MOFs and MOF-Derived Materials for Antibacterial Application
Abstract
1. Introduction
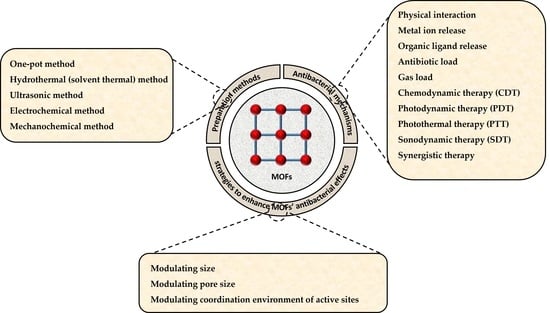
2. Preparation Methods of MOFs
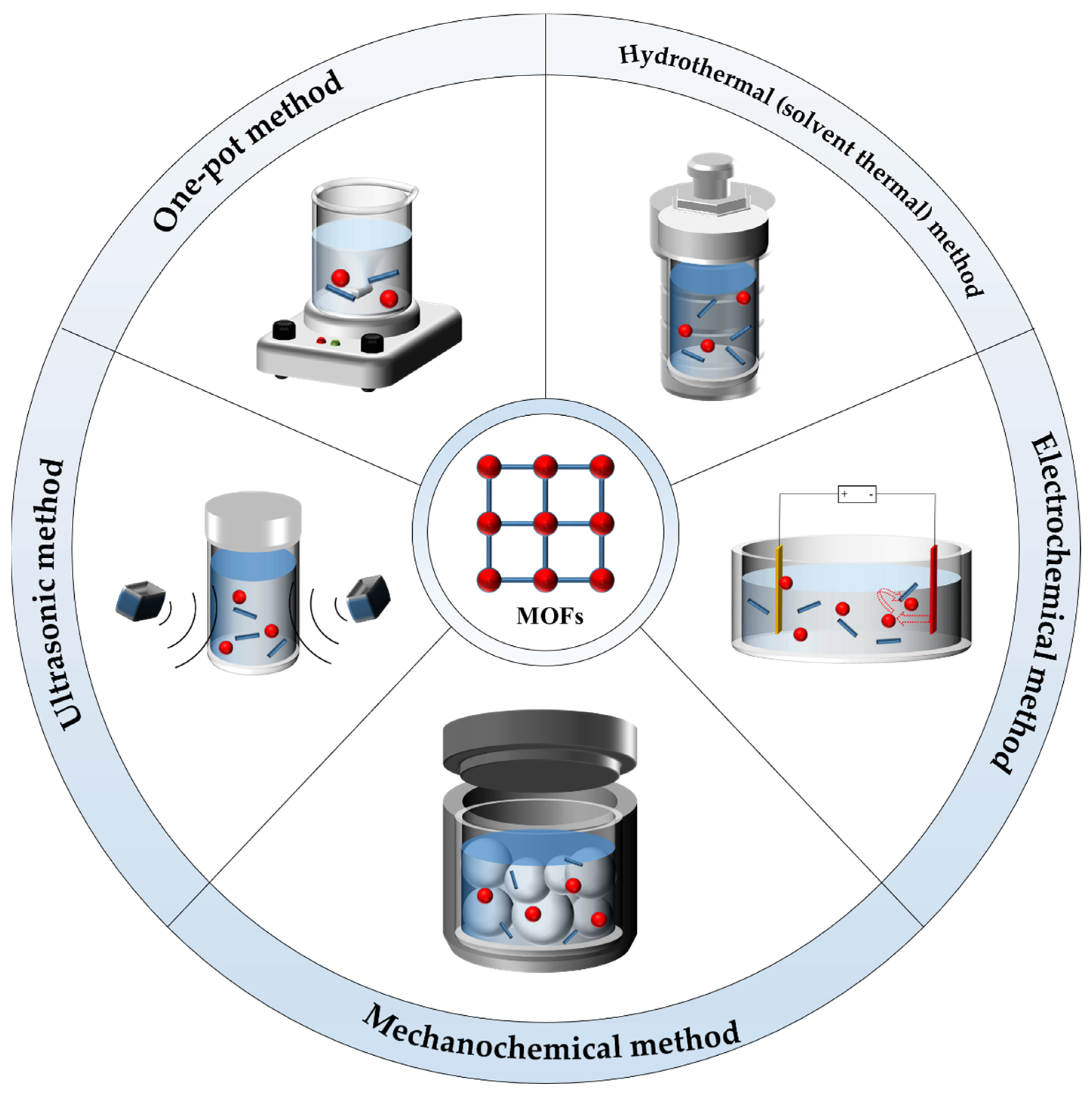
2.1. One-Pot Method
2.2. Hydrothermal (Solvent Thermal) Method
2.3. Ultrasonic Method
2.4. Electrochemical Method
2.5. Mechanochemical Method
3. Antibacterial Mechanisms of MOFs
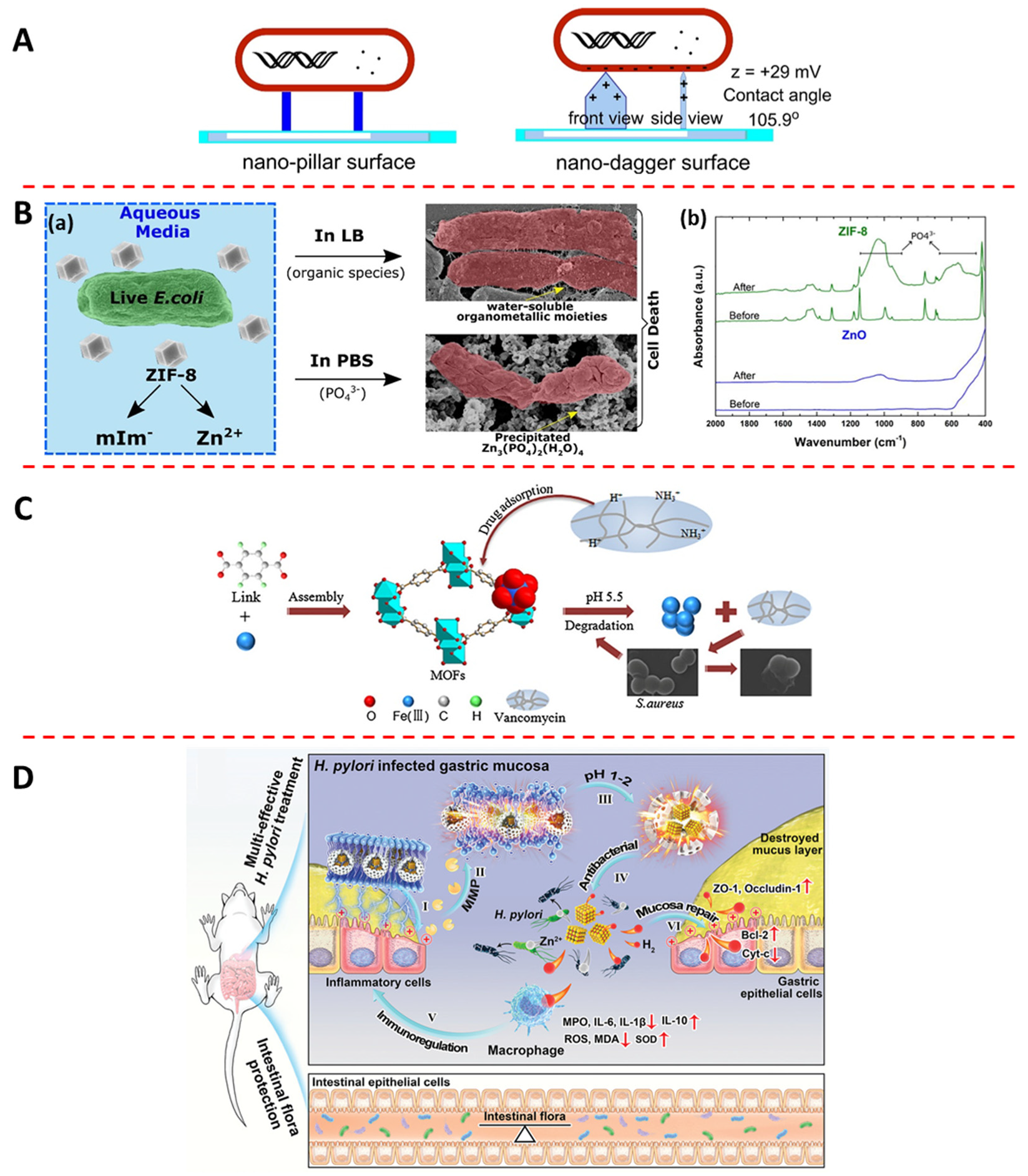
3.1. Physical Interaction
3.2. Metal Ion Release
3.3. Organic Ligand Release
3.4. Antibiotic Load
3.5. Gas Load
3.6. CDT
3.7. PDT
3.8. PTT
3.9. SDT
3.10. Synergistic Therapy
4. Strategies to Enhance the Antibacterial Ability of MOFs
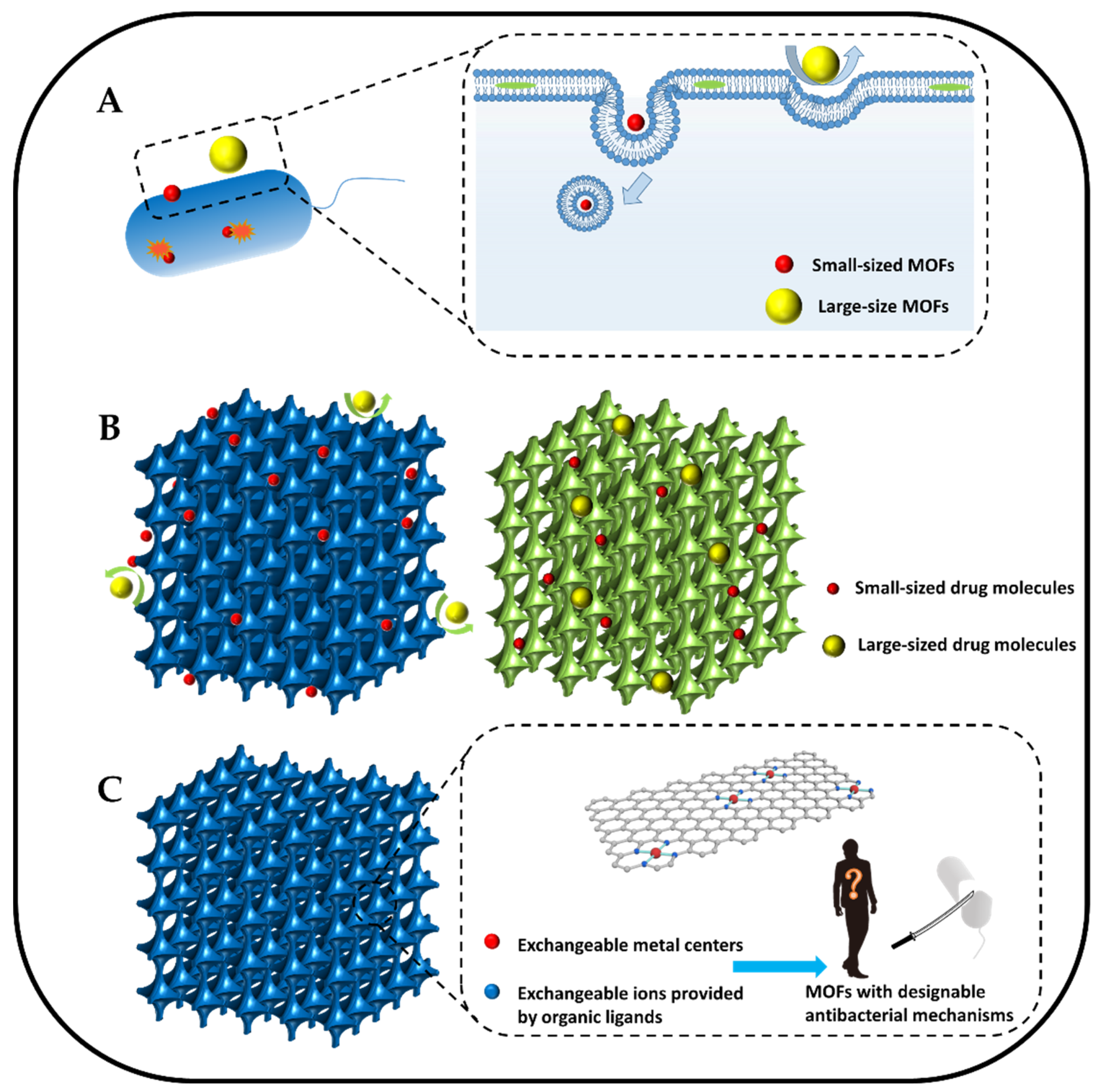
4.1. Size Modulation
4.2. Pore Size Modulation
4.3. Modulating Coordination Environment of Active Sites
4.4. Constructing MOF-Based Composites
4.4.1. MOF@metal and Oxidation Products
4.4.2. MOF@carbon
4.4.3. MOF@MOF
4.4.4. Targeted and Stimulus-Responsive MOFs
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| MOF | metal organic framework |
| IBU | inorganic building unit |
| OBU | organic building unit |
| IRMOF | isoreticular metal−organic framework |
| ZIF | zeolitic imidazolate framework |
| MIL | Matériaux de l′Institut Lavoisier |
| CPL | coordination pillared layer |
| UIO | University of Oslo |
| PCN | porous coordination network |
| HKUST | Hong Kong University of Science and Technology |
| ROS | reactive oxygen species |
| CDT | chemical dynamic therapy |
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
| SDT | sonodynamic therapy |
| MIC | minimum inhibition concentration |
| PS | photosensitizer |
| PTA | photothermal agent |
| NPs | nanoparticles |
| LSPR | local surface plasma resonance |
| LMCT | ligand–metal charge transfer |
| US | ultrasound |
| NIR | near−infrared light |
| UV | ultraviolet light |
| DFT | density functional theory |
| CB | conduction band |
| VB | valence band |
| BTC | benzenetricarboxylic acid |
| BDC | twelve benzenedicarboxylic acid |
| Hmim | methylimidazole |
| H3BTC | trimesic acid |
| BA | benzoic acid |
| H2BDC | terephthalic acid |
| TEA | triethylamine |
| DMF | dimethylformamide |
| ZIF-L | ZIF−8 nanoblade arrays |
| 4-HZBA | 4−hydrazinobenzoic acid |
| VAN | vancomycin |
| GSNO | S−Nitrosoglutathione |
| GSH | glutathione |
| L-Arg | L−arginine (L−Arg) |
| 2I-BODIPY | 5λ4−dipyrrolo[1,2−c:2′,1′−f][1,3,2]diazaborinine |
| H2O2 | hydrogen peroxide |
| ⋅OH | hydroxyl radicals |
| ⋅O2− | superoxide anions |
| 1O2 | singlet oxygen |
| GOD | glucose oxidase |
| POD | peroxidase |
| SOD | superoxide dismutase |
| CAT | catalase |
| H2DBP | 5,15−bis(p−phenylazo)−porphyrin |
| TCPP | tetrakis (4−carboxyphenyl) porphyrin |
| BODIPY | boron dipyrromethene |
| I2-BDP | carboxyl-functionalized diiodo−substituted BODIPYs |
| H2BDC | benzenedicarboxylate |
| PB | Prussian blue |
| Fc(COOH)2 | 1,10−ferrocene carboxylic acid |
| GA | gallic acid |
| PMCS | porous MOF−derived carbons |
| DHMS | double-layer hollow manganese silicate nanoparticle |
| TRBs | thermoresponsive brushes |
| PDA | 2,6−pyridinedicarboxylic acid |
| OXD | oxidase |
| HPO | halogen peroxidase |
| CQD | carbon quantum dots |
| GQD | graphene quantum dots |
| CNT | carbon nanotubes |
| GO | graphene oxide |
| RGO | reduced graphene oxide |
| AC | activated carbon |
| BQ | black phosphorus quantum dots |
| RFP | rifampicin |
| o-NBA | 2−nitrobenzaldehyde |
| E. coli | Escherichia coli |
| S.aureus | Staphylococcus aureus |
| B. subtilis | Bacillus subtilis |
| P. vulgaris | Proteus vulgaris |
| P. aeruginosa | Pseudomonas aeruginosa |
| S. enteritidis | Salmonella enteritidis |
| S. cerevisiae | Saccharomyces cerevisiae |
| P. putida | Pseudomonsa putida |
| H. pylori | Helicobacter pylori |
| MRSA | methicillin−resistant Staphylococcus aureus |
| A. baumannii | Acinetobacter baumannii |
| K. pneumoniae | Klebsiella pneumoniae |
| S. entica | Salmonella enterica |
References
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Csh Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Li, Z.; Xie, Z. BODIPY-containing nanoscale metal-organic frameworks for photodynamic therapy. Chem. Commun. 2016, 52, 5402–5405. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquacult. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Canica, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Granato, E.T.; Meiller-Legrand, T.A.; Foster, K.R. The Evolution and Ecology of Bacterial Warfare. Curr. Biol. 2019, 29, R521–R537. [Google Scholar] [CrossRef]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Edit. 2013, 52, 10766–10770. [Google Scholar] [CrossRef]
- Sun, J.; Yin, Y.; Sheng, G.-H.; Yang, Z.-B.; Zhu, H.-L. Synthesis, molecular modeling and structural characterization of vanillin derivatives as antimicrobial agents. J. Mol. Struct. 2013, 1039, 214–218. [Google Scholar] [CrossRef]
- Wynne, J.H.; Fulmer, P.A.; McCluskey, D.M.; Mackey, N.M.; Buchanan, J.P. Synthesis and Development of a Multifunctional Self-Decontaminating Polyurethane Coating. ACS Appl. Mater. Interfaces 2011, 3, 2005–2011. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, D.; Liang, Q.; Yong, D.; Han, X. Size controllable synthesis and antimicrobial activity of poly-N,N ‘- (4,5-dihydroxy-1,2-phenylene)bis(methylene) bisacrylamide microspheres. Rsc Adv. 2014, 4, 57891–57898. [Google Scholar] [CrossRef]
- Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. Photocatalytic disinfection efficiency of 2D structure graphitic carbon nitride-based nanocomposites: A review. J. Mater. Sci. 2019, 54, 12206–12235. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Yan, Z.; Zhao, C.; Ren, J.; Qu, X. Combating Biofilm Associated Infection In Vivo: Integration of Quorum Sensing Inhibition and Photodynamic Treatment based on Multidrug Delivered Hollow Carbon Nitride Sphere. Adv. Funct. Mater. 2019, 29, 1808222. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Li, Y.; Dong, L.; Mu, Z.; Liu, L.; Yang, J.; Wu, Z.; Pan, D.; Liu, L. Research Advances of Lactoferrin in Electrostatic Spinning, Nano Self-Assembly, and Immune and Gut Microbiota Regulation. J. Agric. Food Chem. 2022, 70, 10075–10089. [Google Scholar] [CrossRef]
- Bu, Y.; Hu, Q.; Bao, T.; Xie, X.; Wang, S. Recent advances in cell membrane-coated technology for drug discovery from natural products. Trac-Trends Anal. Chem. 2022, 151, 116601. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Raza, F.; Wang, X.; Zhang, S.; Rong, R.; Qiu, M.; Su, J. Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy. Pharmaceutics 2021, 13, 99. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Wang, B.; Cote, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Okubo, T.; Asami, A.; Noro, S.i.; Yoshitomi, T.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Seki, K. Rational synthesis of stable channel-like cavities with methane gas adsorption properties:[{Cu2 (pzdc) 2 (L)} n](pzdc= pyrazine-2, 3-dicarboxylate; L= a pillar ligand). Angew. Chem. Int. Edit. 1999, 38, 140–143. [Google Scholar] [CrossRef]
- Yang, Q.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serred, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, D.; Simmons, J.M.; Collier, C.D.; Yuan, D.; Zhou, H.-C. Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake. J. Am. Chem. Soc. 2008, 130, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Ferey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surble, S.; Dutour, J.; Margiolaki, I. A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction. Angew. Chem. Int. Edit. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing Microporosity in Open MetalOrganic Frameworks: Gas Sorption Isotherms for Zn(BDC) (BDC = 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 8571–8572. [Google Scholar] [CrossRef]
- Li, H.L.; Eddaoudi, M.M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, 19064846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; Zheng, H.; Bao, J.; Tang, Y.; Zhou, K. Laser-Assisted Printing of Electrodes Using Metal-Organic Frameworks for Micro-Supercapacitors. Adv. Funct. Mater. 2021, 31, 2009057. [Google Scholar] [CrossRef]
- Cun, J.-E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-based metal-organic frameworks for biomedical applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Cui, L. Progress in Preparation of Metal Organic Frameworks HKUST-1 and Its Application. Mater. Rev. 2016, 30, 114–119, 139. [Google Scholar]
- Li, R.; Chen, T.; Pan, X. Metal-Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal-organic frameworks and their composites. Compr. Rev. Food. Sci. F. 2020, 19, 1397–1419. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszalek, B.; Gil, B.; Dorozynski, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Aguado, S.; Quiros, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial activity of cobalt imidazolate metal-organic frameworks. Chemosphere 2014, 113, 188–192. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Xia, Q.; Yuan, G.; He, Q.; Cui, Y. Multiple topological isomerism of three-connected networks in silver-based metal-organoboron frameworks. Chem. Commun. 2010, 46, 2608–2610. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.; Miller, S.R.; Elkaim, E.; Horcajada, P.; Blanco-Prieto, M.J. A Zn azelate MOF: Combining antibacterial effect. CrystEngComm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Restrepo, J.; Serroukh, Z.; Santiago-Morales, J.; Aguado, S.; Gomez-Sal, P.; Mosquera, M.E.G.; Rosal, R. An Antibacterial Zn-MOF with Hydrazinebenzoate Linkers. Eur. J. Inorg. Chem. 2017, 2017, 574–580. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomed-Nanotechnol 2017, 13, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Geng, Z.; Yin, Y.; Ma, X.; Wang, Z. Morphology effect on the luminescent property and antibacterial activity of coordination polymer particles with identical crystal structures. CrystEngComm 2011, 13, 5100–5104. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Motaghi, M.; Chauhan, N.P.S.; Sargazi, G. A novel synthesis of new antibacterial nanostructures based on Zn-MOF compound: Design, characterization and a high performance application. Heliyon 2020, 6, e03231. [Google Scholar] [CrossRef]
- Han, D.; Han, Y.; Li, J.; Liu, X.; Yeung, K.W.K.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl. Catal. B Environ. 2020, 261, 118248. [Google Scholar] [CrossRef]
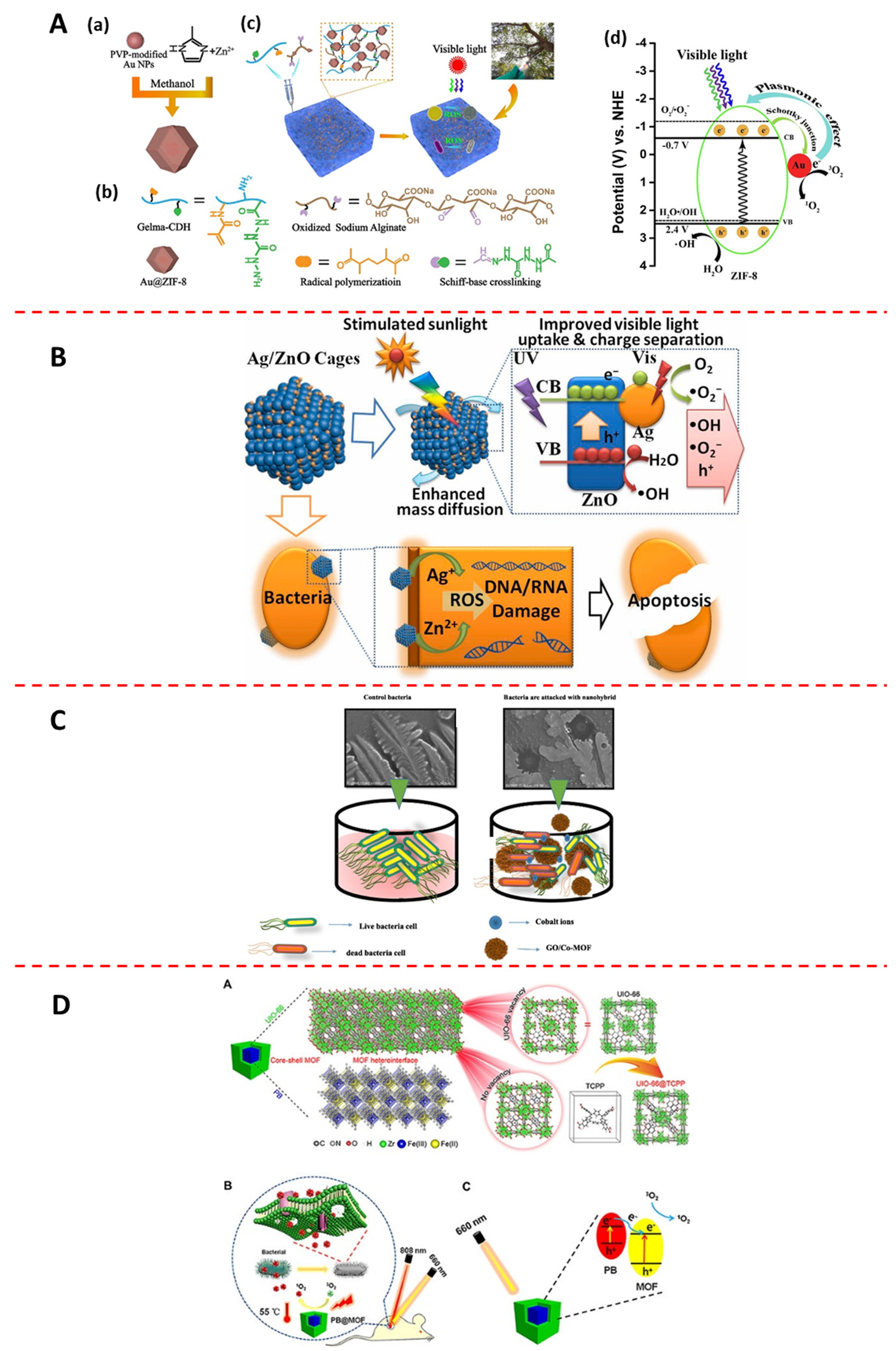
- Deng, Z.; Li, M.; Hu, Y.; He, Y.; Tao, B.; Yuan, Z.; Wang, R.; Chen, M.; Luo, Z.; Cai, K. Injectable biomimetic hydrogels encapsulating Gold/metal-organic frameworks nanocomposites for enhanced antibacterial and wound healing activity under visible light actuation. Chem. Eng. J. 2021, 420, 129668. [Google Scholar] [CrossRef]
- Yang, X.; Chai, H.; Guo, L.; Jiang, Y.; Xu, L.; Huang, W.; Shen, Y.; Yu, L.; Liu, Y.; Liu, J. In situ preparation of porous metal-organic frameworks ZIF-8@Ag on poly-ether-ether-ketone with synergistic antibacterial activity. Colloid Surface B 2021, 205, 111920. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.; Zhang, K.; Ma, G.; Zhu, L.; Zhou, L.; Yan, B. Oxygen-defective MnO2/ZIF-8 nanorods with enhanced antibacterial activity under solar light. Chem. Eng. J. 2022, 428, 131349. [Google Scholar] [CrossRef]
- Cui, J.; Wu, D.; Li, Z.; Zhao, G.; Wang, J.; Wang, L.; Niu, B. Mesoporous Ag/ZnO hybrid cages derived from ZIF-8 for enhanced photocatalytic and antibacterial activities. Ceram. Int. 2021, 47, 15759–15770. [Google Scholar] [CrossRef]
- Hatamie, S.; Ahadian, M.M.; Zomorod, M.S.; Torabi, S.; Babaie, A.; Hosseinzadeh, S.; Soleimani, M.; Hatami, N.; Wei, Z.-H. Antibacterial properties of nanoporous graphene oxide/cobalt metal organic framework. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 109862. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, J.; Liu, X.; Tan, L.; Cui, Z.; Feng, X.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. Dual Metal-Organic Framework Heterointerface. ACS Central Sci. 2019, 5, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Nordin, N.A.H.M.; Jaafar, J.; Ismail, A.F.; Ramli, M.K.N.B. Significant improvement in antibacterial property of ZIF-8 decorated graphene oxide by post-synthetic modification process. J. Environ. Chem. Eng. 2021, 9, 105887. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Cao, Q.; Wang, H.; Wang, X.; Han, H. pH-Responsive, Light-Triggered on-Demand Antibiotic Release from Functional Metal-Organic Framework for Bacterial Infection Combination Therapy. Adv. Funct. Mater. 2018, 28, 1800011. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Zhang, W.; Li, X.; Gu, Z.; Li, W.; Zhao, S.; Fu, Y. Preparation of MOF catalysts and simultaneously modulated metal nodes and ligands via a one-pot method for optimizing cycloaddition reactions. New J. Chem. 2020, 44, 9611–9615. [Google Scholar] [CrossRef]
- Huang, L. Synthesis, morphology control, and properties of porous metal–organic coordination polymers. Microporous Mesoporous Mater. 2003, 58, 105–114. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous metal–organic framework by an ultrasonic method and selective sensing of organoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef]
- Petrier, C.; Luche, J.; Luche, J. Synthetic Organic Sonochemistry; Plenum Press: New York, NY, USA, 1998; pp. 53–56. [Google Scholar]
- Suslick, K.S.; Nyborg, W.L. ULTRASOUND: Its Chemical, Physical and Biological Effects. J. Acoust. Soc. Am. 1989, 87, 919–920. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Puskaric, A.; Strukil, V.; Mottillo, C.; Julien, P.A.; Uzarevic, K.; Minh-Hao, P.; Trong-On, D.; Kimber, S.A.J.; Lazic, P.; et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef]
- Freund, R.; Canossa, S.; Cohen, S.M.; Yan, W.; Deng, H.; Guillerm, V.; Eddaoudi, M.; Madden, D.G.; Fairen-Jimenez, D.; Lyu, H.; et al. 25 Years of Reticular Chemistry. Angew. Chem. Int. Edit. 2021, 60, 23946–23974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Alezi, D.; Eddaoudi, M. A reticular chemistry guide for the design of periodic solids. Nat. Rev. Mater. 2021, 6, 466–487. [Google Scholar] [CrossRef]
- Chiericatti, C.; Carlos Basilico, J.; Zapata Basilico, M.L.; Manuel Zamaro, J. Novel application of HKUST-1 metal-organic framework as antifungal: Biological tests and physicochemical characterizations. Microporous Mesoporous Mater. 2012, 162, 60–63. [Google Scholar] [CrossRef]
- Iram, S.; Imran, M.; Kanwal, F.; Iqbal, Z.; Deeba, F.; Iqbal, Q.J. Bismuth(III) based Metal Organic Frameworks: Luminescence, Gas Adsorption, and Antibacterial Studies. Z. Anorg. Allg. Chem. 2019, 645, 50–56. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef]
- Raju, P.; Ramalingam, T.; Nooruddin, T.; Natarajan, S. In vitro assessment of antimicrobial, antibiofilm and larvicidal activities of bioactive nickel metal organic framework. J. Drug Deliv. Sci. Tec. 2020, 56, 101560. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Sun, Y.; Bogale, R.F.; Zhao, L.; Tian, P.; Ning, G. Ligand effects on the structural dimensionality and antibacterial activities of silver-based coordination polymers. Dalton Trans. 2014, 43, 10104–10113. [Google Scholar] [CrossRef]
- Sancet, M.P.A.; Hanke, M.; Wang, Z.; Bauer, S.; Azucena, C.; Arslan, H.K.; Heinle, M.; Gliemann, H.; Woell, C.; Rosenhahn, A. Surface anchored metal-organic frameworks as stimulus responsive antifouling coatings. Biointerphases 2013, 8, 29. [Google Scholar] [CrossRef]
- Taheri, M.; Ashok, D.; Sen, T.; Enge, T.G.; Verma, N.K.; Tricoli, A.; Lowe, A.; Nisbet, D.R.; Tsuzuki, T. Stability of ZIF-8 nanopowders in bacterial culture media and its implication for antibacterial properties. Chem. Eng. J. 2021, 413, 127511. [Google Scholar] [CrossRef]
- Chen, L.-J.; Liu, Y.-Y.; Zhao, X.; Yan, X.-P. Vancomycin-Functionalized Porphyrinic Metal-Organic Framework PCN-224 with Enhanced Antibacterial Activity against Staphylococcus Aureus. Chem. Asian J. 2021, 16, 2022–2026. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Porous Iron-Carboxylate Metal-Organic Framework: A Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.R.; Rubin, H.N.; Rithner, C.D.; Finke, R.G.; Reynolds, M.M. Copper ion vs copper metal organic framework catalyzed NO release from bioavailable S-Nitrosoglutathione en route to biomedical applications: Direct H-1 NMR monitoring in water allowing identification of the distinct, true reaction stoichiometries and thiol dependencies. J. Inorg. Biochem. 2019, 199, 110760. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-S.; Zeng, J.-Y.; Cheng, H.; Zhang, X.-Z. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials 2018, 185, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhou, L.-L.; Li, Y.-A.; Dong, Y.-B. A nanoscale metal-organic framework for combined photodynamic and starvation therapy in treating breast tumors. Chem. Commun. 2019, 55, 14898–14901. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Fan, Y.; Cao, R.; Xu, Y.; Weng, Z.; Ye, J.; He, C.; Zhu, Y.; Wang, X. Metal-Organic-Framework-Based Hydrogen-Release Platform for Multieffective Helicobacter Pylori Targeting Therapy and Intestinal Flora Protective Capabilities. Adv. Mater. 2022, 34, 2105738. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Karimi, F.; Xie, K.; Fu, Q.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. MOF-Mediated Destruction of Cancer Using the Cell’s Own Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2017, 9, 33599–33608. [Google Scholar] [CrossRef]
- Hao, Y.-N.; Qu, C.-C.; Shu, Y.; Wang, J.-H.; Chen, W. Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy. Nanomaterials 2021, 11, 1843. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal-Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.; Zhou, H.-C. Controlled Generation of Singlet Oxygen in Living Cells with Tunable Ratios of the Photochromic Switch in Metal-Organic Frameworks. Angew. Chem. Int. Edit. 2016, 55, 7188–7193. [Google Scholar] [CrossRef]
- Fu, G.; Liu, W.; Feng, S.; Yue, X. Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem. Commun. 2012, 48, 11567–11569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Liang, C.; Feng, L.; Fu, T.; Dong, Z.; Chao, Y.; Li, Y.; Lu, G.; Chen, M.; et al. Nanoscale Metal-Organic Particles with Rapid Clearance for Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Nano 2016, 10, 2774–2781. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.-J.; Peng, X. One Stone Two Birds: Zr-Fc Metal-Organic Framework Nanosheet for Synergistic Photothermal and Chemodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20321–20330. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, X.; Chen, H.; Zhang, J.; Zhang, H.; Wang, Z. Gram-scale synthesis of coordination polymer nanodots with renal clearance properties for cancer theranostic applications. Nat. Commun. 2015, 6, 8003. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bai, L.; Wang, H.; Wu, Q.; Wang, H.; Liu, S.; Xu, B.; Shi, X.; Liu, H. Metal-Organic-Framework-Derived Carbon Nanostructure Augmented Sonodynamic Cancer Therapy. Adv. Mater. 2018, 30, 1800180. [Google Scholar] [CrossRef]
- Pan, X.; Wang, W.; Huang, Z.; Liu, S.; Guo, J.; Zhang, F.; Yuan, H.; Li, X.; Liu, F.; Liu, H. MOF-Derived Double-Layer Hollow Nanoparticles with Oxygen Generation Ability for Multimodal Imaging-Guided Sonodynamic Therapy. Angew. Chem. Int. Edit. 2020, 59, 13557–13561. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, L.; Li, Z.; Liu, X.; Zheng, Y.; Feng, X.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. Single-atom catalysis for efficient sonodynamic therapy of methicillin-resistant Staphylococcus aureus-infected osteomyelitis. ACS Nano 2021, 15, 10628–10639. [Google Scholar] [CrossRef]
- Bouhidel, Z.; Cherouana, A.; Durand, P.; Doudouh, A.; Morini, F.; Guillot, B.; Dahaoui, S. Synthesis, spectroscopic characterization, crystal structure, Hirshfeld surface analysis and antimicrobial activities of two triazole Schiff bases and their silver complexes. Inorg. Chim. Acta 2018, 482, 34–47. [Google Scholar] [CrossRef]
- Liu, A.; Wang, C.-C.; Wang, C.-z.; Fu, H.-f.; Peng, W.; Cao, Y.-L.; Chu, H.-Y.; Du, A.-F. Selective adsorption activities toward organic dyes and antibacterial performance of silver-based coordination polymers. J. Colloid Interface Sci. 2018, 512, 730–739. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res. 2003, 533, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Seyedpour, S.F.; Rahimpour, A.; Najafpour, G. Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: A novel approach toward long-lasting biofouling mitigation. J. Membr. Sci. 2019, 573, 257–269. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, H.-C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, H.; Lu, H.; Zheng, Y.; Ying, J.Y.; Zhang, Y. ZIF nano-dagger coated gauze for antibiotic-free wound dressing. Chem. Commun. 2019, 55, 699–702. [Google Scholar] [CrossRef]
- Zhuang, W.; Yuan, D.; Li, J.-R.; Luo, Z.; Zhou, H.-C.; Bashir, S.; Liu, J. Highly Potent Bactericidal Activity of Porous Metal-Organic Frameworks. Adv. Healthc. Mater. 2012, 1, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, J.; Yin, X.; Wei, X. Modification of Bentonite and Its Application in Antimicrobial Material. Bentonite Modif. 2020, 5, 54–61. [Google Scholar]
- Xu, Y.-T.; Ye, Z.-M.; Ye, J.-W.; Cao, L.-M.; Huang, R.-K.; Wu, J.-X.; Zhou, D.-D.; Zhang, X.-F.; He, C.-T.; Zhang, J.-P.; et al. Non-3d Metal Modulation of a Cobalt Imidazolate Framework for Excellent Electrocatalytic Oxygen Evolution in Neutral Media. Angew. Chem. Int. Edit. 2019, 58, 139–143. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Zheng, H.; Li, R.; Tang, Y.; Li, B.; Zhu, C.; You, L.; Gao, M.-R.; Liu, Z.; et al. Embedding Ultrafine Metal Oxide Nanoparticles in Monolayered Metal-Organic Framework Nanosheets Enables Efficient Electrocatalytic Oxygen Evolution. ACS Nano 2020, 14, 1971–1981. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Li, M.; Yu, P.; Chu, X.; Li, L.; Tan, G.; Wang, Y.; Chen, X.; Zhang, Y.; et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J. Inorg. Biochem. 2016, 163, 214–220. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Fu, L.; Zhu, J.; Fan, M.; Chen, J.; Yang, C.; Yang, G.; Wu, L.; Mao, G.; et al. Ultra-efficient Antibacterial System Based on Photodynamic Therapy and CO Gas Therapy for Synergistic Antibacterial and Ablation Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 22479–22491. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Zhao, P.; Cheng, Q.; He, Q.; Ma, D.; Xue, W. NIR-Laser-Controlled Hydrogen-Releasing PdH Nanohydride for Synergistic Hydrogen-Photothermal Antibacterial and Wound-Healing Therapies. Adv. Funct. Mater. 2019, 29, 1905697. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Liu, Y.; Hu, J.; He, Q.; et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Edit. 2017, 56, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Schmidt, M.; Boehmer, A.; Zoerner, A.A.; Gutzki, F.-M.; Jordan, J. UPLC-MS/MS measurement of S-nitrosoglutathione (GSNO) in human plasma solves the S-nitrosothiol concentration enigma. J. Chromatogr. B 2013, 927, 147–157. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Li, S.; Tan, J.; Xu, C.; Huang, Y. MOF-derived Co3O4@Co-Fe oxide double-shelled nanocages as multi- functional specific peroxidase-like nanozyme catalysts for chemo/biosensing and dye degradation. Chem. Eng. J. 2020, 395, 125130. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Z.; Zhang, Y.; Liu, Z.; Sun, Y.; Ren, J.; Qu, X. Two-Dimensional Metal-Organic Framework/Enzyme Hybrid Nanocatalyst as a Benign and m Self-Activated Cascade Reagent for in Vivo Wound Healing. ACS Nano 2019, 13, 5222–5230. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, L.; Yan, H.; Wu, Y.; Chen, L.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Glucose Oxidase-Integrated Metal-Organic Framework Hybrids as Biomimetic Cascade Nanozymes for Ultrasensitive Glucose Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 22096–22101. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Song, G.; Yang, Y.; Zhao, Y.; Wang, P.; Zhu, L.-M.; Yin, X.; Zhang, X.-B. Persistent Regulation of Tumor Microenvironment via Circulating Catalysis of MnFe2O4@Metal-Organic Frameworks for Enhanced Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1901417. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Liu, C.-Y.; Zeng, X.-Y.; Chen, J.; Lu, J.; Lin, R.-G.; Cao, R.; Lin, Z.-J.; Su, J.-W. MOF-808: A Metal-Organic Framework with Intrinsic Peroxidase-Like Catalytic Activity at Neutral pH for Colorimetric Biosensing. Inorg. Chem. 2018, 57, 9096–9104. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Wang, S.; Sun, X.; Wang, L.; Wang, W.; Shen, H.; Liu, H. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci. China-Life Sci. 2018, 61, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Hou, H.; Huang, X.; Wei, G.; Xu, F.; Wang, Y.; Zhou, S. Fenton Reaction-Assisted Photodynamic Therapy for Cancer with Multifunctional Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 29579–29592. [Google Scholar] [CrossRef]
- Huang, Y.; Skripka, A.; Labrador-Paez, L.; Sanz-Rodriguez, F.; Haro-Gonzalez, P.; Jaque, D.; Rosei, F.; Vetrone, F. Upconverting nanocomposites with combined photothermal and photodynamic effects. Nanoscale 2018, 10, 791–799. [Google Scholar] [CrossRef]
- Jia, T.; Xu, J.; Dong, S.; He, F.; Zhong, C.; Yang, G.; Bi, H.; Xu, M.; Hu, Y.; Yang, D.; et al. Mesoporous cerium oxide-coated upconversion nanoparticles for tumor-responsive chemo-photodynamic therapy and bioimaging. Chem. Sci. 2019, 10, 8618–8633. [Google Scholar] [CrossRef]
- Pan, C.; Ou, M.; Cheng, Q.; Zhou, Y.; Yu, Y.; Li, Z.; Zhang, F.; Xia, D.; Mei, L.; Ji, X. Z-Scheme Heterojunction Functionalized Pyrite Nanosheets for Modulating Tumor Microenvironment and Strengthening Photo/Chemodynamic Therapeutic Effects. Adv. Funct. Mater. 2020, 30, 1906466. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, J.; You, T.; Sun, D. Metal-organic frameworks for improving wound healing. Coord. Chem. Rev. 2021, 439, 213929. [Google Scholar] [CrossRef]
- Cai, X.; Gao, W.; Ma, M.; Wu, M.; Zhang, L.; Zheng, Y.; Chen, H.; Shi, J. A Prussian Blue-Based Core-Shell Hollow-Structured Mesoporous Nanoparticle as a Smart Theranostic Agent with Ultrahigh pH-Responsive Longitudinal Relaxivity. Adv. Mater. 2015, 27, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hu, W.; Zhao, H.; Miao, X.; Guan, Y.; Cai, W.; Zeng, Z.; Fan, Q.; Tan, T.T.Y. Generating New Cross Relaxation Pathways by Coating Prussian Blue on NaNdF4 for Enhanced Photothermal Agents. Angew. Chem. 2019, 58, 8624–8628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ji, Y.; Shi, J.; Wang, L. NIR-Driven Water Splitting H-2 Production Nanoplatform for H-2-Mediated Cascade-Amplifying Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 23677–23688. [Google Scholar] [CrossRef]
- Fan, X.; Yang, F.; Huang, J.; Yang, Y.; Nie, C.; Zhao, W.; Ma, L.; Cheng, C.; Zhao, C.; Haag, R. Metal-Organic-Framework-Derived 2D Carbon Nanosheets for Localized Multiple Bacterial Eradication and Augmented Anti-infective Therapy. Nano Lett. 2019, 19, 5885–5896. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Xia, L.; Cui, C.; Wan, S.; Jiang, Y.; Yang, Y.; Wu, Q.; Qiu, L.; Tan, W. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem. Sci. 2018, 9, 7505–7509. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Jiang, Z.; Xu, J.; Chen, L.; Xue, Y.; Nie, A.; Xie, Z.; Kuang, Q.; Zheng, L. Atomically dispersed hierarchically ordered porous Fe-N-C electrocatalyst for high performance electrocatalytic oxygen reduction in Zn-Air battery. Nano Energy 2020, 71, 104547. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, L.; Wang, L.; Huang, J.; Wang, S.; Xie, X.; Zhu, J.; Ding, T.; Cai, K.; Zhang, J. Flower-Like Nanozymes with Large Accessibility of Single Atom Catalysis Sites for ROS Generation Boosted Tumor Therapy. Adv. Funct. Mater. 2022, 32, 2111171. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, D.; Zhang, X.; Gu, L.; Yuan, Y. Ascorbic acid-assisted hydrothermal route to create mesopores in polymeric carbon nitride for increased photocatalytic hydrogen generation. Int. J. Hydrog. Energy 2021, 46, 38310–38318. [Google Scholar] [CrossRef]
- Jensen, W.B. The Lewis acid-base definitions: A status report. Chem. Rev. 1978, 78, 1–22. [Google Scholar] [CrossRef]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.A.; Lin, J. Yolk-Shell Structured Au Nanostar@Metal-Organic Framework for Synergistic Chemo-photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef]
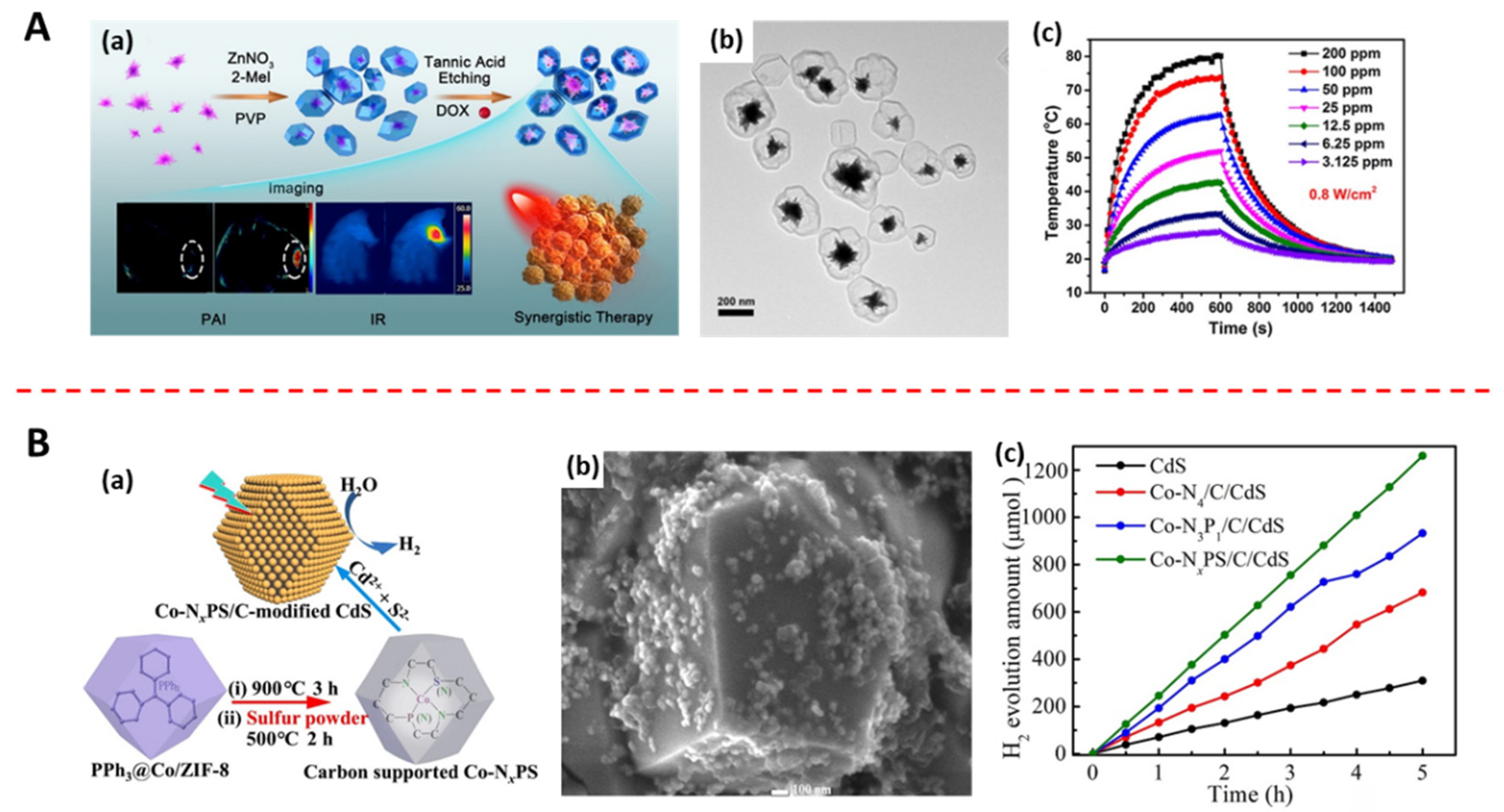
- Ji, Q.; Xu, J.; Wang, C.; Wang, L. Controlling the coordination environment of Co atoms derived from Co/ZIF-8 for boosting photocatalytic H-2 evolution of CdS. J. Colloid Interface Sci. 2021, 596, 139–147. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Wei, Y.; Zhang, J.; Tao, C. Recent advances in metal-organic framework-based materials for anti-staphylococcus aureus infection. Nano Res. 2022, 15, 6220–6242. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt-1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Edit. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Han, J.; Meng, X.; Lu, L.; Bian, J.; Li, Z.; Sun, C. Single-Atom Fe-N-x-C as an Efficient Electrocatalyst for Zinc-Air Batteries. Adv. Funct. Mater. 2019, 29, 1800872. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Z.; Zheng, Y.; Wu, Z.; Ma, T.; Zhu, B.; Yang, C.; Xiang, X.; Ma, L.; Han, X.; et al. A Library of ROS-Catalytic Metalloenzyme Mimics with Atomic Metal Centers. Adv. Mater. 2022, 34, 2200255. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, C.; Yan, Y.; Yu, Y.; Zhao, H.; Zhou, Z.; Wang, F.; Feng, Y. Engineering DNA/Fe-N-C single-atom nanozymes interface for colorimetric biosensing of cancer cells. Anal. Chim. Acta 2021, 1180, 338856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ina, T.; Chen, W.-T.; Shang, L.; Sun, F.; Wei, S.; Sun-Waterhouse, D.; Telfer, S.G.; Zhang, T.; Waterhouse, G.I.N. Evolution of Zn(II) single atom catalyst sites during the pyrolysis-induced transformation of ZIF-8 to N-doped carbons. Sci. Bull. 2020, 65, 1743–1751. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lazaro-Martinez, J.M.; Rodriguez-Castellon, E.; Bandosz, T.J. Carbon Quantum Dot Surface-Chemistry-Dependent Ag Release Governs the High Antibacterial Activity of Ag-Metal-Organic Framework Composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Du, P.; Zhang, L.; Lei, J. Metal-Organic Framework (MOF) Hybrid as a Tandem Catalyst for Enhanced Therapy against Hypoxic Tumor Cells. Angew. Chem. Int. Edit. 2019, 58, 7808–7812. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Qi, J.; Dong, R.; Liu, H.; Wu, D.; Shao, H.; Jiang, X. Boronic Acid-Decorated Multivariate Photosensitive Metal-Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano 2022, 16, 7732–7744. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Das, B.; Kumar, A.; Tripathy, S.; Roy, S.; Sahu, S.K. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant Staphylococcus aureus. Nanotechnology 2017, 28, 095102. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, G.; Ruan, Z.; Tang, N.; Song, C.; Li, Q.; Zhou, W.; Jin, J.; Haick, H.; Chen, Y.; et al. Biomimetic Self-Assembling Metal–Organic Architectures with Non-Iridescent Structural Coloration for Synergetic Antibacterial and Osteogenic Activity of Implants. ACS Nano 2022, 16, 16584–16597. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.-H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Enhancing therapeutic efficacy through designed aggregation of nanoparticles. Biomaterials 2014, 35, 7860–7869. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Aghayi-Anaraki, M.; Safarifard, V. Fe3O4@MOF Magnetic Nanocomposites: Synthesis and Applications. Eur. J. Inorg. Chem. 2020, 2020, 1916–1937. [Google Scholar] [CrossRef]
- Li, S.; Huo, F. Metal-organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Long, R.; Mao, K.; Gong, M.; Zhou, S.; Hu, J.; Zhi, M.; You, Y.; Bai, S.; Jiang, J.; Zhang, Q.; et al. Tunable Oxygen Activation for Catalytic Organic Oxidation: Schottky Junction versus Plasmonic Effects. Angew. Chem. Int. Edit. 2014, 53, 3205–3209. [Google Scholar] [CrossRef]
- Han, W.; Wu, Z.; Li, Y.; Wang, Y. Graphene family nanomaterials (GFNs)-promising materials for antimicrobial coating and film: A review. Chem. Eng. J. 2019, 358, 1022–1037. [Google Scholar] [CrossRef]
- Lakshmi, S.D.; Avti, P.K.; Gurumurthy, H. Activated carbon nanoparticles from biowaste as new generation antimicrobial agents: A review. Nano Struct. Nano Objects 2018, 16, 306–321. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The recent progress on metal-organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar] [CrossRef] [PubMed]

| MOF | Microbial Strain | Antibacterial Activity | Ref. | |||
|---|---|---|---|---|---|---|
| Average Inhibition Diameter (mm) | MBC (μg mL−1) | MIC (μg mL−1) | Other Method | |||
| ZIF-67 | S. cerevisiae P. putida E. coli | 15 15 15 | – | – – 5 | [49] | |
| Co-SIM-1 | S. cerevisiae P. putida E. coli | 15 15 15 | – | – – 5 | ||
| [(AgL)NO3]·2H2O | E. coli S. aureus | 13 16 | – | 300 297 | [50] | |
| [(AgL)CF3SO3]·2H2O | E. coli S. aureus | 15 16 | – | 300 307 | ||
| [(AgL)ClO4]·2H2O | E. coli S. aureus | 15 19 | – | 308 293 | ||
| BioMOF-5 | S. aureus | – | 1700 | 4300 | [51] | |
| {[Zn(μ-4-HZBA)2]2·4(H2O)}n | S. aureus | – | – | – | a half maximal effective antibacterial concentration of about 20 mg L–1 | [52] |
| ZIF-L | E. coli S. aureus | – | – | – | log reduction > 7 for E. coli and S. aureus; SEM images | [53] |
| CPPs | B. subtilis P. vulgaris S. aureus P. aeruginosa S. enteritidis | – | – | <25 | [54] | |
| Zn-PDA | S. aureus B. subtilis A. baumannii K. pneumoniae S. entica E. coli | 17 16 11 9.7 9.7 8.6 | – | 300–308 | [55] | |
| Cu2+-doped PCN-224 | S. aureus | – | – | – | antibacterial efficacy (99.71%) | [56] |
| Au@ZIF-8 | S. aureus E. coli | – | – | – | inhibition ratio against S. aureus and E. coli was >99.9% when the dosage of Au@ZIF-8 was 0.2 mg mL−1 | [57] |
| SPZA | S. aureus E. coli | – | – | – | The number of viable bacterial cells on SPZA is zero; SEM images | [58] |
| MnO2/ZIF-8 | E. coli | – | 3.24 | – | complete inactivation against E. coli at low concentrations (3.24 μg mL−1) | [59] |
| Ag/ZnO | E. coli S. aureus | – | 12.5 6.25 | 6.25 3.12 | [60] | |
| GO/Co-PTA | E. coli S. aureus | – | – | – | inhibited the growth of E. coli and S. aureus by up to 99% | [61] |
| Dual MOFs | E. coli S. aureus | – | – | – | the antibacterial efficiency against S. aureus and E. coli exceeded 99.31 and 98.68%, respectively. | [62] |
| ZGO-NH | E. coli S. aureus | 2.59 3.82 | - | - | [63] | |
| ZIF-8@RFP | MRSA | - | 10 | - | [64] | |
| IBUs | OBUs | Functional Materials | Main Role of MOFs in Antibacterial Application | Ref. |
|---|---|---|---|---|
| Ag+ | 3−(Biphenyl−4−yl)−5−(4−tertbutylphenyl)−4−phenyl-4H−1,2,4−triazole | AgTAZ | Ag+ release | [49] |
| Ag+ | Tris−(4−pyridylduryl)borane(L) | [(AgL)NO3]·2H2O | Ag+ release | [50] |
| Ag+ | 1−Butylimidazole | [Ag(Bim)] | Ag+ release | [78] |
| Co2+ | Hmim | ZIF−67 | Co2+ release | [49] |
| Cu2+ | Trimesic acid | HKUST−1 | Co2+ release | [74] |
| Cu2+ | 1,4−Benzendicarboxylic acid | Cu−SURMOF−2 | Cu2+ release | [79] |
| Zn2+ | Azathioprine | BioMOF−5 | Zn2+ release | [51] |
| Zn2+ | Hmim | ZIF−8 | Organic compound generation | [80] |
| Ni2+ | Hmim | Ni−Hmim | Hmin release | [77] |
| Zn2+ | 4−HZBN | [Zn(μ−4−HZBA)2]2·4(H2O)}n | HZBN release | [52] |
| Zr4+ | BA, TCPP | VAN | VAN release | [81] |
| Fe3+ | H2BDC | VAN | VAN release | [82] |
| Cu1+ | 1,3,5−Tribromobenzene + diethylamine → H3BTTri | GSNO | NO release | [83] |
| Zr4+ | BA, TCPP | L−Arg | NO release | [84] |
| Hf4+ | 2−Hydroxyterephthalic acid, acetic acid | MnCO | CO release | [85] |
| Zn2+ | Hmim | Pd(H) | H2 release | [86] |
| Fe3+ | Acrylic acid | MIL−88B | CDT | [87] |
| Cu2+ | 1,3,5−Tricarboxybenzene | HKUST−1 | CDT | [88] |
| Zr4+ | H2BDC → I2−BDP | UIO−PDT | PDT | [6] |
| Hf4+ | H2DBP | DBP−UIO | PDT | [89] |
| Hf4+ | TCPP | Hf−TCPP NMOF | PDT | [90] |
| Zr4+ | H2TCPP | PCN−224 | PDT | [91] |
| Fe2+/ Fe3+ | K4[Fe(CN)6] | PB | PTT | [92] |
| Mn2+ | IR825 | Mn−IR825 | PTT | [93] |
| Zr4+ | (Fc(COOH)2) | Zr−Fc MOF | PTT | [94] |
| Fe3+ | GA | Fe−CPND | PTT | [95] |
| Zn2+ | Hmim | ZIF−8 → PMCS | SDT | [96] |
| Zn2+, Mn2+ | Hmim | ZIF−8 → DHMS | SDT | [97] |
| Zr4+ Pt4+ Au3+ | BA | HNTM → HNTM−Pt@Au | SDT | [98] |
| Material 1 | Material 2 | Compound Mode | Main Role of MOFs in Antibacterial Application | Ref. |
|---|---|---|---|---|
| g-C3N4 | Cu3P | Heterojunction | H2 loaded PTT PDT CDT | [136] |
| ZIF-8 | Au NStar | Core–shell | Ag+ release PTT | [146] |
| PCN-224 | Cu2+ | stem grafting | PDT PTT | [56] |
| ZIF-8 | Au NPs | Heterojunction | PDT | [57] |
| ZIF-8 | Ag NPs | Heterojunction | Ag+ release PDT | [58] |
| ZIF-8 | MnO2 | Heterojunction | Ag+ release PDT | [59] |
| ZIF-8 → ZnO | Ag NPs | Heterojunction | Zn2+ release Ag+ release PDT | [60] |
| Ag-BTC-S/N | CQDS | Heterojunction | Ag+ release physical interaction CDT | [157] |
| Co-PTA | GO | Heterojunction | Co2+ release physical interaction | [61] |
| MIL-101 | BQ | Core–shell | PDT PTT | [158] |
| UIO-66 | PB | Core–shell | PDT PTT | [62] |
| multivariate MOF | Photosensitized porphyrin, BA | Core–shell | PDT | [159] |
| ZIF-8 | -NH2 | stem grafting | Zn2+ release | [63] |
| ZIF-8@RFP | o-NBA | Core–shell | Zn2+ release Antibiotic loaded | [64] |
| VAN@ZIF-8 | FA | Core–shell | Zn2+ release Antibiotic loaded | [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Peng, F.; Wang, D. MOFs and MOF-Derived Materials for Antibacterial Application. J. Funct. Biomater. 2022, 13, 215. https://doi.org/10.3390/jfb13040215
Zhang X, Peng F, Wang D. MOFs and MOF-Derived Materials for Antibacterial Application. Journal of Functional Biomaterials. 2022; 13(4):215. https://doi.org/10.3390/jfb13040215
Chicago/Turabian StyleZhang, Xin, Feng Peng, and Donghui Wang. 2022. "MOFs and MOF-Derived Materials for Antibacterial Application" Journal of Functional Biomaterials 13, no. 4: 215. https://doi.org/10.3390/jfb13040215
APA StyleZhang, X., Peng, F., & Wang, D. (2022). MOFs and MOF-Derived Materials for Antibacterial Application. Journal of Functional Biomaterials, 13(4), 215. https://doi.org/10.3390/jfb13040215