Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges
Abstract
:1. Introduction to Brain Anatomy and Pathology
1.1. Brain Tissue Composition and Structure
1.2. Brain Tissue Alterations under Pathological Conditions
2. Introduction to Preclinical Models of Human Brain Tissue
2.1. 3D Brain Tissue Models
2.1.1. Main cell Populations in Brain Tissue Models
Primary Cells and Immortalized Cell Lines
Stem Cells
2.1.2. Brain Tissue Models
3. Focus on TE Brain Models
3.1. General Design Criteria for TE Brain Models
| Properties | Target Specification/Values | Ref. |
|---|---|---|
| Biomimetic composition | Scaffolds should contain proteins (or their peptide motifs) and/or polysaccharides naturally present in the brain ECM or biomimetic with respect to brain ECM composition. Examples include: collagen, laminin, gelatin, fibrin, hyaluronic acid, chitosan, bacterial cellulose, RGD peptide, TATVHL peptide, poly(lysine), poly(ornithine). Surface charge and wettability influence cell behavior on scaffolds. Synthetic polymers, such as poly(lactic-co-glycolic acid) (PLGA) and poly(caprolactone) (PCL), have also been used to improve the stability of scaffolds for brain TE, in combination with natural polymers or adhesive peptides, forming “bioartificial” biomaterials. | [178,179,180,181,182,183,184,185,186,187] |
| Biomimetic stiffness | Target value of scaffold stiffness is brain tissue stiffness (0.5–14 kPa). Soft gels with moduli <1 kPa were found to enhance neural stem cells (NSCs) differentiation. | [188,189,190] |
| Biomimetic architecture | Fibrous scaffolds with aligned fibers are suitable for the engineering of white matter, which contains aligned and myelinated axonal fibers, and is mechanically anisotropic. Fibrous scaffolds with randomly oriented fibers have been demonstrated to favor cortical NSC proliferation and differentiation. | [191,192] |
| Electrical conductivity | Electrically conductive scaffolds containing electrically conductive polymers (e.g., polyaniline (PANi), poly(3,4-ethylenedioxythiophene) (PEDOT), and polypyrrole (PPy)) or polymer composites (e.g., containing graphene or carbon nanotubing (CNT)) can enhance neural regeneration. | [188,193] |
| Porosity | Scaffold pore size and porosity degree influence cell infiltration and tissue ingrowth, as well as scaffold mechanical properties and degradation rate. The optimal range for scaffold porosity should be compatible with the size of an adult stem cell (20 µm approximately). Small pores (<100 um) favor stem cell adhesion and local niche formation, while larger pores (120 um) are ideal for nutrient and oxygen delivery. Additionally, small pores reduce penetration of morphogenetic factors, influencing cell differentiation. | [174,194] |
3.2. Relevant 3D Brain TE Models Using iPSCs
3.2.1. Hydrogel-Based Models
3.2.2. Biofabricated Brain Models
3.2.3. Decellularized Scaffolds for Brain Modeling
3.2.4. Engineered Porous Scaffolds for 3D Culture and Brain Modeling
4. Discussion
| 3D SUBSTRATE PROPERTIES | ||
|---|---|---|
| Scaffold Properties | Target Characteristics | Ref. |
| Architecture | Fibrous scaffold architecture embedded into a soft hydrogel | [267,268,273] |
| Composition | Fibrous scaffold based on a synthetic polymer (e.g., PCL, PLGA) or natural polymer (silk fibroin). Hydrogel filler based on: Collagen type I Functionalizing molecules: Laminin, poly-L-ornithin | [267,268,273] |
| Stiffness | Tailored by composite scaffold composition (target value: brain tissue stiffness of 0.5–14 kPa). | [267,268,273] |
| Electrical conductivity | Optimal electrical conductivity: 3 × 10−4 to 6 × 10−2 S/cm | [267,268,273] |
| Porosity | Optimal porosity: 84–98% range. | [287] |
| Degradation time | At least a few months to allow construct maturation and further experiments | [288] |
| CELLS | ||
| Cells | Optimal cell Culture Procedure on Scaffolds | Ref. |
| hiPSC | hiPSC differentiation into some of the brain cells directly on the scaffolds (e.g., neurons and astrocytes), followed by the addition of other cell types. | [278,280] |
| MODEL CHARACTERIZATION AND VALIDATION | ||
| Model properties | Validation of the Model | Ref. |
| Cell population | Stem cell and differentiation markers expression by PCR, immunofluorescence and Western blot analysis. | [267,268,273] |
| Brain structure recapitulation | Cell morphology and cell–cell assembly by immunofluorescence analysis. | [267,268,273] |
| ECM | Characterization of decellularized brain ECM through liquid chromatography–mass spectrometry (LC–MS); GAGs compositional analysis through fluorescence-assisted carbohydrate electrophoresis (FACE). | [267] |
| Functionality | Functional validation of the model by ion flux and electrical conductance analysis. | [267,268,273] |
| Predictivity | Predictivity using model drugs, already approved or tested in the clinics. | [267] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| αβ42 | Amyloid-beta 42 |
| AD | Alzheimer’s disease |
| ALGMA | Methacrylated alginate |
| ASCs | Adult stem cells |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived growth factor |
| BMECs | Brain microvascular endothelial cells |
| BoC | Brain-on-chip |
| CMC | Carboxymethyl chitosan |
| CNS | Central nervous system |
| CNT | Carbon nanotube |
| COL | Collagen |
| COLMA | Methacrylated collagen |
| CS | Chondroitin sulphate |
| CSPGs | Chondroitin sulphate proteoglycans |
| DA | Dopaminergic |
| DAC | 2,3–dialdehyde |
| dbcAMP | Dibutyryl cyclic adenosine monophosphate |
| EBs | Embryoid bodies |
| ECM | Extracellular matrix |
| ENO2 | Enolase 2 |
| ESCs | Embryonic stem cells |
| FACE | Fluorescence-assisted carbohydrate electrophoresis |
| GAGs | Glycosaminoglycans |
| GFAP | Glial fibrillary acidic protein |
| HA | Hyaluronic acid |
| HA-CA | Cathecol (functionalized) hyaluronic acid |
| HAMA | Methacrylated hyaluronic acid |
| hfNSCs | Human fetal neural stem cells |
| HSPGs | Heparan sulphate proteoglycans |
| ICC | Inverted colloidal crystal |
| iPSCs | Induced pluripotent stem cells |
| LC–MS | Liquid chromatography–mass spectrometry |
| LOP | Lab-on-a-printer |
| MAP2 | Microtubule-associated protein 2 |
| MMP | Metalloproteinases |
| MSCs | Mesenchymal stem cells |
| NGF | Nerve growth factor |
| NPCs | Neural progenitor cells |
| NSCs | Neural stem cells |
| NPs | Nanoparticles |
| OLs | Oligodendrocytes |
| OPCs | Oligodendrocyte precursor cells |
| OPN | Osteopontin |
| Pγ | Pyrrole |
| PAAM–CH | Polyacrylamide–chitosan |
| PANi | Polyaniline |
| PBT | Polybutylene terephthalate |
| PCL | Polycaprolactone |
| PD | Parkinson’s disease |
| PEDOT | Poly-3,4-ethylenedioxythiophene |
| PEGDA | Polyethylene glycol diacrylate |
| γ-PGA | Poly-γ-glutamic acid |
| PGS | Pyrolytic graphite sheet |
| PLA | Polylactic acid |
| PLO | Poly-L-ornithine |
| PLGA | Polylactic-co-glycolic-acid |
| polyHIPE | Polymerized high internal phase emulsions |
| PPγ | Polypyrrole |
| RA | Retinoic acid |
| S100β | S100 calcium-binding protein β |
| SEM | Scanning electron microscopy |
| SF | Silk fibroin |
| SPI | Spinal cord injury |
| TE | Tissue engineering |
| TMPTA | Trimethylolpropane triacrylate |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| Tuj1 | β-III-tubulin |
References
- Pogoda, K.; Janmey, P.A. Glial Tissue Mechanics and Mechanosensing by Glial Cells. Front. Cell. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Barnes, A.; Bassett, D.S.; Fornito, A.; Kitzbichler, M.; Meunier, D.; Suckling, J. Generic aspects of complexity in brain imaging data and other biological systems. NeuroImage 2009, 47, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Kopell, N.; Ermentrout, B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc. Natl. Acad. Sci. USA 2004, 101, 15482–15487. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Suzuki, R.; Zhu, X.; Bonfanti, L. NG2 cells (polydendrocytes) in brain physiology and repair. Front. Cell. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef]
- Kirby, L.; Jin, J.; Cardona, J.G.; Smith, M.D.; Martin, K.A.; Martin, J.; Wang, J.; Strasburger, H.; Herbst, L.; Alexis, M.; et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 2019, 10, 3887. [Google Scholar] [CrossRef]
- Barres, B.A. Perspective The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Stimpson, C.D.; Raghanti, M.A.; Wildman, D.E.; Uddin, M.; Grossman, L.I.; Goodman, M.; Redmond, J.C.; Bonar, C.J.; Erwin, J.M.; et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 13606–13611. [Google Scholar] [CrossRef]
- Peteri, U.K.; Niukkanen, M.; Castrén, M.L. Astrocytes in Neuropathologies Affecting the Frontal Cortex. Front. Cell. Neurosci. 2019, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Nave, K.A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- McKanzie, I.A.; Ohayon, D.; Li, H.; De Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, A.; Holtman, I.R.; Krueger, M.; Yogev, N.; Bruttger, J.; Khorooshi, R.; Benmamar-Badel, A.; Boer-Bergsma, J.J.; Martin, N.A.; Karram, K.; et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 2017, 36, 3292–3308. [Google Scholar] [CrossRef]
- Bignami, A.; Hosley, M.; Dahl, D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. 1993, 188, 419–433. [Google Scholar] [CrossRef]
- George, N.; Geller, H.M. Extracellular matrix and traumatic brain injury. J. Neurosci. Res. 2018, 96, 573–588. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Lin, J.; Nowsheen, S.; Liu, T.; Zhao, Y.; Villalta, P.W.; Sicard, D.; Tschumperlin, D.J.; Lee, S.B.; Kim, J.J.; et al. Extracellular matrix stiffness determines DNA repair efficiency and cellular sensitivity to genotoxic agents. Sci. Adv. 2020, 6, eabb2630. [Google Scholar] [CrossRef]
- Zimmermann, D.R.; María Dours-Zimmermann, T. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008, 130, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Kwok, J.C.F. The Extracellular Matrix in the Nervous System: The Good and the Bad Aspects. In Composition and Function of the Extracellular Matrix in the Human Body; IntechOpen: London, UK, 2016. [Google Scholar]
- Frischknecht, R.; Gundelfinger, E.D. The brain’s extracellular matrix and its role in synaptic plasticity. Adv. Exp. Med. Biol. 2012, 970, 153–171. [Google Scholar] [PubMed]
- Franco, S.J.; Müller, U. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Dev. Neurobiol. 2011, 71, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Franze, K.; Janmey, P.A.; Guck, J. Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 2013, 15, 227–251. [Google Scholar] [CrossRef]
- Morwood, S.R.; Nicholson, L.B. Modulation of the immune response by extracellular matrix proteins. Arch. Immunol. Ther. Exp. 2006, 54, 367–374. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Y.; Lewis, M.; Hsieh, J.C.; Reing, J.; Chaillet, J.R.; Howell, C.Y.; Melhem, M.; Inoue, S.; Kuszak, J.R.; et al. Neurologic Defects and Selective Disruption of Basement Membranes in Mice Lacking Entactin-1/Nidogen-1. Lab. Investig. 2002, 82, 1617–1630. [Google Scholar] [CrossRef]
- Morris, A.W.J.; Sharp, M.M.; Albargothy, A.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef]
- Timpl, R. Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 1989, 180, 487–502. [Google Scholar] [CrossRef]
- Giamanco, K.A.; Morawski, M.; Matthews, R.T. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience 2010, 170, 1314–1327. [Google Scholar] [CrossRef]
- Härtig, W.; Mages, B.; Aleithe, S.; Nitzsche, B.; Altmann, S.; Barthel, H.; Krueger, M.; Michalski, D.; Pantazopoulos, H.; Fox, M. Damaged Neocortical Perineuronal Nets Due to Experimental Focal Cerebral Ischemia in Mice, Rats and Sheep. Front. Integr. Neurosci. 2017, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Brückner, M.K.; Riederer, P.; Brückner, G.; Arendt, T. Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U. Brain matrix: Structure, turnover and necessity. Biochem. Soc. Trans. 2007, 35, 656–660. [Google Scholar] [CrossRef]
- Barone, E.; Reagan, L.; Lloret, A.; Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell. Physiol. 2018, 315, 343–356. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Zanier, E.R.; Negri, M.; Lindskog, M.; González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.B.; ZhuGe, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial Scar Formation Occurs in the Human Brain after Ischemic Stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurother 2016, 13, 685–701. [Google Scholar] [CrossRef]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Progr. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef]
- Lynch, M.A. The Multifaceted Profile of Activated Microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef]
- Nakajima, K.; Kohsaka, S. Microglia: Neuroprotective and neurotrophic cells in the central nervous system. Cardiovasc. Hematol. Disord. Drug Targets. 2004, 4, 65–84. [Google Scholar] [CrossRef]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia Actively Regulate the Number of Functional Synapses. PLoS ONE 2013, 8, 56293. [Google Scholar] [CrossRef]
- Fogdell-Hahn, A.; Institutet, K.; Hua Su, S.; Yenari, M.A.; Zhang, J.H.; Zhang, J.; Xu, S.; Lu, J.; Shao, A. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar]
- Shi, Y.; Yamada, K.; Liddelow, S.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Mellai, M.; Bovio, E.; Bisogno, I.; Casalone, C.; Annovazzi, L. Glioblastoma niches: From the concept to the phenotypical reality. Neurol. Sci. 2018, 39, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Laskaris, L.E.; Biase, M.A.D.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C.; Laskaris, L.; Biase, M.D.; et al. Microglial activation and progressive brain changes in schizophrenia. Br. J. Pharmacol. 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Dewar, D.; Underhill, M.; Goldberg, P. Oligodendrocytes and Ischemic Brain Injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef]
- Zhang, R.; Chopp, M.; Zhang, Z.G.; Cellerino, A.; Yip, H.K.; Yang, S. Oligodendrogenesis after cerebral ischemia. Front. Cell. Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef]
- Plemel, J.R.; Keough, M.B.; Duncan, G.J.; Sparling, J.S.; Yong, V.W.; Stys, P.K.; Tetzlaff, W. Remyelination after spinal cord injury: Is it a target for repair? Prog. Neurobiol. 2014, 117, 54–72. [Google Scholar] [CrossRef] [PubMed]
- El Waly, B.; Macchi, M.; Cayre, M.; Durbec, P.; De Chevigny, A.; Represa, A.; Dimou, L. Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 2014, 8, 145. [Google Scholar] [CrossRef]
- Benarroch, E.E. Extracellular matrix in the CNS. Neurology 2015, 85, 1417–1427. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Gonzalez, A.L. Stroke Repair via Biomimicry of the Subventricular Zone. Front. Mater. 2018, 5, 15. [Google Scholar] [CrossRef]
- Zhang, H.; Uchimura, K.; Kadomatsu, K. Brain keratan sulfate and glial scar formation. Ann. N. Y. Acad. 2006, 1086, 81–90. [Google Scholar] [CrossRef]
- Soleman, S.; Filippov, M.A.; Dityatev, A.; Fawcett, J.W. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013, 253, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; French-Constant, C.; Edgar, J.M.; Smith, K.J. Neuroprotection and repair in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 624–634. [Google Scholar] [CrossRef]
- Chun, S.J.; Rasband, M.N.; Sidman, R.L.; Habib, A.A.; Vartanian, T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J. Cell Biol. 2003, 163, 397–408. [Google Scholar] [CrossRef]
- Van Horssen, J.; Dijkstra, C.D.; De Vries, H.E. The extracellular matrix in multiple sclerosis pathology. J. Neurochem. 2007, 103, 1293–1301. [Google Scholar] [CrossRef]
- Stoffels, J.M.J.; De Jonge, J.C.; Stancic, M.; Nomden, A.; Van Strien, M.E.; Ma, D.; Išková, Z.S.; Maier, O.; French-Constant, C.; Franklin, R.J.M.; et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 2013, 136, 116–131. [Google Scholar] [CrossRef]
- Back, S.A.; Tuohy, T.M.F.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef]
- Lau, L.W.; Keough, M.B.; Haylock-Jacobs, S.; Cua, R.; Sloka, S.; Stirling, D.P.; Rivest, S.; Wee Yong, V. Chondroitin Sulfate Proteoglycans in Demyelinated Lesions Impair Remyelination. Ann. Neurol. 2012, 72, 419–432. [Google Scholar] [CrossRef]
- Mcrae, P.A.; Baranov, E.; Rogers, S.L.; Porter, B.E. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur. J. Neurosci. 2012, 36, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kwak, H.J.; Lee, S.H. Role of hyaluronan in glioma invasion. Cell Adhes. Migr. 2008, 2, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.S.; Huang, P.H. The Pathobiology of Collagens in Glioma. Mol. Cancer Res. 2013, 11, 1129–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Kanda, N.; Ericsson, J.E.; Karnstrom, L.; Mattsson, B.; Parkin, D.M.; Stiller, C.A.; Draper, G.J.; Bieber, C.A.; Terracini, B.; et al. Extracranial primitive neuroectodermal tumours. The Memorial Sloan-Kettering Cancer Center experience. Cancer 1991, 67, 1825–1829. [Google Scholar]
- Xiong, A.; Kundu, S.; Forsberg-Nilsson, K.; Forsberg-Nilsson, C.K. Heparan sulfate in the regulation of neural differentiation and glioma development. FEBS J. 2014, 281, 4993–5008. [Google Scholar] [CrossRef]
- Ponz-Sarvise, M.; Alberto, P.M.; Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro Models of Neurodegenerative Diseases. Front. Cell. Dev. Biol. 2020, 8, 328. [Google Scholar]
- Colquitt, R.B.; Colquhoun, D.A.; Thiele, R.H. In silico modelling of physiologic systems. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 499–510. [Google Scholar] [CrossRef]
- Destexhe, A. In Silico, Computer Simulations from Neurons up to the Whole Brain. Eneuro 2021, 8, ENEURO.0124-21.2021. [Google Scholar] [CrossRef]
- Kolenda, T.; Kapałczyńska, M.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. State of the art paper 2D and 3D cell cultures-a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef]
- Carter, M.; Shieh, J. Chapter 14—Cell Culture Techniques. In Guide to Research Techniques in Neuroscience, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 295–310. [Google Scholar]
- Abrahamsson, E.S.; Brewster, B.; Brouwers, M.; Butler, J.; Carlert, J.; Dickinson, S.A.; Dressman, P.A.; Holm, J.; Klein, R.; Mann, S.; et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2013, 16, 342–366. [Google Scholar]
- Huszthy, P.C.; Daphu, I.; Niclou, S.P.; Stieber, D.; Nigro, J.M.; Sakariassen, P.Ø.; Miletic, H.; Thorsen, F.; Bjerkvig, R. In vivo models of primary brain tumors: Pitfalls and perspectives. Neuro Oncol. 2012, 14, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Daphu, I.; Sundstrøm, T.; Horn, S.; Huszthy, P.C.; Niclou, S.P.; Sakariassen, P.Ø.; Immervoll, H.; Miletic, H.; Bjerkvig, R.; Thorsen, F. In vivo animal models for studying brain metastasis: Value and limitations. Clin. Exp. Metastasis. 2013, 30, 695–710. [Google Scholar] [CrossRef]
- Lorrio, S.; Gómez-Rangel, V.; Negredo, P.; Egea, J.; Leon, R.; Romero, A.; Dal-Cim, T.; Villarroya, M.; Rodriguez-Franco, M.I.; Conde, S.; et al. Novel multitarget ligand ITH33/IQM9.21 provides neuroprotection in in vitro and in vivo models related to brain ischemia. Neuropharmacology 2013, 67, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Shuler, M.L.; Hickman, J.J. Toward in vitro models of brain structure and function. Proc. Natl. Acad. Sci. USA 2014, 111, 13682–13683. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 20 October 2010).
- Morrison, B.; Cater, H.L.; Benham, C.D.; Sundstrom, L.E. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J. Neurosci. Methods 2006, 150, 192–201. [Google Scholar] [CrossRef]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in vitro modeling of the central nervous system. Progr. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef]
- Agostinho Machado-Neto, J.; Lima, K.; Marconi Roversi, F.; Suchorska, W.M.; Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell. Dev. Biol. 2021, 9, 711381. [Google Scholar]
- Ferrari, E.; Cardinale, A.; Picconi, B.; Gardoni, F. From cell lines to pluripotent stem cells for modelling Parkinson’s Disease. J. Neurosci. Methods. 2020, 340, 108741. [Google Scholar] [CrossRef]
- Kovalevich, J.; Abstract, D.L. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [PubMed]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Magalingam, K.B.; Radhakrishnan, A.K.; Somanath, S.D.; Md, S.; Haleagrahara, N. Influence of serum concentration in retinoic acid and phorbol ester induced differentiation of SH-SY5Y human neuroblastoma cell line. Mol. Biol. Rep. 2020, 47, 8775–8788. [Google Scholar] [CrossRef]
- Scott, I.G.; Akerman, K.E.O.; Heikkila, J.E.; Kaila, K.; Andersson, L.C. Development of a Neural Phenotype in Differentiating Ganglion Cell-Derived Human Neuroblastoma Cells. J. Cell. Physiol. 1986, 128, 128285–128292. [Google Scholar] [CrossRef]
- Kume, T.; Kawato, Y.; Osakada, F.; Izumi, Y.; Katsuki, H.; Nakagawa, T.; Kaneko, S.; Niidome, T.; Takada-Takatori, Y.; Akaike, A. Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neurosci. Lett. 2008, 443, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sarkanen, J.-R.; Nykky, J.; Siikanen, J.; Selinummi, J.; Ylikomi, T.; Jalonen, T.O. Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J. Neurochem. 2007, 102, 1941–1952. [Google Scholar] [CrossRef]
- Recio-Pinto, E.; Ishii, D.N. Effects of Insulin, Insulin-like Growth Factor-II and Nerve Growth Factor on Neurite Outgrowth in Cultured Human Neuroblastoma Cells. Brain Res. 1984, 302, 323–334. [Google Scholar] [CrossRef]
- Kovalevich, J.; Santerre, M.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. In Neuronal Cell Culture. Methods in Molecular Biology; Amini, S., White, M.K., Eds.; Humana: New York, NY, USA, 2021; Volume 2311. [Google Scholar]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Liu, Y.; Eaton, E.D.; Wills, T.E.; McCann, S.K.; Antonic, A.; Howells, D.W. Human Ischaemic Cascade Studies Using SH-SY5Y Cells: A Systematic Review and Meta-Analysis. Transl. Stroke Res. 2018, 9, 564–574. [Google Scholar] [CrossRef]
- Laudati, G.; Mascolo, L.; Guida, N.; Sirabella, R.; Pizzorusso, V.; Bruzzaniti, S.; Serani, A.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology 2019, 71, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Malagelada Grau, C.; Greene, L.A. Use of PC12 Cells and Rat Superior Cervical Ganglion Sympathetic Neurons as Models for Neuroprotective Assays Relevant to Parkinson’s Disease. Methods Mol. Biol. 2012, 846, 201–211. [Google Scholar]
- Westerink, R.H.S.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, X.; Yang, C.; Chen, L.; Chen, Z. Resolvin D1 Attenuates Mpp+-Induced Parkinson Disease via Inhibiting Inflammation in PC12 Cells. Med. Sci. Monit. 2017, 23, 2684–2691. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.; Huh, E.; Oh, M.S.; Kim, Y.S. Neuroprotection against 6-OHDA toxicity in PC12 cells and mice through the Nrf2 pathway by a sesquiterpenoid from Tussilago farfara. Redox Biol. 2018, 18, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rick, C.E.; Ebert, A.; Virag, T.; Bohn, M.C.; Surmeier, D.J. Differentiated Dopaminergic MN9D Cells Only Partially Recapitulate the Electrophysiological Properties of Midbrain Dopaminergic Neurons. Dev. Neurosci. 2006, 28, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Joannides, A.J.; Fiore-Hérich, C.; Hériché, H.; Battersby, A.A.; Athauda-Arachchi, P.; Bouhon, I.A.; Williams, L.; Westmore, K.; Kemp, P.J.; Compston, A.; et al. A Scaleable and Defined System for Generating Neural Stem Cells from Human Embryonic Stem Cells. Stem. Cells 2007, 25, 731–737. [Google Scholar] [CrossRef]
- Hong, S.G.; Winkler, T.; Wu, C.; Guo, V.; Pittaluga, S.; Nicolae, A.; Donahue, R.E.; Metzger, M.E.; Price, S.D.; Uchida, N.; et al. Resource Path to the Clinic: Assessment of iPSC-Based Cell Therapies In Vivo in a Nonhuman Primate Model. Cell Rep. 2014, 7, 1298–1309. [Google Scholar] [CrossRef]
- Hamazaki, T.; Rouby, N.E.L.; Fredette, N.C.; Santostefano, K.E.; Terada, N. Concise Review: Induced Pluripotent Stem Cell Research in the Era of Precision Medicine. Stem Cells 2017, 35, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Nemec, S.; Roohani, I. iPSC Bioprinting: Where are We at? Materials 2019, 12, 2453. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Rao, M.S. A Review of the Methods for Human iPSC Derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar] [PubMed]
- Bessis, N.; Garciacozar, F.J.; Boissier, M.C. Immune responses to gene therapy vectors: Influence on vector function and effector mechanisms. Gene Ther. Suppl. 2004, 11, S10–S17. [Google Scholar] [CrossRef]
- Steward, M.M.; Sridhar, A.; Meyer, J.S.; Steward, M.M.; Sridhar, Á.A.; Meyer, J.S. Neural Regeneration production of mature neuronal cell types from a patient-specific somatic. Curr. Top. Microbiol. Immunol. 2013, 367, 163–191. [Google Scholar]
- Inoue, H.; Nagata, N.; Kurokawa, H.; Yamanaka, S. IPS cells: A game changer for future medicine. EMBO J. 2014, 33, 409–417. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Krencik, R.; Zhang, S.C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc. 2011, 6, 1710–1717. [Google Scholar] [CrossRef]
- Roybon, L.; Lamas, N.J.; Garcia-Diaz, A.; Yang, E.J.; Sattler, R.; Jackson-Lewis, V.; Kim, Y.A.; Kachel, C.A.; Rothstein, J.D.; Przedborski, S.; et al. Human Stem Cell-Derived Spinal Cord Astrocytes with Defined Mature or Reactive Phenotypes. Cell Rep. 2013, 4, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Brownjohn, P.W.; Smith, J.; Solanki, R.; Lohmann, E.; Houlden, H.; Hardy, J.; Dietmann, S.; Livesey, F.J. Functional Studies of Missense TREM2 Mutations in Human Stem Cell-Derived Microglia. Stem Cell Rep. 2018, 10, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Xie, M.; Laurent, T.; Ding, S. Progress in the Reprogramming of Somatic Cells. Circ. Res. 2013, 112, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, D.R.; Takizawa, T.; Li, X.; Brüstle, O.; Flitsch, L.J.; Laupman, K.E. Transcription Factor-Based Fate Specification and Forward Programming for Neural Regeneration. Front. Cell. Neurosci. 2020, 14, 121. [Google Scholar]
- Fennema, E.; Rivron, N.; Rouwkema, J.; Van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, A.X.; An, J.; Kai Chua, C.; Chew, Y. 3D neural tissue models: From spheroids to bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef]
- Jorfi, M.; D’avanzo, C.; Tanzi, R.E.; Kim, D.Y.; Irimia, D. Human Neurospheroid Arrays for In Vitro Studies of Alzheimer’s Disease. Sci. Rep. 2018, 8, 2450. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Messina, A.; Morelli, S.; Forgacs, G.; Barbieri, G.; Drioli, E.; De Bartolo, L. Self-assembly of tissue spheroids on polymeric membranes. J. Tissue Eng. Regen. Med. 2017, 11, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wong, H.L.; Tian, F.R.; Huang, Y.D.; Xu, J.; Yang, J.J.; Chen, P.P.; Fan, Z.L.; Lu, C.T.; Zhao, Y.Z. Enhanced neuroprotection with decellularized brain extracellular matrix containing bFGF after intracerebral transplantation in Parkinson’s disease rat model. Int. J. Pharm. 2017, 517, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Song, L.; Madinya, J.; Ma, T.; Li, Y.; Song, M.L.; Madinya, M.J. Derivation of Cortical Spheroids from Human Induced Pluripotent Stem Cells in a Suspension Bioreactor Running title: Bioreactor-derived Cortical Spheroids from Human Stem Cells. Tissue Eng. Part A 2018, 24, 418–431. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2016, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Chen, H.I.; Song, H.; Ming, G.-L. Applications of Human Brain Organoids to Clinical Problems. Dev. Dyn. 2019, 248, 53–64. [Google Scholar] [CrossRef]
- Kelava, I.; Lancaster, M.A. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 2016, 420, 199–209. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G.-L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Bhaduri, A.; Andrews, M.G.; Leon, W.M.; Jung, D.; Shin, D.; Allen, D.; Jung, D.; Schmunk, G.; Haeussler, M.; Salma, J.; et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 1962, 578, 142–148. [Google Scholar] [CrossRef]
- Jorfi, M.; Avanzo, C.D.; Kim, D.Y.; Irimia, D. Three-Dimensional Models of the Human Brain Development and Diseases. Adv. Healthc. Mater. 2017, 7, 1700723. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, B.A.; Brekke, J.H.; Vegoe, A.L.; Ulrich, C.B.; Haider, K.T.; Subramaniam, S.; Venhuizen, S.L.; Eide, C.R.; Orchard, P.J.; Chen, W.; et al. Rapid Induction of Cerebral Organoids from Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl. Med. 2016, 5, 970–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelkamp, H.H.T.; Van Der Meer, A.D.; Hummel, J.M.; Stamatialis, D.F.; Mummery, C.L.; Passier, R.; Ijzerman, M.J. Organs-on-Chips in Drug Development: The Importance of Involving Stakeholders in Early Health Technology Assessment. Appl. Vitr. Toxicol. 2016, 2, 74–81. [Google Scholar] [CrossRef]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, A.D.; Van den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr Biol. 2012, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.A.; Bosak, A.; Hogberg, H.T.; Hartung, T.; Moore, M. Advances in 3D neuronal microphysiological systems: Towards a functional nervous system on a chip. Vitr. Cell Dev. Biol. Anim. 2021, 57, 191–206. [Google Scholar] [CrossRef]
- Pamies, D.; Hartung, T.; Hogberg, H.T. Experimental Biology and Medicine Biological and medical applications of a brain-on-a-chip. Exp. Biol. Med. 2014, 239, 1096–1107. [Google Scholar] [CrossRef]
- Oleaga, C.; Bernabini, C.; Smith, A.S.T.; Srinivasan, B.; Jackson, M.; Mclamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N.; et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef]
- Bang, S.; Jeong, S.; Choi, N. Brain-on-a-chip: A history of development and future perspective. Biomicrofluidics 2019, 13, 51301. [Google Scholar] [CrossRef]
- Park, J.; Kyeong Lee, B.; Seok Jeong, G.; Keun Hyun, J.; Lee, J.C.; Lee, S.-H. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip. 2014, 15, 141–150. [Google Scholar] [CrossRef]
- Lu, X.; Kim-Han, J.S.; O’malley, K.L.; Sakiyama-Elbert, S.E. A microdevice platform for visualizing mitochondrial transport in aligned dopaminergic axons. J. Neurosci. Methods. 2012, 209, 35–39. [Google Scholar] [CrossRef]
- Miccoli, B.; Braeken, D.; Li, Y.E. Brain-on-a-chip Devices for Drug Screening and Disease Modeling Applications. Curr. Pharm. Des. 2018, 24, 5419–5436. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, S.-R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Li Jeon, N. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Morrill, C.; Huang, Y. 3D tissue engineering, an emerging technique for pharmaceutical research. Acta Pharm. Sin. B. 2018, 8, 756–766. [Google Scholar] [CrossRef]
- Breuls, R.G.M.; Jiya, T.U.; Smit, T.H. Scaffold Stiffness Influences Cell Behavior: Opportunities for Skeletal Tissue Engineering. Open J. Orthop. 2008, 2, 103–109. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the blood–brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2008, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Ogunshola, O. In Vitro Modeling of the Blood-Brain Barrier: Simplicity Versus Complexity. Curr. Pharm. Des. 2012, 17, 2755–2761. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Mattei, G.; Sartori, S.; Caddeo, S.; Boffito, M. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar]
- Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D Organ Models-Revolution in Pharmacological Research? Pharmacol. Res. 2018, 139, 446–451. [Google Scholar]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Visai, L.; Kerlero De Rosbo, N.; Raimondi, I.; Izzo, L.; Tunesi, M.; Comar, M.; Albani, D.; Giordano, C. Organ-On-A-Chip in vitro Models of the Brain and the Blood-Brain Barrier and Their Value to Study the Microbiota-Gut-Brain Axis in Neurodegeneration. Front. Bioeng. Biotechnol. 2020, 1, 435. [Google Scholar]
- Orcheston-Findlay, L.; Bax, S.; Utama, R.; Engel, M.; Govender, D.; O’neill, G. Molecular Sciences Advanced Spheroid, Tumouroid and 3D Bioprinted In-Vitro Models of Adult and Paediatric Glioblastoma. Int. J. Mol. Sci. 2021, 22, 2962. [Google Scholar] [CrossRef]
- Tay, A.; Schweizer, F.E.; Di Carlo, D. Micro- and nano-technologies to probe the mechano-biology of the brain. Lab Chip. 2016, 16, 1962–1977. [Google Scholar] [CrossRef]
- Mammadov, B.; Sever, M.; Guler, M.O.; Tekinay, A. Neural differentiation on synthetic scaffold materials. Biomater. Sci. 2013, 1, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Shah, S.; Memoli, K.A.; Park, S.Y.; Hong, S.; Lee, K.-B. Controlling Differentiation of Neural Stem Cells Using Extracellular Matrix Protein Patterns. Small 2010, 6, 2509–2513. [Google Scholar] [CrossRef]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2020, 27, 1187–1230. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio. 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Dumsile Mahumane, G.; Kumar, P.; Claire Du Toit, L.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812. [Google Scholar] [CrossRef]
- Feng, Y.; Okamoto, R.J.; Namani, R.; Genin, G.M.; Bayly, P.V. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. J. Mech. Behav. Biomed. Mater. 2013, 23, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, A.; Abousleiman, Y.N.; Mapstone, T.B.; El-Amm, C.A. Dual-porosity poroviscoelasticity and quantitative hydromechanical characterization of the brain tissue with experimental hydrocephalus data. J. Theor. Biol. 2015, 384, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Keskar, V.; Marion, N.W.; Mao, J.J.; Gemeinhart, R.A. In Vitro Evaluation of Macroporous Hydrogels to Facilitate Stem Cell Infiltration, Growth, and Mineralization. Tissue Eng. Part A 2009, 15, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Stabenfeldt, S.E.; Garcia, A.J.; LaPlaca, M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res.-Part A 2006, 77, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.T.; Tian, W.M.; Yu, X.; Cui, F.Z.; Hou, S.P.; Xu, Q.Y.; Lee, I.S. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed. Mater. 2007, 2, S142–S146. [Google Scholar] [CrossRef]
- Farrukh, A.; Ortega, F.; Fan, W.; Marichal, N.; Paez, J.I.; Berninger, B.; Del Campo, A.; Salierno, M.J. Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis. Stem Cell Rep. 2017, 9, 1432–1440. [Google Scholar] [CrossRef]
- Karpiak, J.V.; Ner, Y.; Almutairi, A. Density gradient multilayer polymerization for creating complex tissue. Adv. Mater. 2012, 24, 1466–1470. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Hsueh, C.H. Neuronal production from induced pluripotent stem cells in self-assembled collagen-hyaluronic acid-alginate microgel scaffolds with grafted GRGDSP/Ln5-P4. Mater. Sci. Eng. C 2017, 76, 760–774. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef]
- Pietrucha, K.; Zychowicz, M.; Podobinska, M.; Buzanska, L. Functional properties of different collagen scaffolds to create a biomimetic niche for neurally committed human induced pluripotent stem cells (iPSC). Folia Neuropathol. 2017, 55, 110–123. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, X.; Yao, D.; Peng, G.; Liu, H.; Fan, Y. Enhancing neural differentiation of induced pluripotent stem cells by conductive graphene/silk fibroin films. J. Biomed. Mater. Res. A. 2018, 106, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- KarbalaeiMahdi, A.; Shahrousvand, M.; Javadi, H.R.; Ghollasi, M.; Norouz, F.; Kamali, M.; Salimi, A. Neural differentiation of human induced pluripotent stem cells on polycaprolactone/gelatin bi-electrospun nanofibers. Mater. Sci. Eng. C 2017, 78, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chen, C.W. Neuroregeneration of Induced Pluripotent Stem Cells in Polyacrylamide-Chitosan Inverted Colloidal Crystal Scaffolds with Poly(lactide-co-glycolide) Nanoparticles and Transactivator of Transcription von Hippel-Lindau Peptide. Tissue Eng. Part A 2017, 23, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Hynes, S.R.; Rauch, M.F.; Bertram, J.P.; Lavik, E.B. A library of tunable poly(ethylene glycol)/poly(L-lysine) hydrogels to investigate the material cues that influence neural stem cell differentiation. J. Biomed. Mater. Res. A 2009, 89, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, Z.; Park, I.H.; Zhu, Y.; Patel, S.; Daley, G.Q.; Li, S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials 2011, 32, 5023–5032. [Google Scholar] [CrossRef]
- Sirkkunan, D.; Pingguan-Murphy, B.; Muhamad, F. Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons. Gels 2021, 8, 25. [Google Scholar] [CrossRef]
- Horne, M.K.; Nisbet, D.R.; Forsythe, J.S.; Parish, C.L. Three-Dimensional Nanofibrous Scaffolds Incorporating Immobilized BDNF Promote Proliferation and Differentiation of Cortical Neural Stem Cells. Stem Cells Dev. 2010, 19, 843–852. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface. 2020, 17, 20190505. [Google Scholar] [CrossRef]
- Jurga, M.; Dainiak, B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A.; et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef]
- Aurand, E.R.; Wagner, J.; Lanning, C.; Bjugstad, K.B. Functional Biomaterials Building Biocompatible Hydrogels for Tissue Engineering of the Brain and Spinal Cord. J. Funct. Biomater. 2012, 3, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- de la Vega, L.; Lee, C.; Sharma, R.; Amereh, M.; Willerth, S.M. 3D bioprinting models of neural tissues: The current state of the field and future directions. Brain Res. Bull. 2019, 150, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T. Scaffold-Based or Scaffold-Free Bioprinting: Competing or Complementing Approaches? J. Nanotechnol. Eng. Med. 2015, 6, 024701. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Salaris, F.; Rosa, A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: Challenges and opportunities. Brain Res. 2019, 1723, 146393. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized scaffolds for tissue engineering: Current status and future perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- De Waele, J.; Reekmans, K.; Daans, J.; Goossens, H.; Berneman, Z.; Ponsaerts, P. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 2015, 41, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Tottey, S.; Slivka, P.F.; Badylak, S.F. Effects of Biologic Scaffolds on Human Stem Cells and Implications for CNS Tissue Engineering. Tissue Eng. Part A 2014, 20, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Huh, K.M.; Kang, S.W. Applications of Biomaterials in 3D Cell Culture and Contributions of 3D Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Biol. Macromol. 2021, 22, 2491. [Google Scholar] [CrossRef]
- Smith, L.A.; Liu, X.; Ma, P.X. Tissue engineering with nano-fibrous scaffolds. Soft Matter. 2008, 4, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Springer: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- Kuo, Y.C.; Chung, C.Y. TATVHL peptide-grafted alginate/poly(γ-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials 2012, 33, 8955–8966. [Google Scholar] [CrossRef]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef]
- Kanno, H.; Nakano, S.; Kubo, A.; Mimura, T.; Tajima, N.; Sugimoto, N. Neuronal differentiation of neural progenitor cells by intracellular delivery of synthetic oligopeptide derived from Von Hippel-Lindau protein. Protein Pept. Lett. 2009, 16, 1291–1296. [Google Scholar] [CrossRef]
- João, C.F.C.; Vasconcelos, J.M.; Silva, J.C.; Borges, J.P. An overview of inverted colloidal crystal systems for tissue engineering. Tissue Eng. Part B Rev. 2015, 20, 437–454. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Ku, I.-N. Cartilage Regeneration by Novel Polyethylene Oxide/Chitin/ Chitosan Scaffolds. Biomacromolecules 2008, 9, 2662–2669. [Google Scholar] [CrossRef]
- Lee, J.; Shanbhag, S.; Kotov, N.A. Inverted colloidal crystals as three-dimensional microenvironments for cellular co-cultures. J. Mater. Chem. 2006, 16, 3558–3564. [Google Scholar] [CrossRef]
- Shanbhag, S.; Woo Lee, J.; Kotov, N. Diffusion in three-dimensionally ordered scaffolds with inverted colloidal crystal geometry. Biomaterials 2005, 26, 5581–5585. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chen, X.; Gong, S.; Yu, P.; Yau, S.; Su, Z.; Zhou, L.; Yu, J.; Pan, G.; Shi, L. Characteristic analyses of a neural differentiation model from iPSC-derived neuron according to morphology, physiology, and global gene expression pattern. Sci. Rep. 2017, 7, 12233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Herai, R.H.; Anh, V.; Huu, N.; Wen, J.H.; et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef]
- Weaving, L.S.; Ellaway, C.J.; Gécz, J. Rett syndrome: Clinical review and genetic update. J. Med. Genet. 2005, 42, 1–7. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.N.; Cervasio, D.A.; Laughlin, S.T. Visualizing the brain’s astrocytes. Methods Enzymol. 2019, 622, 129–151. [Google Scholar] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano. 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Pietrucha, K.; Marzec, E.; Kudzin, M. Pore structure and dielectric behaviour of the 3D collagen-DAC scaffolds designed for nerve tissue repair. Int. J. Biol. Macromol. 2016, 92, 1298–1306. [Google Scholar] [CrossRef]
- Pietrucha, K. Physicochemical properties of 3D collagen-CS scaffolds for potential use in neural tissue engineering. Int. J. Biol. Macromol. 2015, 80, 732–739. [Google Scholar] [CrossRef]
- Oh, J.E.; Jang, D.H.; Kim, H.; Kang, H.K.; Chung, C.P.; Park, W.H.; Min, B.M. α3β1 integrin promotes cell survival via multiple interactions between 14-3-3 isoforms and proapoptotic proteins. Exp. Cell Res. 2009, 315, 3187–3200. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Malda, J.; Klein, T.J.; Upton, Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007, 13, 2153–2162. [Google Scholar] [CrossRef]
- Murphy, A.R.; Ghobrial, I.; Jamshidi, P.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Tailored emulsion-templated porous polymer scaffolds for iPSC-derived human neural precursor cell culture. Polym. Chem. 2017, 8, 6617–6627. [Google Scholar] [CrossRef]
- Kuang, Y.L.; Munoz, A.; Nalula, G.; Santostefano, K.E.; Sanghez, V.; Sanchez, G.; Terada, N.; Mattis, A.N.; Iacovino, M.; Iribarren, C.; et al. Evaluation of commonlyused ectoderm markers in iPSC trilineage differentiation. Stem Cell Res. 2019, 37, 101434. [Google Scholar] [CrossRef] [PubMed]
- Nardo, T.; Carmagnola, I.; Ruini, F.; Caddeo, S.; Calzone, S.; Chiono, V.; Ciardelli, G. Chapter 65—Synthetic Biomaterial for Regenerative Medicine Applications; Orlando, G., Remuzzi, G., Williams, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 901–921. [Google Scholar]
- Hoven, V.P.; Tangpasuthadol, V.; Angkitpaiboon, Y.; Vallapa, N.; Kiatkamjornwong, S. Surface-charged chitosan: Preparation and protein adsorption. Ind. Eng. Chem. Res. 2021, 60, 9159–9166. [Google Scholar] [CrossRef]
- Saranya, N.; Moorthi, A.; Saravanan, S.; Devi, M.P.; Selvamurugan, N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromol. 2011, 48, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release. 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Green, M.A.; Bilston, L.E.; Sinkus, R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008, 7, 755–764. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef]
- Salaris, F.; Colosi, C.; Brighi, C.; Soloperto, A.; De Turris, V.; Benedetti, M.C.; Ghirga, S.; Rosito, M.; Di Angelantonio, S.; Rosa, A. 3D Bioprinted Human Cortical Neural Constructs Derived from Induced Pluripotent Stem Cells. J. Clin. Med. 2019, 8, 1595. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Livesey, F. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 2012, 7, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Abelseth, E.; Abelseth, L.; De La Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- De la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef]
- Georges, J.; Barthès, D.; Patterson, J.; Leuven, K.U.; Jose, B.; Granjeiro, M.; Willerth, S.M.; Sharma, R.; Smits, I.P.M.; De, L.; et al. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar]
- Gonzalez, R.; Garitaonandia, I.; Abramihina, T.; Wambua, G.K.; Ostrowska, A.; Brock, M.; Noskov, A.; Boscolo, F.S.; Craw, J.S.; Laurent, L.C.; et al. Deriving dopaminergic neurons for clinical use. A practical approach. Sci. Rep. 2013, 3, 1463. [Google Scholar] [CrossRef]
- Robinson, M.; Yau, S.-Y.; Sun, L.; Gabers, N.; Bibault, E.; Christie, B.R.; Willerth, S.M. Optimizing Differentiation Protocols for Producing Dopaminergic Neurons from Human Induced Pluripotent Stem Cells for Tissue Engineering Applications. Biomark. Insights 2015, 10, 61–70. [Google Scholar] [CrossRef]
- Cho, A.N.; Jin, Y.; Kim, S.; Kumar, S.; Shin, H.; Kang, H.C.; Cho, S.W. Aligned Brain Extracellular Matrix Promotes Differentiation and Myelination of Human-Induced Pluripotent Stem Cell-Derived Oligodendrocytes. ACS Appl. Mater. Interfaces. 2019, 11, 15344–15353. [Google Scholar] [CrossRef]
- Lin, H.; Li, Q.; Lei, Y. Three-dimensional tissues using human pluripotent stem cell spheroids as biofabrication building blocks. Biofabrication 2017, 9, 025007. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, Y.; Guo, Z.; Meng, H.; Huang, J.; Zhang, L.; Zhao, B.; Zhao, Q.; Zheng, Y.; Peng, J. Cauda Equina-Derived Extracellular Matrix for Fabrication of Nanostructured Hybrid Scaffolds Applied to Neural Tissue Engineering. Tissue Eng. Part A 2015, 21, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Forostyak, S.; Koci, Z.; Zaviskova, K.; Vackova, I.; Vyborny, K.; Sandvig, I.; Sandvig, A.; Medberry, C.J.; Badylak, S.F.; et al. Injectable Extracellular Matrix Hydrogels as Scaffolds for Spinal Cord Injury Repair. Tissue Eng. Part A 2016, 22, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Peault, B.; Woei Ng, K.; Ahmed Khan, A.; Sab, P.; Kumar Vishwakarma, S.; Bardia, A.; Lakkireddy, C.; Ameer Basha Paspala, S. Bioengineering Human Neurological Constructs Using Decellularized Meningeal Scaffolds for Application in Spinal Cord Injury. Front. Bioeng. Biotechnol. 2018, 6, 150. [Google Scholar]
- Ranjan, V.D.; Qiu, L.; Lee, J.W.L.; Chen, X.; Jang, S.E.; Chai, C.; Lim, K.L.; Tan, E.K.; Zhang, Y.; Huang, W.M.; et al. A microfiber scaffold-based 3D: In vitro human neuronal culture model of Alzheimer’s disease. Biomater. Sci. 2020, 8, 4861–4874. [Google Scholar] [CrossRef] [PubMed]
- Garrudo, F.F.F.; Nogueira, D.E.S.; Rodrigues, C.A.V.; Ferreira, F.A.; Paradiso, P.; Colaço, R.; Marques, A.C.; Cabral, J.M.S.; Morgado, J.; Linhardt, R.J.; et al. Electrical stimulation of neural-differentiating iPSCs on novel coaxial electroconductive nanofibers. Biomater. Sci. 2021, 9, 5359–5382. [Google Scholar] [CrossRef]
- Revkova, V.A.; Sidoruk, K.V.; Kalsin, V.A.; Melnikov, P.A.; Konoplyannikov, M.A.; Kotova, S.; Frolova, A.A.; Rodionov, S.A.; Smorchkov, M.M.; Kovalev, A.V.; et al. Spidroin Silk Fibers with Bioactive Motifs of Extracellular Proteins for Neural Tissue Engineering. ACS Omega 2021, 6, 15264–15273. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Serio, A.; Amdursky, N.; Besnard, C.; Stevens, M.M. Fabrication of Hemin-Doped Serum Albumin-Based Fibrous Scaffolds for Neural Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 5305–5317. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin fiber scaffolds for engineering functional cardiac tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; He, J.H.; Lu, C.J.; Gu, Q.F.; Wu, L.X.; Liu, Q.; Li, H.; Xu, Q.F.; Lu, J.M. Changing the stability of polymer-based memory devices in high conductivity state via tuning the red-ox property of Hemin. Polymer 2015, 70, 343–350. [Google Scholar] [CrossRef]
- Mohtaram, N.K.; Ko, J.; King, C.; Sun, L.; Muller, N.; Jun, M.B.G.; Willerth, S.M. Electrospun biomaterial scaffolds with varied topographies for neuronal differentiation of human-induced pluripotent stem cells. J. Biomed. Mater. Res. A 2015, 103, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Tonge, P.D.; Andrews, P.W. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation 2010, 80, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Wu, S.C.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015, 72, 1559–1576. [Google Scholar] [CrossRef]
- Hoveizi, E.; Ebrahimi-Barough, S.; Tavakol, S.; Sanamiri, K. In Vitro Differentiation of Human iPS Cells into Neural like Cells on a Biomimetic Polyurea. Mol. Neurobiol. 2016, 54, 601–607. [Google Scholar] [CrossRef]
- Bhuvanesh, G.; Nilesh, R.; Jöns, H. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar]
- Echave, M.C.; Sánchez, P.; Pedraz, J.L.; Orive, G. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017, 42, 63–74. [Google Scholar] [CrossRef]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. Part C 2014, 34, 402–409. [Google Scholar] [CrossRef]
- Cantley, W.L.; Du, C.; Lomoio, S.; Depalma, T.; Peirent, E.; Kleinknecht, D.; Hunter, M.; Tang-Schomer, M.D.; Tesco, G.; Kaplan, D.L. Functional and Sustainable 3D Human Neural Network Models from Pluripotent Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 4278–4288. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Cairns, D.M.; Dabbi, J.M.; Ramakrishnan, C.; Deisseroth, K.; Black Iii, L.D.; Santaniello, S.; Kaplan, D.L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Johnson, T.D.; Dequach, J.A.; Gaetani, R.; Ungerleider, J.; Elhag, D.; Nigam, V.; Behfar, A.; Christman, K.L. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014, 2, 735–744. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.; Katagiri, Y.; Geller, H.M. An in vitro model of reactive astrogliosis and its effect on neuronal growth. Methods Mol. Biol. 2012, 814, 327–340. [Google Scholar]
- Rouleau, N.; Cantley, W.L.; Liaudanskaya, V.; Berk, A.; Du, C.; Rusk, W.; Peirent, E.; Koester, C.; Nieland, T.J.F.; Kaplan, D.L. A Long Living Bioengineered Neural Tissue Platform to Study Neurodegeneration. Macromol. Biosci. 2020, 20, e2000004. [Google Scholar] [CrossRef]
- Bennett, A.J.; Ringach, D.L. Animal Research in Neuroscience: A Duty to Engage. Neuron 2016, 92, 653–657. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic Organ-on-a-Chip System for Disease Modeling and Drug Development. Biosensors 2022, 12, 370. [Google Scholar] [CrossRef]
- Peng, B.; Leach, J.; Zhao, Y.; Zhu, H.; Qiao, X.; Liu, W.; Wang, C. Microglia Play an Essential Role in Synapse Development and Neuron Maturation in Tissue-Engineered Neural Tissues. Front Neurosci. 2020, 14, 586452. [Google Scholar]
- Bassil, R.; Shields, K.; Granger, K.; Zein, I.; Ng, S.; Chih, B. Improved modeling of human AD with an automated culturing platform for iPSC neurons, astrocytes and microglia. Nat. Commun. 2021, 12, 5220. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, I.; Tunesi, M.; Forloni, G.; Albani, D.; Giordano, C. 3D brain tissue physiological model with co-cultured primary neurons and glial cells in hydrogels. J. Tissue Eng. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, S.M.; Davaz, S.; Wanner, D.; Milleret, V.; Ehrbar, M.; Gietl, A.; Tackenberg, C. Increased maturation of iPSC-derived neurons in a hydrogel-based 3D culture. J. Neurosci. Methods. 2021, 360, 109254. [Google Scholar] [CrossRef] [PubMed]
- Nazari, B.; Kazemi, M.; Kamyab, A.; Nazari, B.; Ebrahimi-Barough, S.; Hadjighassem, M.; Norouzi-Javidan, A.; Ai, A.; Ahmadi, A.; Ai, J. Fibrin hydrogel as a scaffold for differentiation of induced pluripotent stem cells into oligodendrocytes. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 192–200. [Google Scholar] [CrossRef]
- Patel, B.B.; Sharifi, F.; Stroud, D.P.; Montazami, R.; Hashemi, N.N.; Sakaguchi, D.S. 3D Microfibrous Scaffolds Selectively Promotes Proliferation and Glial Differentiation of Adult Neural Stem Cells: A Platform to Tune Cellular Behavior in Neural Tissue Engineering. Macromol. Biosci. 2019, 19, 1800236. [Google Scholar] [CrossRef] [PubMed]
- Flagelli, A.; Candini, O.; Frabetti, S.; Dominici, M.; Giardino, L.; Calzà, L.; Baldassarro, V.A. A Novel Three-Dimensional Culture Device Favors a Myelinating Morphology of Neural Stem Cell-Derived Oligodendrocytes. Front. Cell Dev. Biol. 2021, 9, 759982. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Bianchini, P.; Velusamy, J.; Zeng, S.; Parodi, V.; Jacchetti, E.; Osellame, R.; Cerullo, G.; Polli, D.; Raimondi, M.T. Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging. Front. Bioeng. Biotechnol. 2020, 8, 585363. [Google Scholar]
- Moysidou, C.M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Nazarov, R.; Jin, H.-J.; Kaplan, D.L. Porous 3-D Scaffolds from Regenerated Silk Fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef] [PubMed]




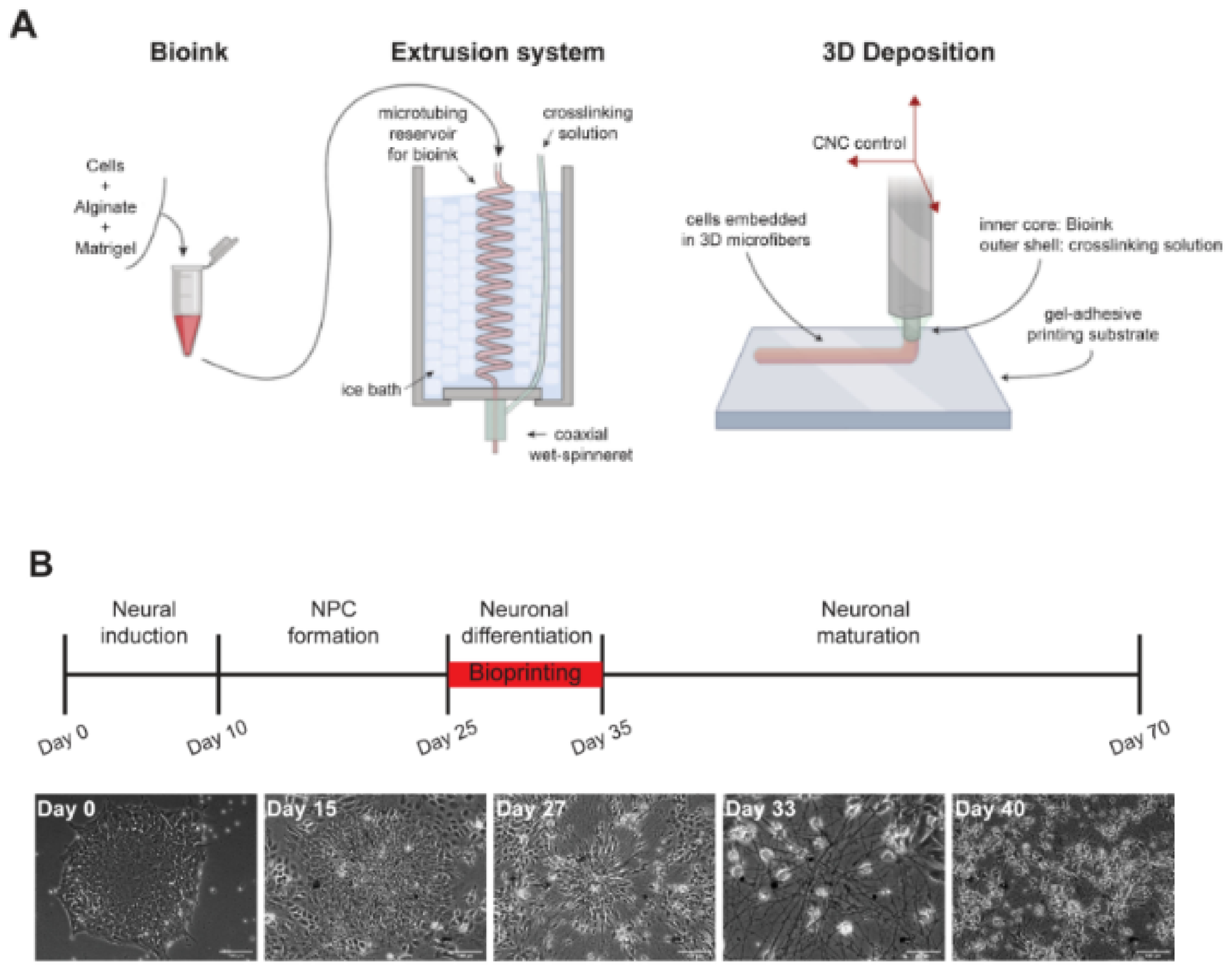
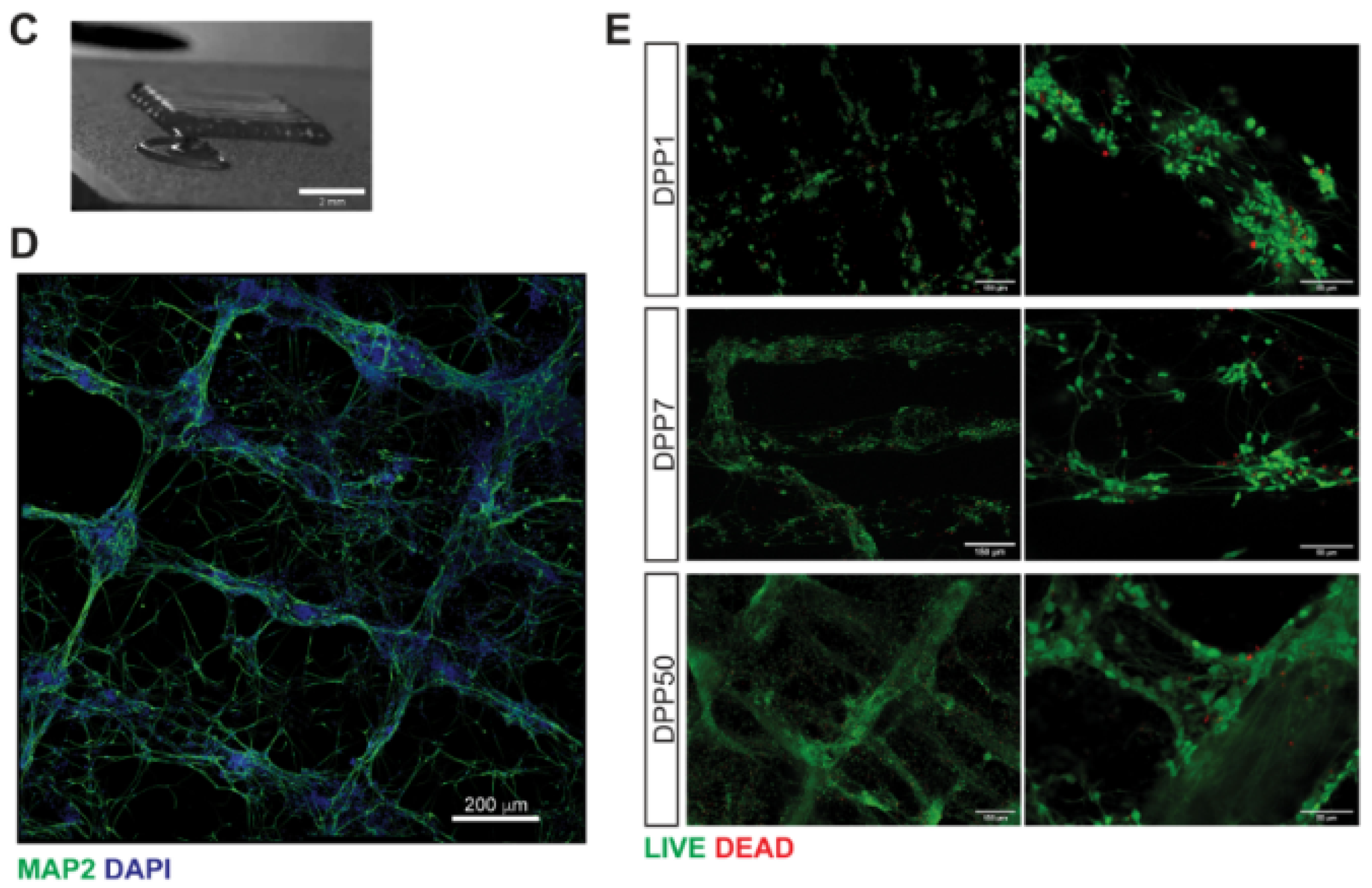

| Cell Types | Characteristics and Functions | References |
|---|---|---|
| Neurons |
| [3] |
| Astrocytes |
| [8,9,10,11] |
| Oligodendrocytes |
| [12,13,14] |
| Microglia |
| [15,16,17] |
| NG2 |
| [5] |
| Oligodendrocyte precursor cells |
| [6,7] |
| 3D Platform | Characteristics | References |
|---|---|---|
| Hydrogels | Hydrophilic networks with outstanding physical and chemical properties. Maximum flexibility and ease in modifying material characteristics. Positive influence on physical guidance and molecule incorporation for localized release. | [195,196] |
| 3D bioprinting | Bioinks loaded with cells and deposited layer-by-layer, obtained with scaffold-based or scaffold-free approaches. Cell differentiation can occur at pre-printing (1) or post-printing (2) stages. Drawbacks include: long time period needed for cell differentiation, inability to control the relative distribution, effects on cell viability. | [197,198,199,200,201] |
| Decellularized scaffolds | Obtained after removal of cellular components from tissues or organs with different chemical, biological and mechanical methods employed. Advantages include recellularization and cell remodeling due to ECM structure (low immunogenicity and biologically recognizable). | [202,203,204,205,206] |
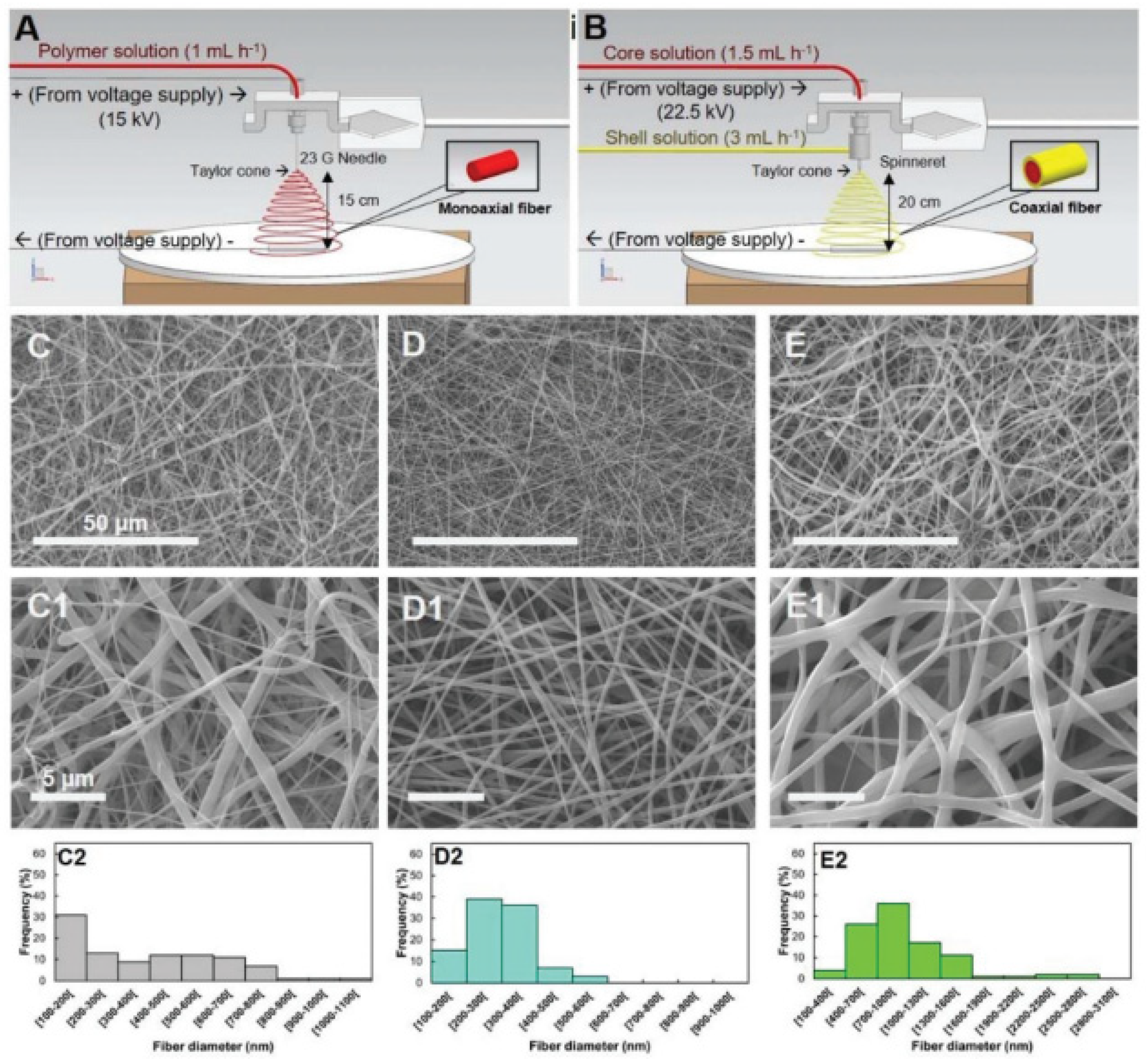
| Fibrous scaffolds | Tight control over fiber orientation, high surface-to-volume ratio, effect on cell adhesion, proliferation and differentiation. Produced with techniques such as self-assembly, template synthesis, phase separation, electrospinning. | [207,208,209] |
| Main Hydrogel-Based Brain Models | |||||
|---|---|---|---|---|---|
| Materials | Scaffold Type | Cells Used | Culture Times | Main Outputs | Ref. |
| Alginate/γ-PGA with TATVHL peptide | Inverted colloidal crystal scaffold. | iPSCs | 7 days | High cell viability (90%). iPSC differentiation into neurons (βIII tubulin expression). | [210] |
| HAMA | Density gradient multilayer polymerization technique. | iPSC-NPCs; NPC-derived neurons and astrocytes | 6 weeks | Favored neural over glial cells differentiation. Accelerated neuron maturation compared vs. 2D cultures. | [218] |
| Cathecol-functionalized HA with CNTs and PPy | Oxidative polymerization of cathecol-functionalized HA and in situ PPY polymerization and CNT incorporation. | hfNSCs and hiPSC-NPCs | 7 days | hfNSCs differentiation into neurons, astrocytes and oligodendrocytes at 5 days. hiPSC-NPCs differentiation into neurons at 7 days. | [183] |
| COL-CS; COL-DAC | (1) COL-CS: multiple freeze drying steps followed by EDC crosslinking. (2) COL-DAC: DAC preparation by cellulose oxidation, fabrication of 3D COL sponge shapes and crosslinking in a DAC-containing solution | hiPSC-NPCs | 6 days | High proliferation and viability of hiPSC-NPCs. Differentiation into neural cells, astrocytes and oligodendrocytes. | [185] |
| COLMA/HAMA/ALGMA with GRGDSP and Ln5-P4. | Photocrosslinking using a mask. | iPSCs | 3 days | iPSC neural differentiation increased to 98% after induction by NGF | [182] |
| Laminin-coated PEGDA; control TMPTA | PolyHIPE scaffolds | hiPSC-NSCs | 14 days | Upregulation of glial cell markers especially on TMPTA scaffolds; Increased spontaneous calcium activity within laminin-coated PEGDA scaffolds. | [228] |
| PAAM-CH, PLGA NPs | Inverted colloidal crystal scaffolds | iPSCs | 3 days of culture | Enhanced neural differentiation | [187] |
| Bioprinted Models of Brain Tissue | |||||
|---|---|---|---|---|---|
| Bioink Hydrogel | Bioprinting Technique | Cells Used | Culture Times | Main Outputs | Ref. |
| Alginate (5%), carboxymethyl chitosan (5%) and agarose (1.5%) | Microextrusion bioprinting | hNSCs | 10 days | Differentiation of hNSCs into neurons and glial cells post-printing. | [234] |
| As above | Microextrusion bioprinting | iPSCs | 10 days in proliferative medium; 11–20 days in a differentiation BDNF-containing medium | Neuronal and glial cells differentiation with spontaneous and bicuculline-induced calcium responses at 20 days. | [236] |
| Matrigel and alginate (1:1 weight ratio; 2% alginate concentration | Microextrusion bioprinting | iPSCs differentiated into cortical neurons | 1, 7, 50 and 70 days. | Expression of neuronal (TRB1 for mature cortical neurons) and glial (GFAP) markers, and calcium activity at 7 days. Mature cortical neurons were maintained in neuronal differentiation medium up to 70 days. | [237] |
| Fibrinogen, alginate, chitosan, calcium chloride, thrombin and genipin. | Microextrusion bioprinting and lab-on-a-printer technology | hiPSC aggregates | 41 days: at 17 days addition of RA to induce differentiation into dopaminergic neurons | Expression of Tuj1 (an early neuronal marker) at day 41 by immunostaining. | [240] |
| As above with addition of guggulsterone-loaded microspheres to promote cell differentiation. | Microfluidics-based RX1 bioprinter | hiPSC-NPCs | Up to 30 days | At 15 and 30 days: neural markers detected by immunostaining; cells expressing glial (GFAP) and oligodendrocyte markers (O4) assessed by flow cytometry. At 30 days: expression of dopaminergic markers (TUj1, NURR1, LMX1B, TH and PAX6). | [243] |
| Decellularized ECM-Based Scaffolds for Brain Modeling or Supporting Nerve/Glial Cells | |||||
|---|---|---|---|---|---|
| Substrate | Other Stimuli | Cells Used | Culture Times | Main Outputs | Ref. |
| Decellularized human brain tissue | Co-culture with mouse induced neurons (iN) | iPSC-OPCs | 14 days | iPSCs differentiated into myelin-expressing oligodendrocytes | [246] |
| Decellularized human brain tissue | Functionalized with basic FGF | PC-12 | 24 h | In vitro PD model | [247] |
| Electrospun genipin-crosslinked gelatin scaffolds with 1% rat ECM | - | MSCs | 7 days | Differentiation of MSCs towards neural cells | [248] |
| Electrospun PLGA blended with decellularized porcine cauda equina | - | Schwann cells derived from sciatic nerve | 7 days | Scaffolds favored the proliferation and the orientation of Schwann cells | [249] |
| Decellularized porcine spinal cord and urinary bladder injectable hydrogels | - | hWJ-MSCs | 7 days | Stimulation of neovascularization and axonal growth in an in vivo model of spinal cord injury | [250] |
| Decellularized human meningeal scaffolds | - | hNPCs | 21 days | Differentiation of hNPCs | [251] |
| Main TE Models of Brain Tissue Reported in the Literature | |||||
|---|---|---|---|---|---|
| Materials | Substrate | Cells Used | Culture Times | Main Outputs | Ref. |
| PLGA | Wet electrospun fibrous scaffolds | iPSC-NPCs | 7 days | AD model: neuronal differentiation with pathogenic Aβ42 and phospho-tau levels | [252] |
| Soft core layer in pyrolytic graphite sheet (PGS), combined with an electroconductive PCL-PANI layer. | Coaxial electrospun fibrous scaffolds | iPSC-NPCs | 21 days | Increased expression of neural markers (MAP2) and genes related to excitatory pathways (glutamatergic and voltage-sensitive channel genes). Downregulation of GABAergic markers. | [253] |
| Spindroin-PCL enriched with extracellular matrix peptide motifs (RGD, IKVAV and VAEIDGIEL). | Electrospun scaffolds with aligned fibers | Directly reprogrammed NPCs | Proliferation during first 3 days and differentiation during 4–14 days. | RGD promoted a lower number of neurons with longer neurites; IKVAV supported a higher number of NF200-positive neurons with shorter neurites. Obtainment of neuroglial stem cell niche with preservation of stem cells. | [254] |
| SA with hemin, laminin coating and functionalization with FGF2 | Electrospun scaffolds | iPSC-NSCs | 7 days | Neural maturation of iPSC-NSCs assessed by β-III-tubulin expression. | [255] |
| PCL + PCL-RA | Melt electrospun PCL scaffolds (loop mesh and biaxially aligned microscale topographies), coated with electrospun PCL/RA | iPSC-NPCs | 12 days | iPSC-NP differentiation into neurons, evaluated by the expression of the neuronal marker β-III-tubulin. Maximum neurite outgrowth on biaxial scaffolds. | [259] |
| Polylactic acid (PLA)/gelatin | Electrospun scaffolds | hiPSCs embryoid bodies (EBs) | 21 days using media with differentiating factors: FGF and NGF | Expression of β-III-tubulin and MAP2 iPSC suggesting differentiation into neurons. | [262] |
| PCL/Gelatin | Bi-electrospun nanofibers | hiPSCs embryoid bodies (EBs) | 14 days | GFAP, β-tubulin-III, neuron-specific enolase (NSE), MAP2 and Olig2 expression demonstrated differentiation into neurons, astrocytes and oligodendrocytes. | [186] |
| Silk fibroin porous structure with poly-L-ornithine and laminin coatings and collagen I hydrogel filler | Salt leaching | iPSCs | 8 months | Expression of MAP2, enolase-2 and β-tubulin-III demonstrated differentiation into astrocytes and neurons. | [267] |
| Silk fibroin porous scaffold with laminin coating and collagen type I, plus decellularized porcine ECM as filler | Salt leaching | hiNSCs | 7 months | Growth of mature astrocytes, downregulation of CSPGs (marker of astrogliosis after 2-month culture. | [268] |
| Silk fibroin scaffolds with a silk-free central window and filled with collagen I during cell seeding. | Salt leaching | hiPSC | 2 years | Glial marker expression in long-term cultures. Structural and functional stability for over 2 years. | [273] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarricone, G.; Carmagnola, I.; Chiono, V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. J. Funct. Biomater. 2022, 13, 146. https://doi.org/10.3390/jfb13030146
Tarricone G, Carmagnola I, Chiono V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. Journal of Functional Biomaterials. 2022; 13(3):146. https://doi.org/10.3390/jfb13030146
Chicago/Turabian StyleTarricone, Giulia, Irene Carmagnola, and Valeria Chiono. 2022. "Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges" Journal of Functional Biomaterials 13, no. 3: 146. https://doi.org/10.3390/jfb13030146





