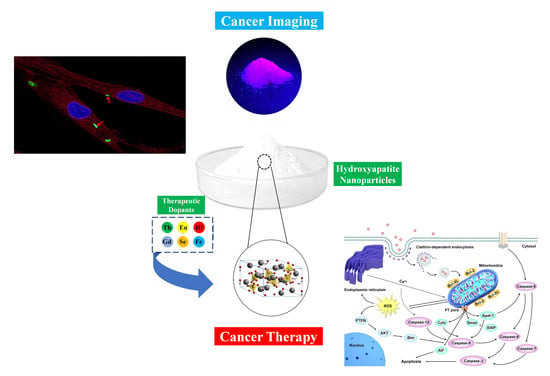
Hydroxyapatite Nanoparticles for Improved Cancer Theranostics
Abstract
:1. Introduction
| Element | Ionic Charge(s) | Advantages | Ref(s) |
|---|---|---|---|
| Fluoride (F) | 1− | -Provides the possibility of medical imaging of soft tissues (e.g., breast cancers) using positron emission tomography (PET)/computer tomography (CT) | [45] |
| Silicon (Si) | 4− 4+ | -Promotes drug loading efficiency, good biocompatibility, and biodegradability | [46] |
| Sulfur (S) | 2−, 2+, 4+, 6+ | -In the form of sulfate, may make a pH-responsive platform for efficient tumor-targeting drug delivery | [44] |
| Selenium (Se) | 2−, 4+, 6+ | -Exerts a strong cytotoxic activity against prostate (PC3) and breast (MDA-MB-231) cancer cells-Inhibits human osteosarcoma cell growth via inducing apoptosis | [47,48] |
| Strontium (Sr) | 2+ | -The radioactive isotope strontium-89 renders luminescent properties | [32] |
| Barium (Ba) | 2+ | -Acts as a suitable computed tomography (CT) contrast agent | [49] |
| Hafnium (Hf) | 4+ | -Induces the formation of ROS and cell apoptosis (in vitro and in vivo) after being exposed to ionizing radiation (e.g., gamma rays) | [50] |
| Zinc (Zn) | 2+ | -Enhances the radiation of breast cancer cells | [51] |
| Copper (Cu) | 1+, 2+ | -In the form of a nanocluster, it can impart luminescent properties to HAp, providing a suitable material for cancer imaging | [52] |
| Iron (Fe) | 2+, 3+ | -Imparts superparamagnetic behavior (useful in MR cancer imaging) and causes damage to mitochondria and cancer cell membranes-Fenton reaction-based cancer treatment. | [42,53] |
| Zirconium (Zr) | 4+ | -Accumulates and internalizes in the lung cancer cell line A549 | [54] |
| Europium (Eu) | 3+ | -Acts as a fluorescent probe and is thereby suitable for tracking cancer cells | [55] |
| Terbium (Tb) | 3+, 4+ | -Provides photoluminescence properties | [55] |
| Samarium (Sm) | 3+ | -Suitable for bioimaging, drug delivery, and as an MRI contrast agent | [56,57,58,59,60] |
| Neodymium (Nd) | 3+, 4+ | -Exhibits near-infrared emission at 670 nm after excitation at 410 nm, unraveling its theranostic capabilities -Excellent compatibility with mammalian cells | [59] |
| Gadolinium (Gd) | 3+ | -Great potential for applications as an MR T1 contrast agent and drug carrier for cancer therapy | [61,62] |
| Vanadium (V) | 2+, 3+, 4+, 5+ | -Causes strong cytotoxicity against human bone cancer cells | [63] |
2. Hallmarks of Cancer: A Call for Theranostic Nanoparticles
3. HAp Structure Fits Therapeutic Applications
4. Interactions of HAp NPs with Cancer Cells
| HAp Nanostructure | Cancer Type | In Vitro/In Vivo | Imaging/Therapy | Remarks | Ref |
|---|---|---|---|---|---|
| Strontium (Sr)-doped HAp nanorods | Bone | In vitro | Therapy | Generation of the radioactive isotopes of strontium-89 and phosphorus-32 through neutron irradiation leads to the treatment of bone tumors while regenerating the affected region | [32] |
| Hafnium (Hf)-doped HAp nanoparticles | Lung | In vitro/In vivo | Therapy | Generation of reactive oxygen species (ROS) after gamma irradiation and inducing apoptosis | [50] |
| Catechins-modified selenium-doped HAp nanoparticles | Bone | In vitro | Therapy | Stimulating the apoptosis of human osteosarcoma MNNG/HOS cell lines via generation of ROS, inducing the overexpression of caspase-3, p53, and Bax while downregulating cox-2 and PTK-2, with no side effects on human bone marrow stem cells | [104] |
| Se-doped HAp/chitosan bio-papers | Bone | In vitro/In vivo | Therapy | Overgeneration and accumulation of ROS, inducing apoptosis of HCS 2/8 chondrosarcoma and SJSA osteosarcoma cells through overexpression of Bax, caspase-3, JNK activation, and STAT3 inhibition and lessening the size of the tumor in a patient-derived xenograft (PDX) mouse model | [103] |
| Selenium (Se)-substituted HAp nanoparticles | Hepatocellular carcinoma | In vitro/In vivo | Therapy | Promoted the survival rate of nude mice, inducing tumor necrosis, reduced toxicity on liver and kidney functions | [105] |
| Europium (Eu)-doped nanoHAp | HeLa cells | In vitro | Imaging/therapy | Strong fluorescence properties, biocompatible, good delivery, and sustained drug (5-fluorouracil) release | [106] |
| Eu-doped calcium-deficient HAp core functionalized with cyclodextrin and cucurbituril | HeLa cells | In vitro | Imaging/therapy | Luminescent properties, controlled and prolonged drug (5-fluorouracil) release, the efficient killing of HeLa cells (over 80%) | [107] |
| Ag/Fe co-doped HA | HeLa cells | In vitro | Therapy | Targeted drug (5-fluorouracil) delivery to the tumor site, controlled drug release, and the killing of the cancerous cells with no cytotoxic effect on the normal cells (HEK-293) and without any infections | [108] |
| Neodymium (Nd)- doped HAp nanoparticles | Colon | In vitro/In vivo | Imaging/therapy | Early stage diagnosis of tumors, targeted colon tumor therapy, and monitoring | [60] |
| Eu-HAp-loaded PLGA NPs with foliate decoration | Breast | In vitro | Imaging | Promoted fluorescent properties, targeted imaging of MCF-7 cells at early stages, biocompatible and photostable | [109] |
| Eu/Gd co-doping HAp nanocrystals | Liver, spleen, heart, lung, kidney | In vitro/In vivo | Imaging | Biocompatible, biodegradable, improved luminescence and fluorescence properties for cell imaging | [90] |
| Eu (or Tb)-doped HAp nanorods | A549 cells (lung cancer) and HeLa cells | In vitro | Imaging | Biocompatible, excellent red or green luminescent properties, converting to hydrophilic particles with surfactant Pluronic F127 and applications for live cell imaging | [110] |
5. HAp Nanostructures for the Delivery of Anticancer Drugs
6. Doped HAp NPs for Cancer Theranostics
7. Surface-Modified HAp Nanosystems for Targeted Therapy
8. Nanohydroxyapatite-Containing Scaffolds
9. Nano-Sized HAp in Cancer Imaging
10. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Ashton-Prolla, P.; Cantor, A.; Mariosa, D.; Brennan, P. The role of genomics in global cancer prevention. Nat. Rev. Clin. Oncol. 2021, 18, 116–128. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Ohuchida, K.; Shindo, K.; Moriyama, T.; Akagawa, S.; Maeyama, R.; Nagai, S.; Nakata, K.; Nabae, T.; Suehara, N.; et al. High frequency of bone recurrence as an initial recurrence site after radical surgery in T1N3 gastric cancer: A propensity score matching analysis. Langenbeck’s Arch. Surg. 2021, 406, 2305–2313. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Ruiz-Patiño, A.; Arrieta, O.; Cardona, A.F.; Martín, C.; Raez, L.E.; Zatarain-Barrón, Z.L.; Barrón, F.; Ricaurte, L.; Bravo-Garzón, M.A.; Mas, L. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non-small cell lung cancer (NSCLC) compared with chemotherapy (Quijote-CLICaP). Thorac. Cancer 2020, 11, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Ali, O.A.; Mooney, D.J. Immunologically active biomaterials for cancer therapy. In Cancer Immunology and Immunotherapy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 279–297. [Google Scholar]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1606036. [Google Scholar] [CrossRef]
- Wang, H.; Agarwal, P.; Zhao, S.; Yu, J.; Lu, X.; He, X. Combined cancer therapy with hyaluronan-decorated fullerene-silica multifunctional nanoparticles to target cancer stem-like cells. Biomaterials 2016, 97, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chang, J.; Wu, C. MoS2-based biomaterials for cancer therapy. In Biomaterials in Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–161. [Google Scholar]
- Yue, S.; Luo, M.; Liu, H.; Wei, S. Recent Advances of Gold Compounds in Anticancer Immunity. Front. Chem. 2020, 8, 543. [Google Scholar] [CrossRef]
- Kostka, L.; Kotrchova, L.; Subr, V.; Libanska, A.; Ferreira, C.A.; Malatova, I.; Lee, H.J.; Barnhart, T.E.; Engle, J.W.; Cai, W.; et al. HPMA-based star polymer biomaterials with tuneable structure and biodegradability tailored for advanced drug delivery to solid tumours. Biomaterials 2020, 235, 119728. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Najibi, A.J.; Shih, T.Y.; Mao, A.S.; Sharda, A.; Scadden, D.T.; Mooney, D.J. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 2020, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Kang, M.; Song, N.; Wang, D.; Tang, B.Z. Boosting the photodynamic therapy efficiency by using stimuli-responsive and AIE-featured nanoparticles. Biomaterials 2020, 232, 119749. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Moztarzadeh, F. Synthesis and characterization of Barium-Iron containing magnetic bioactive glasses: The effect of magnetic component on structure and in vitro bioactivity. Colloids Surf. B Biointerfaces 2019, 176, 27–37. [Google Scholar] [CrossRef]
- Kermani, F.; Kargozar, S.; Dorozhkin, S.V.; Mollazadeh, S. Calcium phosphate bioceramics for improved angiogenesis. In Biomaterials for Vasculogenesis and Angiogenesis; Woodhead Publishing: Cambridge, UK, 2022; pp. 185–203. [Google Scholar] [CrossRef]
- Oliveira, T.M.; Berti, F.C.B.; Gasoto, S.C.; Schneider, B., Jr.; Stimamiglio, M.A.; Berti, L.F. Calcium Phosphate-Based Bioceramics in the Treatment of Osteosarcoma: Drug Delivery Composites and Magnetic Hyperthermia Agents. Front. Med. Technol. 2021, 3, 700266. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.; Kargozar, S.; Tayarani-Najaran, Z.; Yousefi, A.; Beidokhti, S.M.; Moayed, M.H. Synthesis of nano HA/βTCP mesoporous particles using a simple modification in granulation method. Mater. Sci. Eng. C 2019, 96, 859–871. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–19. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Kermani, F.; Gharavian, A.; Mollazadeh, S.; Kargozar, S.; Youssefi, A.; Vahdati Khaki, J. Silicon-doped calcium phosphates; the critical effect of synthesis routes on the biological performance. Mater. Sci. Eng. C 2020, 111, 110828. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Vahdati Khakhi, J. Improved osteogenesis and angiogenesis of theranostic ions doped calcium phosphates (CaPs) by a simple surface treatment process: A state-of-the-art study. Mater. Sci. Eng. C 2021, 124, 112082. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, Z.; Kermani, F.; Mollazadeh, S.; Kargozar, S.; Vahdati Khakhi, J. Crystallization behavior and density functional theory study of solution combustion synthesized silicon doped calcium phosphates. Ceram. Int. 2022, 48, 14349–14359. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltran, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Ceram. Int. 2015, 41, 13913–13966. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Qiao, W.; Lan, X.; Tsoi, J.K.; Chen, Z.; Su, R.Y.; Yeung, K.W.; Matinlinna, J.P. Biomimetic hollow mesoporous hydroxyapatite microsphere with controlled morphology, entrapment efficiency and degradability for cancer therapy. RSC Adv. 2017, 7, 44788–44798. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; He, L.; Zhang, P.; Zhang, J.; Chen, Z.; Ren, X.; Mei, X. Folate-modified hydroxyapatite nanorods induce apoptosis in MCF-7 cells through a mitochondrial-dependent pathway. New J. Chem. 2019, 43, 14728–14738. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- de Rezende, M.R.; Andrade, G.F.; Cipreste, M.F.; Miranda, M.C.; Gomes, D.A.; de Barros Correia Menezes, M.Â.; de Sousa, E.M. 89Sr-doped hydroxyapatite nanoparticles as a potential therapeutic agent for bone tumors. Int. J. Appl. Ceram. Technol. 2019, 16, 1904–1919. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Jia, Q.-Y.; Li, X.-D.; Li, S.-P.; Shen, J.; Lin, J.; Li, D.-X. Synthesis of hollow mesoporous HAp-Au/MTX and its application in drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124231. [Google Scholar] [CrossRef]
- Aval, N.A.; Islamian, J.P.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.-R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ji, D.; Li, C.; Zhu, Y.; Xiong, G.; Wan, Y. Layered nanohydroxyapatite as a novel nanocarrier for controlled delivery of 5-fluorouracil. Int. J. Pharm. 2016, 513, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, X.; Iqbal, M.Z.; Zhao, Q.; Zhao, R.; Farheen, J.; Zhang, Q.; Zhang, P.; Kong, X. siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy. Pharmaceutics 2021, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Ansari, L.; Derakhshi, M.; Bagheri, E.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Folate conjugation improved uptake and targeting of porous hydroxyapatite nanoparticles containing epirubicin to cancer cells. Pharm. Dev. Technol. 2020, 25, 601–609. [Google Scholar] [CrossRef]
- AbouAitah, K.; Stefanek, A.; Higazy, I.M.; Janczewska, M.; Swiderska-Sroda, A.; Chodara, A.; Wojnarowicz, J.; Szałaj, U.; Shahein, S.A.; Aboul-Enein, A.M. Effective Targeting of Colon Cancer Cells with Piperine Natural Anticancer Prodrug Using Functionalized Clusters of Hydroxyapatite Nanoparticles. Pharmaceutics 2020, 12, 70. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth Kumar, B.N.; Sarkar, D.; Azab, B.; Pathak, A.; Kundu, S.C.; Fisher, P.B.; Mandal, M. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Yang, Z.; Zuo, G.; Zhang, Q.; Yao, F.; Wan, Y. Encapsulating doxorubicin-intercalated lamellar nanohydroxyapatite into PLGA nanofibers for sustained drug release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Prasad, S.R.; Jayakrishnan, A.; Kumar, T.S. Hydroxyapatite-poly (vinyl alcohol) core-shell nanoparticles for dual delivery of methotrexate and gemcitabine for bone cancer treatment. J. Drug Deliv. Sci. Technol. 2019, 51, 629–638. [Google Scholar] [CrossRef]
- Kermani, F.; Vojdani-Saghir, A.; Beidokhti, S.M.; Nazarnezhad, S.; Mollaei, Z.; Hamzehlou, S.; El-Fiqi, A.; Baino, F.; Kargozar, S. Iron (Fe)-doped mesoporous 45S5 bioactive glasses: Implications for cancer therapy. Transl. Oncol. 2022, 20, 101397. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G.; et al. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Li, Y.; Xiao, D.; Li, T.; Zhang, T.; Fu, W.; Lin, Y. Hyaluronan-directed fabrication of co-doped hydroxyapatite as a dual-modal probe for tumor-specific bioimaging. J. Mater. Chem. B 2020, 8, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.H., III; Gore, J.C.; Yankeelov, T.E.; Barnes, S.; Peterson, T.E.; True, J.M.; Shokouhi, S.; McIntyre, J.O.; Sanders, M.; Abramson, V.; et al. A novel approach to breast cancer diagnosis via PET imaging of microcalcifications using 18F-NaF. J. Nucl. Med. 2014, 55, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H.; Jia, G.; Liu, D.; Ge, K.; et al. Hybrid Mesoporous Silica-Based Drug Carrier Nanostructures with Improved Degradability by Hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef] [PubMed]
- Barbanente, A.; Palazzo, B.; Esposti, L.D.; Adamiano, A.; Iafisco, M.; Ditaranto, N.; Migoni, D.; Gervaso, F.; Nadar, R.; Ivanchenko, P.; et al. Selenium-doped hydroxyapatite nanoparticles for potential application in bone tumor therapy. J. Inorg. Biochem. 2021, 215, 111334. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, X.; Xu, T.; Sun, T.; Huang, H.; Lu, M.; Yin, Y.; Ge, J.; Weng, J. Selenium-substituted hydroxyapatite particles with regulated microstructures for osteogenic differentiation and anti-tumor effects. Ceram. Int. 2019, 45, 13787–13798. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, R.; Zheng, X.; Wu, L.; Hou, X. Mono-dispersed Ba2+-doped Nano-hydroxyapatite conjugated with near-infrared Cu-doped CdS quantum dots for CT/fluorescence bimodal targeting cell imaging. Microchem. J. 2017, 134, 41–48. [Google Scholar] [CrossRef]
- Chen, M.H.; Hanagata, N.; Ikoma, T.; Huang, J.Y.; Li, K.Y.; Lin, C.P.; Lin, F.H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef]
- Yedekci, Y.; Gedik, E.; Evis, Z.; Dogan, L.; Özyigit, G.; Gürkaynak, M. Radiosensitization induced by zinc-doped hydroxyapatite nanoparticles in breast cancer cells. Int. J. Appl. Ceram. Technol. 2021, 18, 563–572. [Google Scholar] [CrossRef]
- Simon, A.T.; Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Quercetin-Loaded Luminescent Hydroxyapatite Nanoparticles for Theranostic Application in Monolayer and Spheroid Cultures of Cervical Cancer Cell Line In Vitro. ACS Appl. Bio Mater. 2021, 4, 4495–4506. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Monteiro, F.J.; Laranjeira, M.S. Duality of iron (III) doped nano hydroxyapatite in triple negative breast cancer monitoring and as a drug-free therapeutic agent. Ceram. Int. 2020, 46, 16590–16597. [Google Scholar] [CrossRef]
- Febrian, M.B.; Mahendra, I.; Kurniawan, A.; Setiadi, Y.; Ambar Wibawa, T.H.; Lesmana, R.; Syarif, D.G. Zirconium doped hydroxyapatite nanoparticle as a potential design for lung cancer therapy. Ceram. Int. 2021, 47, 27890–27897. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Li, S. Biocompatible europium doped hydroxyapatite nanoparticles as a biological fluorescent probe. Curr. Nanosci. 2010, 6, 178–183. [Google Scholar] [CrossRef]
- Paduraru, A.V.; Oprea, O.; Musuc, A.M.; Vasile, B.S.; Iordache, F.; Andronescu, E. Influence of Terbium Ions and Their Concentration on the Photoluminescence Properties of Hydroxyapatite for Biomedical Applications. Nanomaterials 2021, 11, 2442. [Google Scholar] [CrossRef]
- Tesch, A.; Wenisch, C.; Herrmann, K.-H.; Reichenbach, J.R.; Warncke, P.; Fischer, D.; Müller, F.A. Luminomagnetic Eu3+-and Dy3+-doped hydroxyapatite for multimodal imaging. Mater. Sci. Eng. C 2017, 81, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Kim, H.H.; Seo, H.; Lee, K.D.; Oh, J. Magnetic hydroxyapatite: A promising multifunctional platform for nanomedicine application. Int. J. Nanomed. 2017, 12, 8389–8410. [Google Scholar] [CrossRef] [Green Version]
- Cipreste, M.F.; Peres, A.M.; Cotta, A.A.; Aragón, F.H.; Antunes, A.d.M.; Leal, A.S.; Macedo, W.A.; de Sousa, E.M. Synthesis and characterization of 159Gd-doped hydroxyapatite nanorods for bioapplications as theranostic systems. Mater. Chem. Phys. 2016, 181, 301–311. [Google Scholar] [CrossRef]
- Victor, S.P.; Paul, W.; Vineeth, V.M.; Komeri, R.; Jayabalan, M.; Sharma, C.P. Neodymium doped hydroxyapatite theranostic nanoplatforms for colon specific drug delivery applications. Colloids Surf. B Biointerfaces 2016, 145, 539–547. [Google Scholar] [CrossRef]
- Ignjatović, N.L.; Mančić, L.; Vuković, M.; Stojanović, Z.; Nikolić, M.G.; Škapin, S.; Jovanović, S.; Veselinović, L.; Uskoković, V.; Lazić, S. Rare-earth (Gd3+, Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Tian, Y.; Wu, J.; Sun, J.; Teng, Z.; Wang, S.; Lu, G. Gadolinium-Doped Hydroxyapatite Nanorods as T1 Contrast Agents and Drug Carriers for Breast Cancer Therapy. ACS Appl. Nano Mater. 2019, 2, 1194–1201. [Google Scholar] [CrossRef]
- Kalniņa, D.; Levina, A.; Pei, A.; Gross, K.A.; Lay, P.A. Synthesis, characterization and in vitro anti-cancer activity of vanadium-doped nanocrystalline hydroxyapatite. New J. Chem. 2019, 43, 17891–17901. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Biological hallmarks of cancer. Holl.-Frei Cancer Med. 2016, 1–10. [Google Scholar] [CrossRef]
- VanDyke, D.; Kyriacopulos, P.; Yassini, B.; Wright, A.; Burkhart, E.; Jacek, S.; Pratt, M.; Peterson, C.R.; Rai, P. Nanoparticle Based Combination Treatments for Targeting Multiple Hallmarks of Cancer. Int. J. Nano Stud. Technol. 2016, S4, 1–18. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Wang, Q.; Li, G.; Wan, B.; Sun, Y.; Niu, X.; Chen, D.; Tian, W. Anti-osteosarcoma effect of hydroxyapatite nanoparticles both in vitro and in vivo by downregulating the FAK/PI3K/Akt signaling pathway. Biomater. Sci. 2020, 8, 4426–4437. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, X.; Liu, H.; Chen, S.; Gao, C.; Ge, K.; Zhang, C.; Zhang, J. Oxidative stress-induced apoptosis of osteoblastic MC3T3-E1 cells by hydroxyapatite nanoparticles through lysosomal and mitochondrial pathways. RSC Adv. 2017, 7, 13010–13018. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Du, S.; Zhang, P.; Jiang, Z.; Zhou, J.; Yao, J. Primary tumor and pre-metastatic niches co-targeting "peptides-lego" hybrid hydroxyapatite nanoparticles for metastatic breast cancer treatment. Biomater. Sci. 2018, 6, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1504. [Google Scholar] [CrossRef]
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef]
- Ikoma, T.; Yamazaki, A.; Nakamura, S.; Akao, M. Preparation and structure refinement of monoclinic hydroxyapatite. J. Solid State Chem. 1999, 144, 272–276. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martinez-Pineiro, E.L.; Bres, E.F. Crystallographic structure of human tooth enamel by electron microscopy and x-ray diffraction: Hexagonal or monoclinic? J. Microsc. 2012, 248, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Crystallogr. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Bystrov, V.; Coutinho, J.; Bystrova, A.; Dekhtyar, Y.D.; Pullar, R.; Poronin, A.; Palcevskis, E.; Dindune, A.; Alkan, B.; Durucan, C. Computational study of hydroxyapatite structures, properties and defects. J. Phys. D Appl. Phys. 2015, 48, 195302. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Khakhi, J.V. Solution combustion synthesis (SCS) of theranostic ions doped biphasic calcium phosphates; kinetic of ions release in simulated body fluid (SBF) and reactive oxygen species (ROS) generation. Mater. Sci. Eng. C 1920, 118, 111533. [Google Scholar] [CrossRef]
- Peroos, S.; Du, Z.; de Leeuw, N.H. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials 2006, 27, 2150–2161. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kuwabara, A. First-principles study of vacancy formation in hydroxyapatite. Phys. Rev. B 2007, 75, 014102. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K. Theoretical investigation of the defect formation mechanism relevant to nonstoichiometry in hydroxyapatite. Phys. Rev. B 2008, 77, 104106. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, D.; Zhang, C.; Sun, J.; Feng, W.; Liang, X.J.; Wang, S.; Zhang, J. Defect-Related Luminescent Hydroxyapatite-Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells Via an ATP-Induced cAMP/PKA Pathway. ACS Appl. Mater. Interfaces 2016, 8, 11262–11271. [Google Scholar] [CrossRef]
- Ren, X.; Yi, Z.; Sun, Z.; Ma, X.; Chen, G.; Chen, Z.; Li, X. Natural polysaccharide-incorporated hydroxyapatite as size-changeable, nuclear-targeted nanocarrier for efficient cancer therapy. Biomater. Sci. 2020, 8, 5390–5401. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Das, M.; Balla, V.K. Effect of hydroxyapatite particle size, morphology and crystallinity on proliferation of colon cancer HCT116 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z.; Tang, J.; Yang, X.; Zhou, Y.; Guo, B.; Wang, L.; Zhu, X.; Tu, C.; Zhang, X. The in vitro and in vivo anti-melanoma effects of hydroxyapatite nanoparticles: Influences of material factors. Int. J. Nanomed. 2019, 14, 1177–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, K.; Shaik, M.M.; Ramanan, S.R.; Kowshik, M. Self-Activated Fluorescent Hydroxyapatite Nanoparticles: A Promising Agent for Bioimaging and Biolabeling. ACS Biomater. Sci. Eng. 2016, 2, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.R.; Leite, I.; Inada, N.; Li, M.S.; Da Silva, J.; Andrés, J.; Beltrán-Mir, H.; Cordoncillo, E.; Longo, E. Designing biocompatible and multicolor fluorescent hydroxyapatite nanoparticles for cell-imaging applications. Mater. Today Chem. 2019, 14, 100211. [Google Scholar] [CrossRef]
- Xie, Y.; He, W.; Li, F.; Perera, T.S.H.; Gan, L.; Han, Y.; Wang, X.; Li, S.; Dai, H. Luminescence enhanced Eu3+/Gd3+ co-doped hydroxyapatite nanocrystals as imaging agents in vitro and in vivo. ACS Appl. Mater. Interfaces 2016, 8, 10212–10219. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Qiu, J.; Li, J.; Sang, Y.; Xia, H.; Jiang, H.; Claverie, J.; Liu, H. Eu/Tb codoped spindle-shaped fluorinated hydroxyapatite nanoparticles for dual-color cell imaging. Nanoscale 2016, 8, 11580–11587. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 4, 7134. [Google Scholar] [CrossRef] [Green Version]
- Seyfoori, A.; Naghib, S.M.; Molaabasi, F. Inhibitory effect comparison of the needle, spherical, and mesoporous hydroxyapatite nanoparticles on MCF-7 breast cancer cell line proliferation: An in vitro assay. Adv. Nanochemistry 2020, 2, 11–14. [Google Scholar]
- Gorojod, R.M.; Porte Alcon, S.; Dittler, M.L.; Gonzalez, M.C.; Kotler, M.L. Nanohydroxyapatite Exerts Cytotoxic Effects and Prevents Cellular Proliferation and Migration in Glioma Cells. Toxicol. Sci. 2019, 169, 34–42. [Google Scholar] [CrossRef]
- Yin, M.Z.; Han, Y.C.; Bauer, I.W.; Chen, P.; Li, S.P. Effect of hydroxyapatite nanoparticles on the ultrastructure and function of hepatocellular carcinoma cells in vitro. Biomed. Mater. 2006, 1, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Y.; Ma, X.; Yuan, Y.; Liu, C.; Kohn, J.; Qian, J. Mitochondria-Targeted Hydroxyapatite Nanoparticles for Selective Growth Inhibition of Lung Cancer in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 25680–25690. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ding, T. p53 reaction to apoptosis induced by hydroxyapatite nanoparticles in rat macrophages. J. Biomed. Mater. Res. A 2009, 88, 673–679. [Google Scholar] [CrossRef]
- Tang, W.; Yuan, Y.; Liu, C.; Wu, Y.; Lu, X.; Qian, J. Differential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cells. Nanomedicine 2014, 9, 397–412. [Google Scholar] [CrossRef]
- Chu, S.H.; Feng, D.F.; Ma, Y.B.; Li, Z.Q. Hydroxyapatite nanoparticles inhibit the growth of human glioma cells in vitro and in vivo. Int. J. Nanomed. 2012, 7, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, P.; Li, Z.; Huang, J.; Yang, Z. Oxidative stress and apoptosis induced by hydroxyapatite nanoparticles in C6 cells. J. Biomed. Mater. Res. A 2012, 100, 738–745. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Sun, T.W.; Qin, Y.H.; Zhu, Y.J.; Jiang, Y.Y.; Zhang, Y.; Liu, J.J.; Wu, J.; He, S.S.; Chen, F. Selenium-doped hydroxyapatite biopapers with an anti-bone tumor effect by inducing apoptosis. Biomater. Sci. 2019, 7, 5044–5053. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Liu, Y.; Ullah, I.; Xue, M.; Yang, G.; Hou, H. Catechins-modified selenium-doped hydroxyapatite nanomaterials for improved osteosarcoma therapy through generation of reactive oxygen species. Front. Oncol. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Yanhua, W.; Hao, H.; Li, Y.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Melendez, P.G.; Martinez-Castanon, G.A.; Nino-Martinez, N.; Patino-Marin, N.; Casillas-Santana, M.A.; Castillo-Silva, B.E.; Ruiz, F. Facile Synthesis, Characterization, and Cytotoxic Activity of Europium-Doped Nanohydroxyapatite. Bioinorg. Chem. Appl. 2016, 2016, 1057260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victor, S.P.; Gayathri Devi, M.; Paul, W.; Vijayan, V.M.; Muthu, J.; Sharma, C.P. Europium doped calcium deficient hydroxyapatite as theranostic nanoplatforms: Effect of structure and aspect ratio. ACS Biomater. Sci. Eng. 2017, 3, 3588–3595. [Google Scholar] [CrossRef]
- Veerla, S.C.; Kim, D.R.; Kim, J.; Sohn, H.; Yang, S.Y. Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 98, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wan, D.; Bian, Y.; Sun, H.; Zhang, C.; Jin, F.; Huang, Z.; Gong, J. Fluorescent hydroxyapatite-loaded biodegradable polymer nanoparticles with folate decoration for targeted imaging. AIChE J. 2013, 59, 4494–4501. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Hui, J.; Fan, D.; Ma, H.; Zhang, X.; Wang, Y.; Wei, Y. Ln(3+)-doped hydroxyapatite nanocrystals: Controllable synthesis and cell imaging. Phys. Chem. Chem. Phys. 2015, 17, 20301–20307. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Nezafati, N.; Saber-Samandari, S. The Effective Role of Hydroxyapatite Based Composites in Anticancer Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 41–75. [Google Scholar] [CrossRef]
- Maia, A.L.C.; Ferreira, C.d.A.; Barros, A.L.B.d.; e Silva, A.T.M.; Ramaldes, G.A.; Silva Cunha Júnior, A.d.; Oliveira, D.C.d.P.; Fernandes, C.; Ferreira Soares, D.C. Vincristine-loaded hydroxyapatite nanoparticles as a potential delivery system for bone cancer therapy. J. Drug Target. 2018, 26, 592–603. [Google Scholar] [CrossRef]
- Govindan, B.; Swarna Latha, B.; Nagamony, P.; Ahmed, F.; Saifi, M.A.; Harrath, A.H.; Alwasel, S.; Mansour, L.; Alsharaeh, E.H. Designed Synthesis of Nanostructured Magnetic Hydroxyapatite Based Drug Nanocarrier for Anti-Cancer Drug Delivery toward the Treatment of Human Epidermoid Carcinoma. Nanomaterials 2017, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Meshkini, A.; Oveisi, H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf. B Biointerfaces 2017, 158, 319–330. [Google Scholar] [CrossRef]
- Yasun, E.; Gandhi, S.; Choudhury, S.; Mohammadinejad, R.; Benyettou, F.; Gozubenli, N.; Arami, H. Hollow micro and nanostructures for therapeutic and imaging applications. J. Drug Deliv. Sci. Technol. 2020, 60, 102094. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses (MBGs) in cancer therapy: Full of hope and promise. Mater. Lett. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, S.; Zhang, D. Grafting of thermo-responsive polymer inside mesoporous silica with large pore size using ATRP and investigation of its use in drug release. J. Mater. Chem. 2007, 17, 2428–2433. [Google Scholar] [CrossRef]
- Yang, Y.H.; Liu, C.H.; Liang, Y.H.; Lin, F.H.; Wu, K.C. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Banat, F.; Anwer, S.; Lee, S.; BinSaleh, N.; Latha, S.; Ponpandian, N. Mesoporous hydroxyapatite nanoplate arrays as pH-sensitive drug carrier for cancer therapy. Mater. Res. Express 2019, 6, 085409. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, J.; Ma, H.; Chen, R.; Li, J.; Cao, S. Hierarchical hydroxyapatite/polyelectrolyte microcapsules capped with AuNRs for remotely triggered drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1236–1245. [Google Scholar] [CrossRef]
- Xuan, J.; Pelletier, M.; Xia, H.; Zhao, Y. Ultrasound-Induced Disruption of Amphiphilic Block Copolymer Micelles. Macromol. Chem. Phys. 2011, 212, 498–506. [Google Scholar] [CrossRef]
- Li, D.; Huang, X.; Wu, Y.; Li, J.; Cheng, W.; He, J.; Tian, H.; Huang, Y. Preparation of pH-responsive mesoporous hydroxyapatite nanoparticles for intracellular controlled release of an anticancer drug. Biomater. Sci. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Kim, H.; Oh, J. Nanostructured hollow hydroxyapatite fabrication by carbon templating for enhanced drug delivery and biomedical applications. Ceram. Int. 2019, 45, 17081–17093. [Google Scholar] [CrossRef]
- Chen, R.; Shi, J.; Zhu, B.; Zhang, L.; Cao, S. Mesoporous hollow hydroxyapatite capped with smart polymer for multi-stimuli remotely controlled drug delivery. Microporous Mesoporous Mater. 2020, 306, 110447. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Wu, J.; Sun, J.; Liao, X.; Teng, Z.; Lu, G. Facile synthesis of biodegradable flower-like hydroxyapatite for drug and gene delivery. J. Colloid Interface Sci. 2020, 570, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.P.; Balakrishnan, S.; Kulandaivelu, R.; TSN, S.N.; Lakshmipathy, M.; Sagadevan, S.; Mohammad, F.; Al-Lohedan, H.A.; Paiman, S.; Oh, W.C. Nanoformulations of core–shell type hydroxyapatite-coated gum acacia with enhanced bioactivity and controlled drug delivery for biomedical applications. New J. Chem. 2020, 44, 7175–7185. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Synthesis and luminescence of Eu3+ doped hydroxyapatite nanocrystallines: Effects of calcinations and Eu3+ content. J. Lumin. 2013, 135, 281–287. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Tran, L.H.; Yi, M.; Shin, J.H.; Lee, C.-Y.; Oh, J. Bioactive, luminescent erbium-doped hydroxyapatite nanocrystals for biomedical applications. Ceram. Int. 2020, 46, 16020–16031. [Google Scholar] [CrossRef]
- Trandafir, D.-L.; Mirestean, C.; Turcu, R.; Frentiu, B.; Eniu, D.; Simon, S. Structural characterization of nanostructured hydroxyapatite–iron oxide composites. Ceram. Int. 2014, 40, 11071–11078. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Morales, M.A.; Litterst, F.J. γ-Fe2O3 nanoparticles embedded in nanohydroxyapatite matrix for magnetic hyperthermia and in vitro osteoblast cell studies. Ceram. Int. 2020, 46, 10658–10666. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z.; Zhang, S. Dual functional selenium-substituted hydroxyapatite. Interface Focus 2012, 2, 378–386. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, M.; Li, C. Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnol. 2016, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Chakraborty, S.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. 166Ho-labeled hydroxyapatite particles: A possible agent for liver cancer therapy. Cancer Biother. Radiopharm. 2009, 24, 7–14. [Google Scholar] [CrossRef]
- Hou, C.H.; Hou, S.M.; Hsueh, Y.S.; Lin, J.; Wu, H.C.; Lin, F.H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef]
- Abdel-Hamid, Z.; Rashad, M.; Mahmoud, S.M.; Kandil, A. Electrochemical hydroxyapatite-cobalt ferrite nanocomposite coatings as well hyperthermia treatment of cancer. Mater. Sci. Eng. C 2017, 76, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharm. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedighi, O.; Alaghmandfard, A.; Montazerian, M.; Baino, F. A critical review of bioceramics for magnetic hyperthermia. J. Am. Ceram. Soc. 2022, 105, 1723–1747. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ashok, M.; Girija, E. Development of multifunctional cobalt ferrite/hydroxyapatite nanocomposites by microwave assisted wet precipitation method: A promising platform for synergistic chemo-hyperthermia therapy. Ceram. Int. 2019, 45, 12860–12869. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. Journal of Controlled Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: A systematic review. Pleura Peritoneum 2018, 3, 20180124. [Google Scholar] [CrossRef]
- Yoo, J.O.; Ha, K.S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar]
- Zhang, X.; Ma, J. Photothermal effect of 3D printed hydroxyapatite composite scaffolds incorporated with graphene nanoplatelets. Ceram. Int. 2021, 47, 6336–6340. [Google Scholar]
- Verma, G.; Barick, K.; Shetake, N.G.; Pandey, B.; Hassan, P. Citrate-functionalized hydroxyapatite nanoparticles for pH-responsive drug delivery. RSC Adv. 2016, 6, 77968–77976. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Rohanizadeh, R. Functionalizing the surface of hydroxyapatite drug carrier with carboxylic acid groups to modulate the loading and release of curcumin nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharm. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Shetake, N.G.; Pandrekar, S.; Pandey, B.N.; Hassan, P.A.; Priyadarsini, K.I. Development of surface functionalized hydroxyapatite nanoparticles for enhanced specificity towards tumor cells. Eur. J. Pharm. Sci. 2020, 144, 105206. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Abe, S.; Tagaya, M. Surface-Engineered Design of Efficient Luminescent Europium(III) Complex-Based Hydroxyapatite Nanocrystals for Rapid HeLa Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2019, 11, 8915–8927. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Pathi, S.P.; Lin, D.D.; Dorvee, J.R.; Estroff, L.A.; Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef] [Green Version]
- Sikora, M.; Marcinkowska, K.; Marycz, K.; Wiglusz, R.J.; Smieszek, A. The Potential Selective Cytotoxicity of Poly (L-Lactic Acid)-Based Scaffolds Functionalized with Nanohydroxyapatite and Europium (III) Ions toward Osteosarcoma Cells. Materials 2019, 12, 3779. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Smieszek, A.; Targonska, S.; Walsh, S.A.; Szustakiewicz, K.; Wiglusz, R.J. Three dimensional (3D) printed polylactic acid with nano-hydroxyapatite doped with europium (III) ions (nHAp/PLLA@ Eu3+) composite for osteochondral defect regeneration and theranostics. Mater. Sci. Eng. C 2020, 110, 110634. [Google Scholar] [CrossRef]
- Tornin, J.; Villasante, A.; Sole-Marti, X.; Ginebra, M.P.; Canal, C. Osteosarcoma tissue-engineered model challenges oxidative stress therapy revealing promoted cancer stem cell properties. Free. Radic. Biol. Med. 2021, 164, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Murphy, C.; O’Brien, F.J.; Piskareva, O. Three-dimensional In Vitro Biomimetic Model of Neuroblastoma using Collagen-based Scaffolds. J. Vis. Exp. JoVE 2021. [Google Scholar] [CrossRef]
- Nakayama, M.; Lim, W.Q.; Kajiyama, S.; Kumamoto, A.; Ikuhara, Y.; Kato, T.; Zhao, Y. Liquid-Crystalline Hydroxyapatite/Polymer Nanorod Hybrids: Potential Bioplatform for Photodynamic Therapy and Cellular Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 17759–17765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pan, X.; Yao, M.; Han, L.; Zhang, X.; Jia, Z.; Weng, J.; Chen, W.; Fang, L.; Wang, X. Bioinspired adhesive and tumor microenvironment responsive nanoMOFs assembled 3D-printed scaffold for anti-tumor therapy and bone regeneration. Nano Today 2021, 39, 101182. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous co-substitution of Sr2+/Fe3+ in hydroxyapatite nanoparticles for potential biomedical applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Kataoka, T.; Samitsu, S.; Okuda, M.; Kawagoe, D.; Tagaya, M. Highly Luminescent Hydroxyapatite Nanoparticles Hybridized with Citric Acid for Their Bifunctional Cell-Labeling and Cytostatic Suppression Properties. ACS Appl. Nano Mater. 2019, 3, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chuang, C.-C.; Wang, P.-T.; Tang, C.-M. A comparative study on the direct and pulsed current electrodeposition of cobalt-substituted hydroxyapatite for magnetic resonance imaging application. Materials 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Huang, P.; Zhu, Y.J.; Wu, J.; Zhang, C.L.; Cui, D.X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, L.; Fang, J.; Feng, X. Bio-nanoplatforms based on carbon dots conjugating with F-substituted nano-hydroxyapatite for cellular imaging. Nanoscale 2015, 7, 20033–20041. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. J. Funct. Biomater. 2022, 13, 100. https://doi.org/10.3390/jfb13030100
Kargozar S, Mollazadeh S, Kermani F, Webster TJ, Nazarnezhad S, Hamzehlou S, Baino F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. Journal of Functional Biomaterials. 2022; 13(3):100. https://doi.org/10.3390/jfb13030100
Chicago/Turabian StyleKargozar, Saeid, Sahar Mollazadeh, Farzad Kermani, Thomas J. Webster, Simin Nazarnezhad, Sepideh Hamzehlou, and Francesco Baino. 2022. "Hydroxyapatite Nanoparticles for Improved Cancer Theranostics" Journal of Functional Biomaterials 13, no. 3: 100. https://doi.org/10.3390/jfb13030100