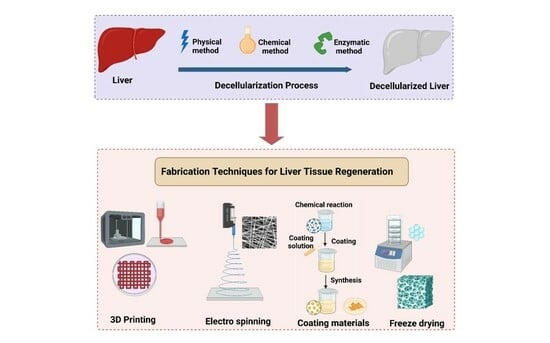
Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration
Abstract
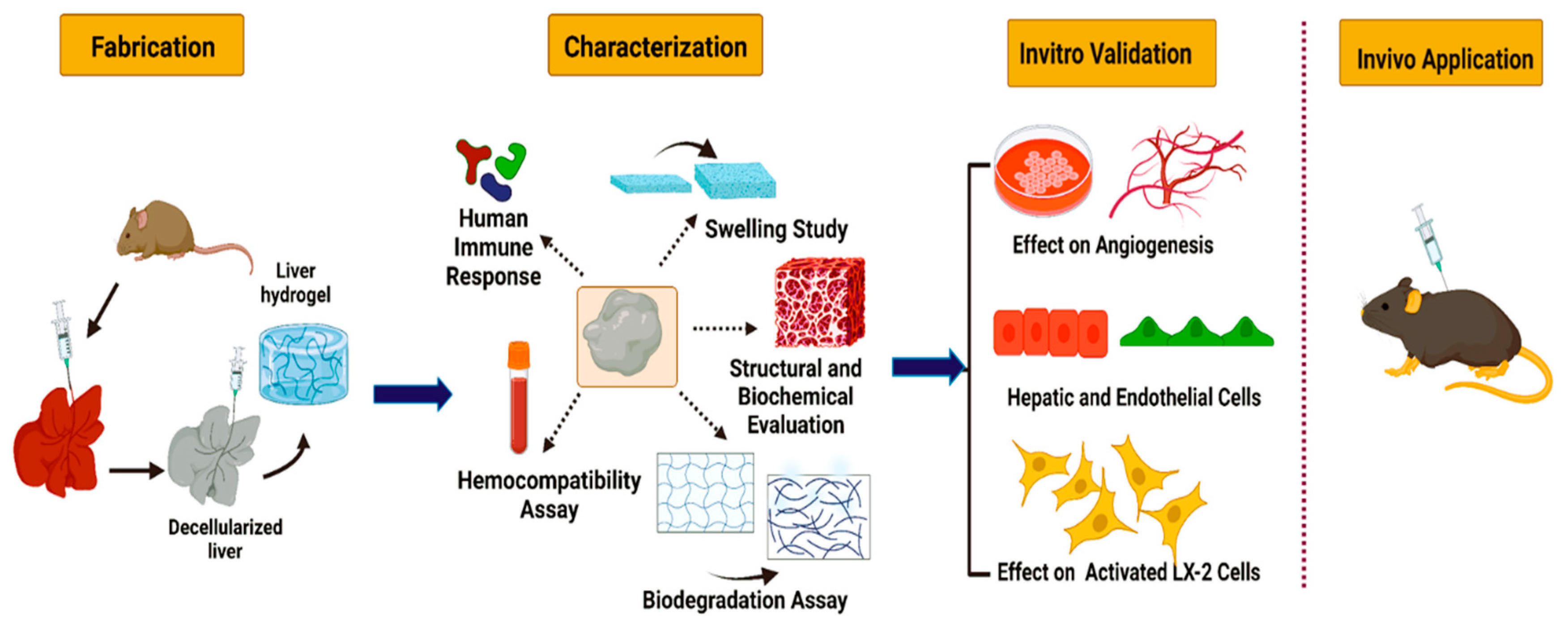
:1. Introduction
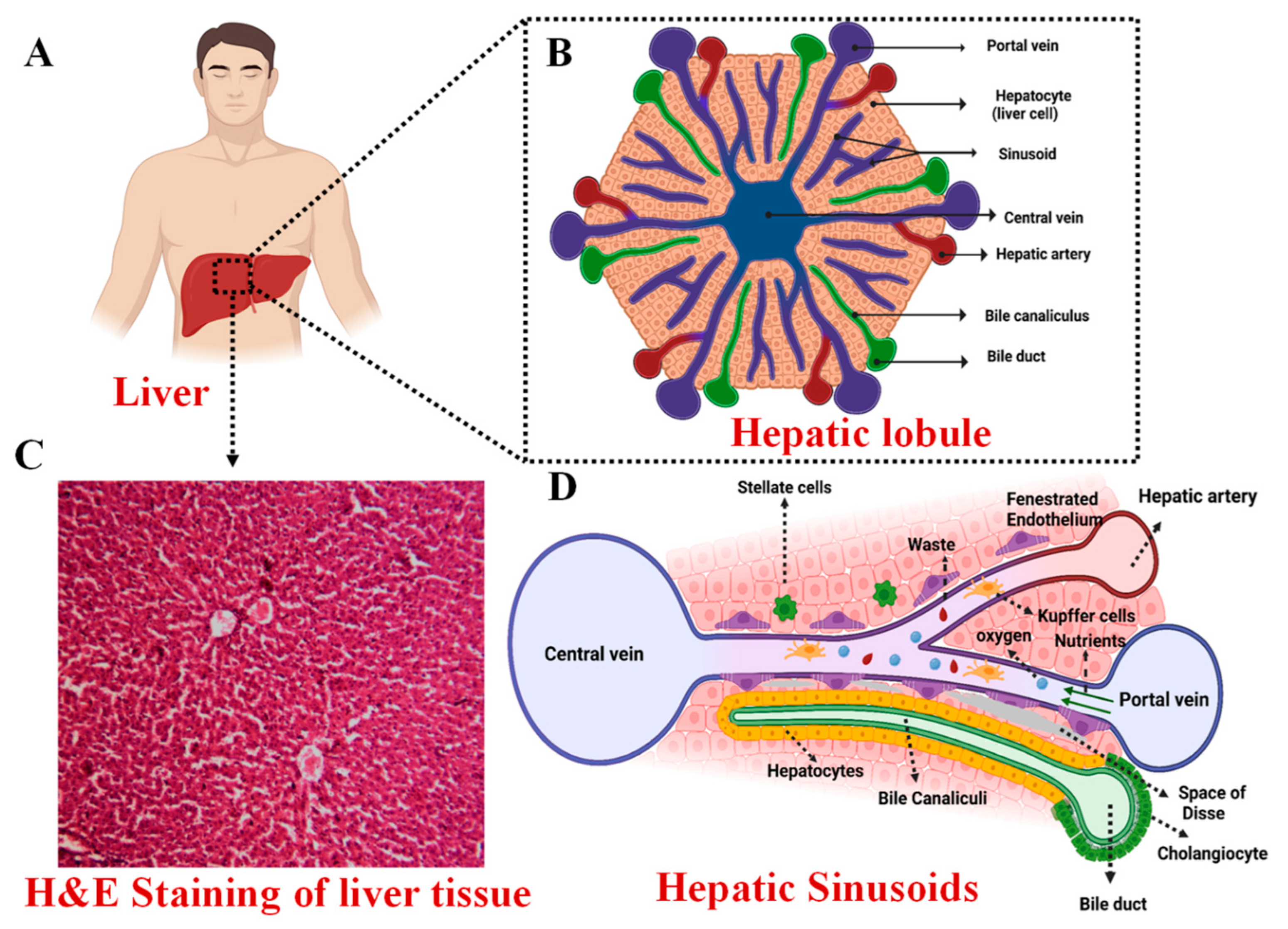
2. Anatomy of the Liver
3. Functions of the Liver
3.1. Metabolic Functions
3.2. Bile Production
3.3. Bilirubin Metabolism
3.4. Other Functions
4. Prevalence of Liver Diseases
5. Decellularized Extracellular Matrix Scaffolds
Hepatic ECM
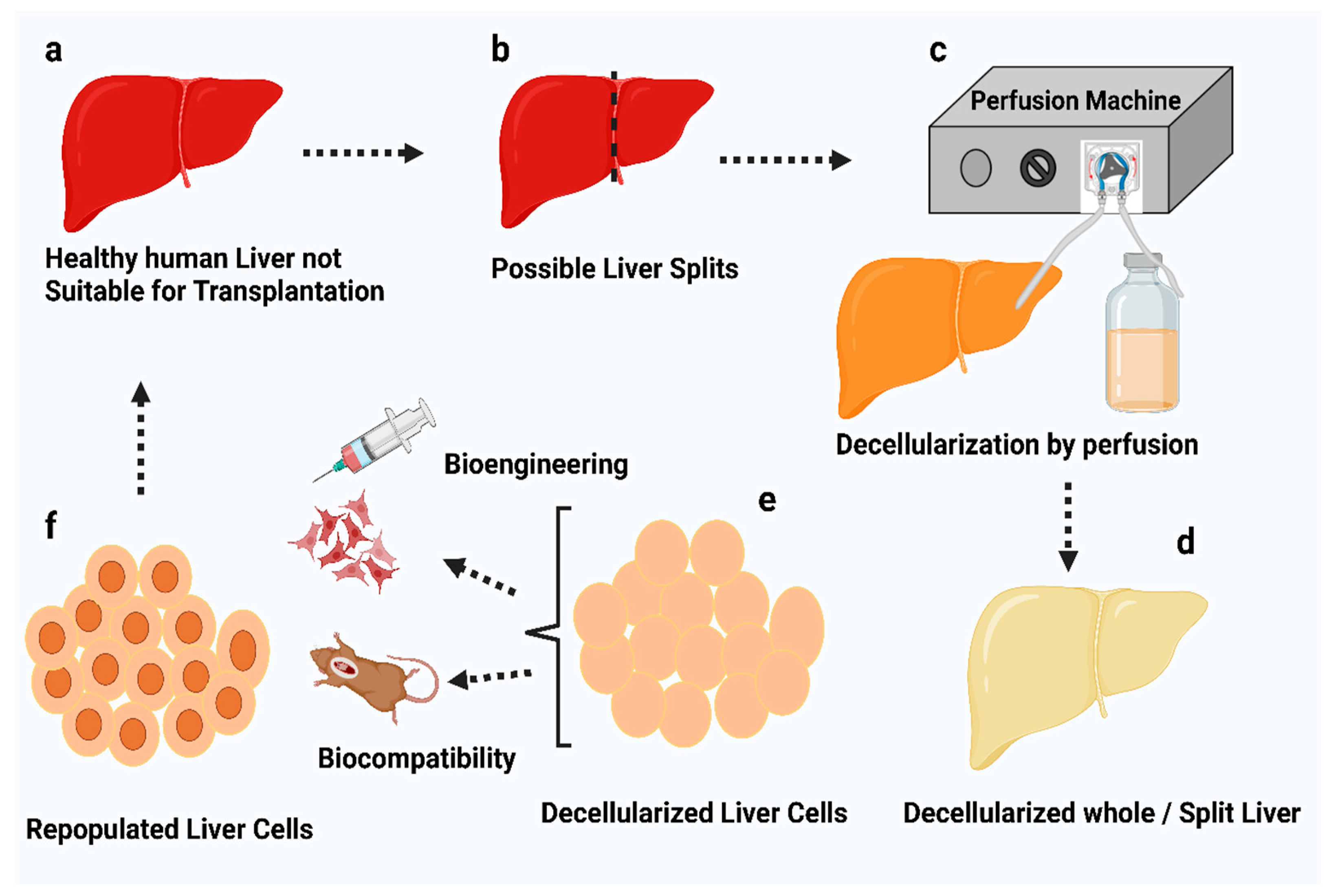
6. General Decellularization Techniques
6.1. Chemical Methods
6.1.1. Surfactants
6.1.2. Acids and Bases
6.2. Enzymatic Methods
6.3. Physical Methods
6.3.1. Freeze–Thawing
6.3.2. Mechanical Loading
6.3.3. Hydrostatic Pressure
6.3.4. Ultrasonication
6.3.5. Electroporation
6.3.6. Perfusion
6.3.7. Supercritical Fluid Treatment
7. Suggested Methodology for Optimal Results in Liver Tissue Decellularization
8. Characterization of Decellularized Liver Samples
9. Effects of Decellularization on ECM
10. Applications of Various Decellularized Liver Matrices and the Techniques Involved
10.1. 3D Bioprinting of Decellularized Hepatic Extracellular Matrix
10.2. Nanoparticles-Incorporated Decellularized Extracellular Matrices
10.3. Electrospinning
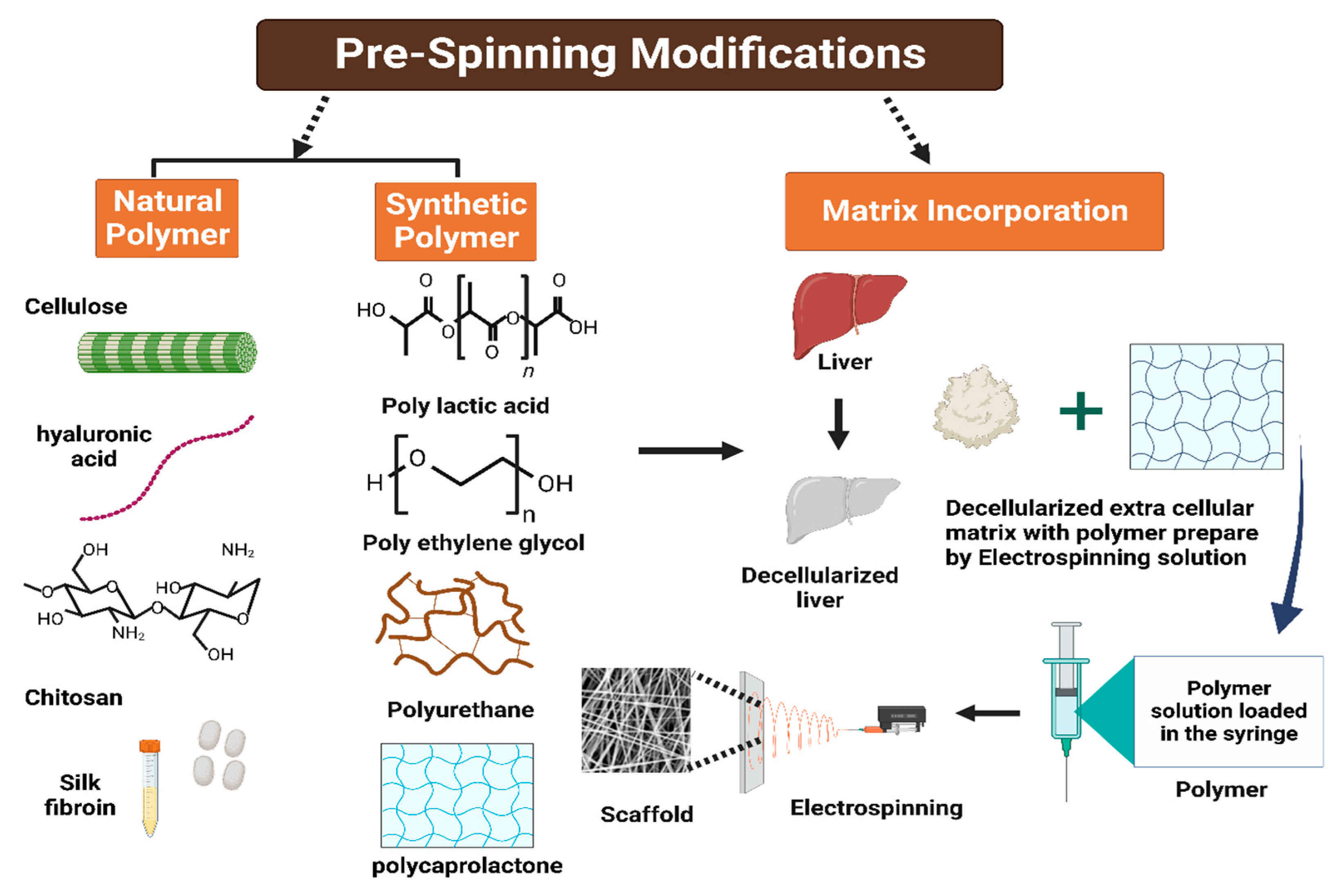
10.4. Lyophilization
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, R.-H.; Wang, Y.-C.; Liu, S.-P.; Shih, T.-R.; Lin, H.-L.; Chen, Y.-M.; Sung, J.-H.; Lu, C.-H.; Wei, J.-R.; Wang, Z.-W.; et al. Decellularization and Recellularization Technologies in Tissue Engineering. Cell Transplant. 2014, 23, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Gratzer, P.F. Decellularized Extracellular Matrix. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 86–96. ISBN 978-0-12-805144-3. [Google Scholar]
- Shimoda, H.; Yagi, H.; Higashi, H.; Tajima, K.; Kuroda, K.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y. Decellularized Liver Scaffolds Promote Liver Regeneration after Partial Hepatectomy. Sci. Rep. 2019, 9, 12543. [Google Scholar] [CrossRef]
- Chen, Y.; Geerts, S.; Jaramillo, M.; Uygun, B.E. Preparation of Decellularized Liver Scaffolds and Recellularized Liver Grafts. In Decellularized Scaffolds and Organogenesis; Turksen, K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1577, pp. 255–270. ISBN 978-1-4939-7655-3. [Google Scholar]
- Dai, Q.; Jiang, W.; Huang, F.; Song, F.; Zhang, J.; Zhao, H. Recent Advances in Liver Engineering with Decellularized Scaffold. Front. Bioeng. Biotechnol. 2022, 10, 831477. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.L.; Paranhos, B.A.; Goldenberg, R.C.D.S. Liver Scaffolds Obtained by Decellularization: A Transplant Perspective in Liver Bioengineering. J. Tissue Eng. 2022, 13, 204173142211053. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the Retention of Tissue Niches. J. Tissue Eng. 2022, 13, 204173142211011. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.Z.; Bloomston, M. Liver Anatomy. Surg. Clin. North. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef]
- Arias, I.M. (Ed.) The Liver: Biology and Pathobiology, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; ISBN 978-1-119-43684-3. [Google Scholar]
- Bram, Y.; Nguyen, D.-H.T.; Gupta, V.; Park, J.; Richardson, C.; Chandar, V.; Schwartz, R.E. Cell and Tissue Therapy for the Treatment of Chronic Liver Disease. Annu. Rev. Biomed. Eng. 2021, 23, 517–546. [Google Scholar] [CrossRef]
- Ishibashi, H.; Nakamura, M.; Komori, A.; Migita, K.; Shimoda, S. Liver Architecture, Cell Function, and Disease. Semin. Immunopathol. 2009, 31, 399–409. [Google Scholar] [CrossRef]
- Kaur, S.; Tripathi, D.M.; Venugopal, J.R.; Ramakrishna, S. Advances in Biomaterials for Hepatic Tissue Engineering. Curr. Opin. Biomed. Eng. 2020, 13, 190–196. [Google Scholar] [CrossRef]
- Sembulingam, K.; Sembulingam, P. Essentials of Medical Physiology, 6th ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2013; ISBN 978-93-5025-936-8. [Google Scholar]
- Akay, E.M.; Okumus, Z.; Yildirim, O.S.; Bokhari, M.A.; Akay, G. Synthetic Organs for Transplant and Bio-Mimic Reactors for Process Intensification Using Nano-Structured Micro-Porous Materials; WIT Press: Riga, Latvia, 2011; pp. 383–394. [Google Scholar]
- Krishna, M. Microscopic Anatomy of the Liver. Clin. Liver Dis. 2013, 2, S4–S7. [Google Scholar] [CrossRef]
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011; ISBN 978-1-4160-4574-8. [Google Scholar]
- Khurana, I. Medical Physiology for Undergraduate Students: Illustrated Synopsis of Dermatology and Sexually Transmitted Diseases, 1st ed.; Elsevier: New Delhi, India, 2014; ISBN 978-81-312-3622-2. [Google Scholar]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver Regeneration. Hepatology 2006, 43, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Maroufi, N.F.; Saghati, S.; Asadi, N.; Darabi, M.; Ahmad, S.N.S.; Hosseinkhani, H.; Rahbarghazi, R. Current Progress in Hepatic Tissue Regeneration by Tissue Engineering. J. Transl. Med. 2019, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Mitra, V.; Metcalf, J. Metabolic Functions of the Liver. Anaesth. Intensive Care Med. 2012, 13, 54–55. [Google Scholar] [CrossRef]
- Dawson, P.A. Bile Formation and the Enterohepatic Circulation. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 931–956. ISBN 978-0-12-809954-4. [Google Scholar]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D. Bilirubin Binding to PPARα Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chowdhury, J.R.; Chowdhury, N.R. Bilirubin Metabolism: Applied Physiology. Curr. Paediatr. 2006, 16, 70–74. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver Tissue Engineering: From Implantable Tissue to Whole Organ Engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and Liver Transplantation: Disease Burden, Current Management and Future Challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global Epidemiology of Cirrhosis—Aetiology, Trends and Predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Sudo, R. Multiscale Tissue Engineering for Liver Reconstruction. Organogenesis 2014, 10, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ Reengineering through Development of a Transplantable Recellularized Liver Graft Using Decellularized Liver Matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized Extracellular Matrix Scaffolds: Recent Trends and Emerging Strategies in Tissue Engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D.; Atzet, S. Engineered Natural Materials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 195–209. ISBN 978-0-12-374626-9. [Google Scholar]
- Chandra, P.K.; Atala, A.A. Use of Matrix and Seeding with Cells for Vasculature of Organs. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; p. B9780128012383110785. ISBN 978-0-12-801238-3. [Google Scholar]
- Wu, I.; Elisseeff, J. Biomaterials and Tissue Engineering for Soft Tissue Reconstruction. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 235–241. ISBN 978-0-12-396983-5. [Google Scholar]
- Gundu, S.; Sahi, A.K.; Varshney, N.; Varghese, J.; Vishwakarma, N.K.; Mahto, S.K. Fabrication and in Vitro Characterization of Luffa-Based Composite Scaffolds Incorporated with Gelatin, Hydroxyapatite and Psyllium Husk for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 2220–2248. [Google Scholar] [CrossRef] [PubMed]
- Allu, I.; Kumar Sahi, A.; Kumari, P.; Sakhile, K.; Sionkowska, A.; Gundu, S. A Brief Review on Cerium Oxide (CeO2NPs)-Based Scaffolds: Recent Advances in Wound Healing Applications. Micromachines 2023, 14, 865. [Google Scholar] [CrossRef]
- Lin, P.; Chan, W.C.W.; Badylak, S.F.; Bhatia, S.N. Assessing Porcine Liver-Derived Biomatrix for Hepatic Tissue Engineering. Tissue Eng. 2004, 10, 1046–1053. [Google Scholar] [CrossRef]
- Choudhury, D.; Yee, M.; Sheng, Z.L.J.; Amirul, A.; Naing, M.W. Decellularization Systems and Devices: State-of-the-Art. Acta Biomater. 2020, 115, 51–59. [Google Scholar] [CrossRef]
- Martinez-Hernandez, A.; Amenta, P.S. The Hepatic Extracellular Matrix: I. Components and Distribution in Normal Liver. Vichows Arch. A Pathol. Anat. 1993, 423, 1–11. [Google Scholar] [CrossRef]
- Laurila, P.; Leivo, I. Basement Membrane and Interstitial Matrix Components Form Separate Matrices in Heterokaryons of PYS-2 Cells and Fibroblasts. J. Cell Sci. 1993, 104, 59–68. [Google Scholar] [CrossRef]
- Bedossa, P.; Paradis, V. Liver Extracellular Matrix in Health and Disease: Liver Extracellular Matrix. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease: Type IV Collagen and Laminin. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Rescan, P.Y.; Loréal, O.; Hassell, J.R.; Yamada, Y.; Guillouzo, A.; Clément, B. Distribution and Origin of the Basement Membrane Component Perlecan in Rat Liver and Primary Hepatocyte Culture. Am. J. Pathol. 1993, 142, 199–208. [Google Scholar] [PubMed]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular Matrix Degradation in Liver Fibrosis: Biochemistry and Regulation. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Rauterberg, J.; Voss, B.; Pott, G.; Gerlach, U. Connective Tissue Components of the Normal and Fibrotic Liver: I. Structure, Local Distribution and Metabolism of Connective Tissue Components in the Normal Liver and Changes in Chronic Liver Diseases. Klin. Wochenschr. 1981, 59, 767–779. [Google Scholar] [CrossRef]
- Xu, B.; Broome, U.; Uzunel, M.; Nava, S.; Ge, X.; Kumagai-Braesch, M.; Hultenby, K.; Christensson, B.; Ericzon, B.-G.; Holgersson, J.; et al. Capillarization of Hepatic Sinusoid by Liver Endothelial Cell-Reactive Autoantibodies in Patients with Cirrhosis and Chronic Hepatitis. Am. J. Pathol. 2003, 163, 1275–1289. [Google Scholar] [CrossRef]
- Klaas, M.; Kangur, T.; Viil, J.; Mäemets-Allas, K.; Minajeva, A.; Vadi, K.; Antsov, M.; Lapidus, N.; Järvekülg, M.; Jaks, V. The Alterations in the Extracellular Matrix Composition Guide the Repair of Damaged Liver Tissue. Sci. Rep. 2016, 6, 27398. [Google Scholar] [CrossRef]
- Thiele, M.; Johansen, S.; Gudmann, N.S.; Madsen, B.; Kjærgaard, M.; Nielsen, M.J.; Leeming, D.J.; Jacobsen, S.; Bendtsen, F.; Møller, S.; et al. Progressive Alcohol-related Liver Fibrosis Is Characterised by Imbalanced Collagen Formation and Degradation. Aliment. Pharmacol. Ther. 2021, 54, 1070–1080. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Lopresti, S.T.; Brown, B.N. Host Response to Naturally Derived Biomaterials. In Host Response to Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 53–79. ISBN 978-0-12-800196-7. [Google Scholar]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and Disinfection Methods for Decellularized Matrix Materials: Review, Consideration and Proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef] [PubMed]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized Scaffolds for Tissue Engineering: Current Status and Future Perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of Decellularized Materials in Tissue Engineering: Advantages, Drawbacks and Current Improvements, and Future Perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Ramella, M.; Fusaro, L.; Catoira, M.C.; Casella, F. Biological Grafts: Surgical Use and Vascular Tissue Engineering Options for Peripheral Vascular Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 310–321. ISBN 978-0-12-805144-3. [Google Scholar]
- Wang, X.; Chan, V.; Corridon, P.R. Decellularized Blood Vessel Development: Current State-of-the-Art and Future Directions. Front. Bioeng. Biotechnol. 2022, 10, 951644. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of Physical Methods in Decellularization of Animal Tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Lange, P.; Greco, K.; Partington, L.; Carvalho, C.; Oliani, S.; Birchall, M.A.; Sibbons, P.D.; Lowdell, M.W.; Ansari, T. Pilot Study of a Novel Vacuum-Assisted Method for Decellularization of Tracheae for Clinical Tissue Engineering Applications: Vacuum-Assisted Decellularization. J. Tissue Eng. Regen. Med. 2017, 11, 800–811. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rezakhani, L.; Soleimannejad, M.; Sharifi, E.; Anjomshoa, M.; Alizadeh, A. Evaluation of Vacuum Washing in the Removal of SDS from Decellularized Bovine Pericardium: Method and Device Description. Heliyon 2019, 5, e02253. [Google Scholar] [CrossRef]
- Butler, C.R.; Hynds, R.E.; Crowley, C.; Gowers, K.H.C.; Partington, L.; Hamilton, N.J.; Carvalho, C.; Platé, M.; Samuel, E.R.; Burns, A.J.; et al. Vacuum-Assisted Decellularization: An Accelerated Protocol to Generate Tissue-Engineered Human Tracheal Scaffolds. Biomaterials 2017, 124, 95–105. [Google Scholar] [CrossRef]
- Lei, C.; Mei, S.; Zhou, C.; Xia, C. Decellularized Tracheal Scaffolds in Tracheal Reconstruction: An Evaluation of Different Techniques. J. Appl. Biomater. Funct. Mater. 2021, 19, 228080002110649. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Rezakhani, L.; Khodaei, M.; Soleimannejad, M.; Alizadeh, A. Evaluating the Effects of Vacuum on the Microstructure and Biocompatibility of Bovine Decellularized Pericardium. J. Tissue Eng. Regen. Med. 2021, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.-M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Das, P.; Singh, Y.P.; Mandal, B.B.; Nandi, S.K. Tissue-Derived Decellularized Extracellular Matrices toward Cartilage Repair and Regeneration. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 157, pp. 185–221. ISBN 978-0-12-820174-9. [Google Scholar]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The Use of High-Hydrostatic Pressure Treatment to Decellularize Blood Vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines 2021, 12, 1486. [Google Scholar] [CrossRef]
- Nouri Barkestani, M.; Naserian, S.; Uzan, G.; Shamdani, S. Post-Decellularization Techniques Ameliorate Cartilage Decellularization Process for Tissue Engineering Applications. J. Tissue Eng. 2021, 12, 204173142098356. [Google Scholar] [CrossRef]
- Smagowska, B.; Pawlaczyk-Łuszczyńska, M. Effects of Ultrasonic Noise on the Human Body—A Bibliographic Review. Int. J. Occup. Saf. Ergon. 2013, 19, 195–202. [Google Scholar] [CrossRef]
- Rubio, F.; Blandford, E.D.; Bond, L.J. Survey of Advanced Nuclear Technologies for Potential Applications of Sonoprocessing. Ultrasonics 2016, 71, 211–222. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsia, K.; Su, C.-K.; Chen, C.-C.; Yeh, C.-C.; Ma, H.; Lu, J.-H. Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers 2021, 13, 1699. [Google Scholar] [CrossRef]
- Phillips, M.; Maor, E.; Rubinsky, B. Nonthermal Irreversible Electroporation for Tissue Decellularization. J. Biomech. Eng. 2010, 132, 091003. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; So, K.; Hwang, N.S. Supercritical Fluid-Based Decellularization Technologies for Regenerative Medicine Applications. Macromol. Biosci. 2021, 21, 2100160. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of Freeze-Thaw Injury and Recovery: A Cool Retrospective and Warming up to New Ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-Thaw Cycles Enhance Decellularization of Large Tendons. Tissue Eng. Methods 2014, 20, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of Freezing and Thawing Rates on Denaturation of Proteins in Aqueous Solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, C.; Gu, Y. Combination of Freeze-Thaw with Detergents: A Promising Approach to the Decellularization of Porcine Carotid Arteries. Bio-Med. Mater. Eng. 2019, 30, 191–205. [Google Scholar] [CrossRef]
- Poornejad, N.; Frost, T.S.; Scott, D.R.; Elton, B.B.; Reynolds, P.R.; Roeder, B.L.; Cook, A.D. Freezing/Thawing without Cryoprotectant Damages Native but Not Decellularized Porcine Renal Tissue. Organogenesis 2015, 11, 30–45. [Google Scholar] [CrossRef]
- Santoso, E.G.; Yoshida, K.; Hirota, Y.; Aizawa, M.; Yoshino, O.; Kishida, A.; Osuga, Y.; Saito, S.; Ushida, T.; Furukawa, K.S. Application of Detergents or High Hydrostatic Pressure as Decellularization Processes in Uterine Tissues and Their Subsequent Effects on In Vivo Uterine Regeneration in Murine Models. PLoS ONE 2014, 9, e103201. [Google Scholar] [CrossRef]
- Azhim, A.; Syazwani, N.; Morimoto, Y.; Furukawa, K.; Ushida, T. The Use of Sonication Treatment to Decellularize Aortic Tissues for Preparation of Bioscaffolds. J. Biomater. Appl. 2014, 29, 130–141. [Google Scholar] [CrossRef]
- Syazwani, N.; Azhim, A.; Morimoto, Y.; Furukawa, K.S.; Ushida, T. Decellularization of Aorta Tissue Using Sonication Treatment as Potential Scaffold for Vascular Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 258–269. [Google Scholar] [CrossRef]
- Yusof, F.; Sha’ban, M.; Azhim, A. Development of Decellularized Meniscus Using Closed Sonication Treatment System: Potential Scaffolds for Orthopedics Tissue Engineering Applications. Int. J. Nanomed. 2019, 14, 5491–5502. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.P. Tissue Engineering with Electroporation. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–21. ISBN 978-3-319-26779-1. [Google Scholar]
- Koo, M.-A.; Jeong, H.; Hong, S.H.; Seon, G.M.; Lee, M.H.; Park, J.-C. Preconditioning Process for Dermal Tissue Decellularization Using Electroporation with Sonication. Regen. Biomater. 2022, 9, rbab071. [Google Scholar] [CrossRef]
- Gerli, M.F.M.; Guyette, J.P.; Evangelista-Leite, D.; Ghoshhajra, B.B.; Ott, H.C. Perfusion Decellularization of a Human Limb: A Novel Platform for Composite Tissue Engineering and Reconstructive Surgery. PLoS ONE 2018, 13, e0191497. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion Decellularization of Whole Organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef]
- Haniel, J.; de Souza Brandão, A.P.M.; Cruz, R.D.C.; Vale, M.; Soares, B.M.; Huebner, R. <b>Development of Equipment for Decellularization Using the Perfusion Method. Acta Sci. Technol. 2018, 40, 35349. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.Q.; Turner, N.J.; Teng, S.F.; Cheng, W.Y.; Zhou, H.Y.; Zhang, L.; Hu, H.W.; Wang, Q.; Badylak, S.F. Perfusion-Decellularized Skeletal Muscle as a Three-Dimensional Scaffold with a Vascular Network Template. Biomaterials 2016, 89, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Casali, D.M.; Handleton, R.M.; Shazly, T.; Matthews, M.A. A Novel Supercritical CO 2 -Based Decellularization Method for Maintaining Scaffold Hydration and Mechanical Properties. J. Supercrit. Fluids 2018, 131, 72–81. [Google Scholar] [CrossRef]
- Chou, P.-R.; Lin, Y.-N.; Wu, S.-H.; Lin, S.-D.; Srinivasan, P.; Hsieh, D.-J.; Huang, S.-H. Supercritical Carbon Dioxide-Decellularized Porcine Acellular Dermal Matrix Combined with Autologous Adipose-Derived Stem Cells: Its Role in Accelerated Diabetic Wound Healing. Int. J. Med. Sci. 2020, 17, 354–367. [Google Scholar] [CrossRef]
- Duarte, M.M.; Silva, I.V.; Eisenhut, A.R.; Bionda, N.; Duarte, A.R.C.; Oliveira, A.L. Contributions of Supercritical Fluid Technology for Advancing Decellularization and Postprocessing of Viable Biological Materials. Mater. Horiz. 2022, 9, 864–891. [Google Scholar] [CrossRef]
- Srinivasan, P.; Hsieh, D.-J. Supercritical Carbon Dioxide Facilitated Collagen Scaffold Production for Tissue Engineering. In Collagen Biomaterials; Mazumder, N., Chakrabarty, S., Eds.; IntechOpen: Rijeka, Croatia, 2022; ISBN 978-1-80355-411-2. [Google Scholar]
- Jiang, Y.; Li, R.; Han, C.; Huang, L. Extracellular Matrix Grafts: From Preparation to Application (Review). Int. J. Mol. Med. 2020, 47, 463–474. [Google Scholar] [CrossRef]
- Yang, W. Nucleases: Diversity of Structure, Function and Mechanism. Quart. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Nokhbatolfoghahaei, H.; Paknejad, Z.; Bohlouli, M.; Rezai Rad, M.; Aminishakib, P.; Derakhshan, S.; Mohammadi Amirabad, L.; Nadjmi, N.; Khojasteh, A. Fabrication of Decellularized Engineered Extracellular Matrix through Bioreactor-Based Environment for Bone Tissue Engineering. ACS Omega 2020, 5, 31943–31956. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of Decellularized Extracellular Matrix in Bone and Cartilage Tissue Engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Narciso, M.; Ulldemolins, A.; Júnior, C.; Otero, J.; Navajas, D.; Farré, R.; Gavara, N.; Almendros, I. Novel Decellularization Method for Tissue Slices. Front. Bioeng. Biotechnol. 2022, 10, 832178. [Google Scholar] [CrossRef]
- Akbari Zahmati, A.H.; Alipoor, R.; Rezaei Shahmirzadi, A.; Khori, V.; Abolhasani, M.M. Chemical Decellularization Methods and Its Effects on Extracellular Matrix. Intern. Med. Med. Investig. J. 2017, 2, 76. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Alaby Pinheiro Faccioli, L.; Suhett Dias, G.; Hoff, V.; Lemos Dias, M.; Ferreira Pimentel, C.; Hochman-Mendez, C.; Braz Parente, D.; Labrunie, E.; Souza Mourão, P.A.; Rogério de Oliveira Salvalaggio, P.; et al. Optimizing the Decellularized Porcine Liver Scaffold Protocol. Cells Tissues Organs 2022, 211, 385–394. [Google Scholar] [CrossRef]
- Croce, S.; Peloso, A.; Zoro, T.; Avanzini, M.A.; Cobianchi, L. A Hepatic Scaffold from Decellularized Liver Tissue: Food for Thought. Biomolecules 2019, 9, 813. [Google Scholar] [CrossRef]
- Hillebrandt, K.H.; Everwien, H.; Haep, N.; Keshi, E.; Pratschke, J.; Sauer, I.M. Strategies Based on Organ Decellularization and Recellularization. Transpl. Int. 2019, 32, 571–585. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized Human Liver as a Natural 3D-Scaffold for Liver Bioengineering and Transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef]
- Coronado, R.E.; Somaraki-Cormier, M.; Natesan, S.; Christy, R.J.; Ong, J.L.; Halff, G.A. Decellularization and Solubilization of Porcine Liver for Use as a Substrate for Porcine Hepatocyte Culture: Method Optimization and Comparison. Cell Transpl. 2017, 26, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Stern, M.M.; Smith, L.; Liu, Y.; Bharadwaj, S.; Liu, G.; Baptista, P.M.; Bergman, C.R.; Soker, S.; Yoo, J.J.; et al. Three-Dimensional Culture of Hepatocytes on Porcine Liver Tissue-Derived Extracellular Matrix. Biomaterials 2011, 32, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.; Verstegen, M.M.A.; Vermeulen, A.; Schurink, I.J.; Roest, H.P.; Van Der Laan, L.J.W.; De Jonge, J. Fast, Robust and Effective Decellularization of Whole Human Livers Using Mild Detergents and Pressure Controlled Perfusion. Mater. Sci. Eng. C 2020, 108, 110200. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bao, J.; Zhou, Y.; Wang, Y.; Du, Z.; Shi, Y.; Li, L.; Bu, H. Optimizing Perfusion-Decellularization Methods of Porcine Livers for Clinical-Scale Whole-Organ Bioengineering. BioMed Res. Int. 2015, 2015, 785474. [Google Scholar] [CrossRef] [PubMed]
- Demko, P.; Hillebrandt, K.H.; Napierala, H.; Haep, N.; Tang, P.; Gassner, J.M.G.V.; Kluge, M.; Everwien, H.; Polenz, D.; Reutzel-Selke, A.; et al. Perfusion-Based Recellularization of Rat Livers with Islets of Langerhans. J. Med. Biol. Eng. 2022, 42, 271–280. [Google Scholar] [CrossRef]
- Fathi, I.; Eltawila, A. Whole-Liver Decellularization: Advances and Insights into Current Understanding. In Xenotransplantation-New Insights; Miyagawa, S., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3355-1. [Google Scholar]
- Nicholls, D.L.; Rostami, S.; Karoubi, G.; Haykal, S. Perfusion Decellularization for Vascularized Composite Allotransplantation. SAGE Open Med. 2022, 10, 205031212211238. [Google Scholar] [CrossRef]
- Shaheen, M.F.; Joo, D.J.; Ross, J.J.; Anderson, B.D.; Chen, H.S.; Huebert, R.C.; Li, Y.; Amiot, B.; Young, A.; Zlochiver, V.; et al. Sustained Perfusion of Revascularized Bioengineered Livers Heterotopically Transplanted into Immunosuppressed Pigs. Nat. Biomed. Eng. 2019, 4, 437–445. [Google Scholar] [CrossRef]
- Toprakhisar, B.; Verfaillie, C.M.; Kumar, M. Advances in Recellularization of Decellularized Liver Grafts with Different Liver (Stem) Cells: Towards Clinical Applications. Cells 2023, 12, 301. [Google Scholar] [CrossRef]
- Maghsoudlou, P.; Georgiades, F.; Smith, H.; Milan, A.; Shangaris, P.; Urbani, L.; Loukogeorgakis, S.P.; Lombardi, B.; Mazza, G.; Hagen, C.; et al. Optimization of Liver Decellularization Maintains Extracellular Matrix Micro-Architecture and Composition Predisposing to Effective Cell Seeding. PLoS One 2016, 11, e0155324. [Google Scholar] [CrossRef]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Methods 2017, 23, 243–250. [Google Scholar] [CrossRef]
- Brézillon, S.; Untereiner, V.; Mohamed, H.T.; Hodin, J.; Chatron-Colliet, A.; Maquart, F.-X.; Sockalingum, G.D. Probing Glycosaminoglycan Spectral Signatures in Live Cells and Their Conditioned Media by Raman Microspectroscopy. Analyst 2017, 142, 1333–1341. [Google Scholar] [CrossRef]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The Use of Whole Organ Decellularization for the Generation of a Vascularized Liver Organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.-M.; Yu, L.; Kwak, H.-H.; Woo, H.-M. Decellularized Hepatic Extracellular Matrix Hydrogel Attenuates Hepatic Stellate Cell Activation and Liver Fibrosis. Mater. Sci. Eng. C 2020, 116, 111160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, J.; Wu, Q.; Zhou, Y.; Li, Y.; Wu, X.; Shi, Y.; Li, L.; Bu, H. Method for Perfusion Decellularization of Porcine Whole Liver and Kidney for Use as a Scaffold for Clinical-Scale Bioengineering Engrafts. Xenotransplantation 2015, 22, 48–61. [Google Scholar] [CrossRef]
- Washabau, R.J.; Day, M.J. Liver. In Canine and Feline Gastroenterology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 849–957. ISBN 978-1-4160-3661-6. [Google Scholar]
- Shupe, T.; Williams, M.; Brown, A.; Willenberg, B.; Petersen, B.E. Method for the Decellularization of Intact Rat Liver. Organogenesis 2010, 6, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Mallarpu, C.S.; Chelluri, L.K. Development of Bioengineered Organ Using Biological Acellular Rat Liver Scaffold and Hepatocytes. Organogenesis 2020, 16, 61–72. [Google Scholar] [CrossRef] [PubMed]
- De Kock, J.; Ceelen, L.; De Spiegelaere, W.; Casteleyn, C.; Claes, P.; Vanhaecke, T.; Rogiers, V. Simple and Quick Method for Whole-Liver Decellularization: A Novel In Vitro Three-Dimensional Bioengineering Tool? Arch. Toxicol. 2011, 85, 607–612. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Hong, Y.; Li, T.; Hu, X.; Sun, L.; Chen, Z.; Chen, Z.; Luo, Z.; Wang, X.; et al. Decellularized Liver as a Translucent Ex Vivo Model for Vascular Embolization Evaluation. Biomaterials 2020, 240, 119855. [Google Scholar] [CrossRef]
- Lu, S.; Cuzzucoli, F.; Jiang, J.; Liang, L.-G.; Wang, Y.; Kong, M.; Zhao, X.; Cui, W.; Li, J.; Wang, S. Development of a Biomimetic Liver Tumor-on-a-Chip Model Based on Decellularized Liver Matrix for Toxicity Testing. Lab. Chip 2018, 18, 3379–3392. [Google Scholar] [CrossRef]
- Caires-Júnior, L.C.; Goulart, E.; Telles-Silva, K.A.; Araujo, B.H.S.; Musso, C.M.; Kobayashi, G.; Oliveira, D.; Assoni, A.; Carvalho, V.M.; Ribeiro-Jr, A.F.; et al. Pre-Coating Decellularized Liver with HepG2-Conditioned Medium Improves Hepatic Recellularization. Mater. Sci. Eng. C 2021, 121, 111862. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Navarro-Alvarez, N.; Yagi, H.; Nahmias, Y.; Yarmush, M.L.; Kobayashi, N. Engineering of an Hepatic Organoid to Develop Liver Assist Devices. Cell Transpl. 2010, 19, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Li, Y.; Jia, L.; Mo, X.; Jiang, G.; Zhou, G. 3D Printing Electrospinning Fiber-Reinforced Decellularized Extracellular Matrix for Cartilage Regeneration. Chem. Eng. J. 2020, 382, 122986. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Zhou, X.; Zhang, J.; Zhou, H.; Hao, H.; Ding, L.; Li, H.; Gu, Y.; Ma, J.; et al. An Overview of Extracellular Matrix-Based Bioinks for 3D Bioprinting. Front. Bioeng. Biotechnol. 2022, 10, 905438. [Google Scholar] [CrossRef]
- Khati, V. Decellularized Liver Extracellular Matrix as a 3D Scaffold for Bioengineering Applications; KTH Royal Institute of Technology: Stockholm, Sweden, 2022. [Google Scholar]
- Zhang, C.-Y.; Fu, C.-P.; Li, X.-Y.; Lu, X.-C.; Hu, L.-G.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering. Molecules 2022, 27, 3442. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B.S.; Cho, D.-W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef]
- Zhe, M.; Wu, X.; Yu, P.; Xu, J.; Liu, M.; Yang, G.; Xiang, Z.; Xing, F.; Ritz, U. Recent Advances in Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting in Tissue Engineering. Materials 2023, 16, 3197. [Google Scholar] [CrossRef]
- Agmon, G.; Christman, K.L. Controlling Stem Cell Behavior with Decellularized Extracellular Matrix Scaffolds. Curr. Opin. Solid. State Mater. Sci. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of Liver Microtissue with Liver Decellularized Extracellular Matrix (dECM) Bioink by Digital Light Processing (DLP) Bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.A.; Gaudenzi, G.; Russom, A. 3D Bioprinting of Multi-Material Decellularized Liver Matrix Hydrogel at Physiological Temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Kang, H.-W. Liver dECM–Gelatin Composite Bioink for Precise 3D Printing of Highly Functional Liver Tissues. J. Funct. Biomater. 2023, 14, 417. [Google Scholar] [CrossRef]
- Mir, T.A.; Nakamura, M.; Sakai, S.; Iwanaga, S.; Wani, S.I.; Alzhrani, A.; Arai, K.; Mir, B.A.; Kazmi, S.; Assiri, A.M.; et al. Mammalian-Specific Decellularized Matrices Derived Bioink for Bioengineering of Liver Tissue Analogues: A Review. Int. J. Bioprint. 2023, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [PubMed]
- Semba, J.A.; Mieloch, A.A.; Tomaszewska, E.; Cywoniuk, P.; Rybka, J.D. Formulation and Evaluation of a Bioink Composed of Alginate, Gelatin, and Nanocellulose for Meniscal Tissue Engineering. Int. J. Bioprint. 2022, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Song, Z.; Wang, Z.; Wang, Z.; Li, Z.; Wang, C.; Liu, H.; Liu, Q.; Wang, J. Printability Optimization of Gelatin-Alginate Bioinks by Cellulose Nanofiber Modification for Potential Meniscus Bioprinting. J. Nanomater. 2020, 2020, 3863428. [Google Scholar] [CrossRef]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- Ahmed, E.; Saleh, T.; Yu, L.; Song, S.; Park, K.; Kwak, H.; Woo, H. Decellularized Extracellular Matrix-rich Hydrogel–Silver Nanoparticle Mixture as a Potential Treatment for Acute Liver Failure Model. J. Biomed. Mater. Res. 2020, 108, 2351–2367. [Google Scholar] [CrossRef]
- Saleh, T.; Ahmed, E.; Yu, L.; Hussein, K.; Park, K.-M.; Lee, Y.-S.; Kang, B.-J.; Choi, K.-Y.; Choi, S.; Kang, K.-S.; et al. Silver Nanoparticles Improve Structural Stability and Biocompatibility of Decellularized Porcine Liver. Artif. Cells Nanomed. Biotechnol. 2018, 46, 273–284. [Google Scholar] [CrossRef]
- Saleh, T.M.; Ahmed, E.A.; Yu, L.; Kwak, H.-H.; Hussein, K.H.; Park, K.-M.; Kang, B.-J.; Choi, K.-Y.; Kang, K.-S.; Woo, H.-M. Incorporation of Nanoparticles into Transplantable Decellularized Matrices: Applications and Challenges. Int. J. Artif. Organs 2018, 41, 421–430. [Google Scholar] [CrossRef]
- Xu, F.; Kang, T.; Deng, J.; Liu, J.; Chen, X.; Wang, Y.; Ouyang, L.; Du, T.; Tang, H.; Xu, X.; et al. Functional Nanoparticles Activate a Decellularized Liver Scaffold for Blood Detoxification. Small 2020, 16, 2001267. [Google Scholar] [CrossRef]
- Sahi, A.K.; Varshney, N.; Poddar, S.; Mahto, S.K. Comparative Behaviour of Electrospun Nanofibers Fabricated from Acid and Alkaline Hydrolysed Gelatin: Towards Corneal Tissue Engineering. J. Polym. Res. 2020, 27, 344. [Google Scholar] [CrossRef]
- Sahi, A.K.; Varshney, N.; Poddar, S.; Gundu, S.; Mahto, S.K. Fabrication and Characterization of Silk Fibroin-Based Nanofibrous Scaffolds Supplemented with Gelatin for Corneal Tissue Engineering. Cells Tissues Organs 2021, 210, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sahi, A.; Gundu, S.; Kumari, P.; Klepka, T.; Sionkowska, A. Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications. Biomimetics 2023, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Santschi, M.; Vernengo, A.; Eglin, D.; D’Este, M.; Wuertz-Kozak, K. Decellularized Matrix as a Building Block in Bioprinting and Electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Liu, J.S.; Madruga, L.Y.C.; Yuan, Y.; Kipper, M.J.; Khetani, S.R. Decellularized Liver Nanofibers Enhance and Stabilize the Long-Term Functions of Primary Human Hepatocytes In Vitro. Adv. Healthc. Mater. 2023, 12, 2202302. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Tripathi, D.M.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S.; Kaur, S. Evolution of Electrospinning in Liver Tissue Engineering. Biomimetics 2022, 7, 149. [Google Scholar] [CrossRef]
- Grant, R.; Hallett, J.; Forbes, S.; Hay, D.; Callanan, A. Blended Electrospinning with Human Liver Extracellular Matrix for Engineering New Hepatic Microenvironments. Sci. Rep. 2019, 9, 6293. [Google Scholar] [CrossRef]
- Grant, R.; Hay, D.C.; Callanan, A. A Drug-Induced Hybrid Electrospun Poly-Capro-Lactone: Cell-Derived Extracellular Matrix Scaffold for Liver Tissue Engineering. Tissue Eng. 2017, 23, 650–662. [Google Scholar] [CrossRef]
- Goecke, T.; Theodoridis, K.; Tudorache, I.; Ciubotaru, A.; Cebotari, S.; Ramm, R.; Höffler, K.; Sarikouch, S.; Vásquez Rivera, A.; Haverich, A.; et al. In Vivo Performance of Freeze-Dried Decellularized Pulmonary Heart Valve Allo- and Xenografts Orthotopically Implanted into Juvenile Sheep. Acta Biomater. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, S.; Chen, Z.; Li, X.; Liu, C.; Bai, J.; Jiang, D.; Nie, Y.; Zhang, J.; Liu, T.; et al. Preparation and Properties of Decellularized Sheep Kidney Derived Matrix Scaffolds. J. Phys. Conf. Ser. 2022, 2160, 012014. [Google Scholar] [CrossRef]
- Gundu, S.; Varshney, N.; Sahi, A.K.; Mahto, S.K. Recent Developments of Biomaterial Scaffolds and Regenerative Approaches for Craniomaxillofacial Bone Tissue Engineering. J. Polym. Res. 2022, 29, 73. [Google Scholar] [CrossRef]
- Ibne Mahbub, M.S.; Bae, S.H.; Gwon, J.-G.; Lee, B.-T. Decellularized Liver Extracellular Matrix and Thrombin Loaded Biodegradable TOCN/Chitosan Nanocomposite for Hemostasis and Wound Healing in Rat Liver Hemorrhage Model. Int. J. Biol. Macromol. 2023, 225, 1529–1542. [Google Scholar] [CrossRef] [PubMed]




| Technique | Mechanism of Action | Effects | Advantages | Limitations | Applications | References |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Freeze–thawing | denatured proteins, resulting in cell necrosis. | eliminated cellular contents completely. retained structural proteins. | adequate cell removal. intact basement membrane. | inefficient removal of genetic material. Ice crystal formation may disrupt the ECM ultrastructure. | tendon, porcine carotid artery, porcine renal tissue. | [78,79,80,81,82] |
| Mechanical Loading | application of physical force caused cell lysis. | removed the surface cellular components of the tissue or the whole organ. | minimal disruption to the ECM architecture. | only suitable for tissues with sufficient mechanical hardness and a less dense ECM. applying force must be performed with the utmost caution to prevent damage to interior structures. | small intestine, urinary bladder | [56,61] |
| Hydrostatic pressure | high hydrostatic pressure caused cell membrane disruption resulting in cell death. | effectively removed cellular and nuclear content. | biomechanical properties of decellularized grafts remain unaltered. relatively short treatment time. | ultra-high pressures can result in protein denaturation. | blood vessels, uterine tissue, and corneal tissue. | [70,71,83] |
| Ultrasonication | caused cell membrane disruption due to induced shear stresses by cavitation. | effective removal of about 90% of cellular content. preserved structural proteins of cells. | retained the ECM microstructure. short treatment time. | demand perfect control over sonication power. | umbilical artery, aorta | [75,84,85,86] |
| Electroporation | distortion of the cell membrane occurred following the application of pulsed electric fields. | efficient cell removal. ECM remained intact. | porosity can be controlled by adjusting the electrical parameters. intact basement membrane. | relatively smaller electrodes limit the tissue surface area decellularized. not preferable for cardiac applications. | porcine liver, skin tissue. | [61,76,87,88] |
| Perfusion | eliminated the cell remnants by allowing a constant flow of decellularizing agents through the tissue. | solubilized cellular and nuclear material. Preserved structural proteins. | generation of biocompatible, non-toxic decellularized scaffolds. maintained ECM ultrastructure. | size shrinkage occurred following the procedure. | porcine renal tissue, heart, and lung tissue. | [89,90,91,92] |
| Supercritical fluid | A relatively inert gas, carbon dioxide at low temperature and pressure conditions facilitated the removal of cellular components. | effectively removed cellular and immunogenic agents. cause tissue dehydration. | preserved the structural integrity of ECM proteins. easily achievable treatment conditions with relatively brief treatment times. | tissue dehydration results in increased scaffold brittleness. | bovine neural tissue, porcine cartilage, adipose tissue, and bovine dermis. | [77,93,94,95,96] |
| (B) | ||||||
| Nucleases (DNases and RNases) | disintegrated nucleic acid sequences by cleaving the phosphodiester bonds. | eliminated cellular remnants. retained collagen and elastin | retained the biomechanical properties of the original tissue | ineffective in the complete removal of nuclear material. Induced immunological response | neural tissue, trachea, adipose tissue, intervertebral discs, porcine heart valves | [7,58,59,67,97,98] |
| Proteases (Trypsin, collagenase and dispase) | cleaved peptide bonds selectively. | detached cells from the tissue. removed matrix proteins such as collagen, elastin, laminins, etc. | efficient in complete cell removal in soft tissues. impedes cell conglomeration. | disrupted the ECM integrity. reduced biomechanical strength of decellularized scaffolds. Ineffective in complete cell removal. | porcine cornea, heart valves, and the dermis. | [7,67,68,69,97,99,100] |
| (C) | ||||||
| Surfactants | ||||||
| Ionic (SDS) | solubilized the cell and nucleic materials Denatured proteins | effectively removed cellular and nuclear material. distorted the structural and signaling proteins. | allowed for complete cell removal and about 90% of host DNA. | disrupted the ECM. decreased GAGs and growth factors. cytotoxic and required an extensive wash process. | porcine cornea, porcine myocardium, porcine kidney, human vein, etc. | [58,59] |
| Non-ionic (Triton X-100) | disrupted DNA-protein, lipid-protein, and lipid-lipid interactions | partially efficient in removing genetic material. Retained elastin. | less harsh than SDS and, therefore, caused less damage to the structural integrity of ECM. | less effective than SDS in removing cells and nuclear material. should be used in conjunction with Ammonium Hydroxide to facilitate complete cell removal. | bovine pericardium, porcine kidney, etc. | [58,59,101] |
| Zwitterionic (CHAPS) | solubilized cell and nuclear membranes Exhibited properties of both ionic and non-ionic surfactants | preserved structural proteins. Effectively removed about 95% of nuclear constituents. | superior cell removal. substantial preservation of ECM architecture. | disrupted the integrity of the basement membrane of ECM | vasculature, neural tissue. | [59,60,102] |
| Acids and Bases | hydrolyzed cytoplasmic constituents of cell | reduced collagen, GAG content, and growth factors. | treatment with an alkaline solution allowed for the complete removal of cellular and nuclear material. acid-mediated decellularization was effective in eliminating residuary genetic constituents. | effects on ECM were found to be strident disrupting the peptide bonds and reducing the overall mechanical properties. | small intestine submucosa, urinary bladder matrix, and dermis samples. | [53,56,58,67,102,103] |
| Protocol | Effects on ECM | References |
|---|---|---|
| 1% Triton X-100 | adequate clearance of cellular debris, a high DNA removal efficiency of about 96%, better collagen retention | [110,124] |
| 0.1% SDS | high cell elimination efficacy complete removal of genetic material retained the structural proteins and integrity of the ECM | [105,124,125] |
| 1% SDS | complete cell removal highly efficient in DNA removal with an efficiency of about 99% disrupted the microvasculature of ECM | [111,113,122] |
| 1% Triton X-100 + 1% SDS | effective removal of cellular components and complete elimination of nuclear material preserved the vasculature and mechanical integrity of the ECM | [113,126] |
| 4% Triton X-100/0.02% EGTA solution and 0.5% SDS aqueous solution | Decellularized whole liver organ as an ex vivo model with a unique native environment and vasculature for vascular embolization evaluation. | [127] |
| Free-thaw + Triton X-100/SDS + DNase/RNase | Sequentially perfusing the organ with SDS and Triton X-100, resulting in the generation of translucent acellular liver matrices within just 9 h. This approach offers a more streamlined and effective method for decellularization. | [128] |
| 1% Triton X-100 + 0.05% EDTA + 30 μg/mL DNase | A unidirectional, one-way perfusion flow improved and accelerated the decellularization approach. Most significantly, decellularization preserved liver extracellular matrix integrity and cell adhesion and proliferation, enabling recellularization. | [129] |
| Enzymatic | Utilizes enzymes (e.g., trypsin, DNase) to digest cellular material while leaving the ECM intact. | [120] |
| Physical | Involves physical disruption of cells through mechanical agitation, shear, or pressure to remove cellular material. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allu, I.; Sahi, A.K.; Koppadi, M.; Gundu, S.; Sionkowska, A. Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration. J. Funct. Biomater. 2023, 14, 518. https://doi.org/10.3390/jfb14100518
Allu I, Sahi AK, Koppadi M, Gundu S, Sionkowska A. Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration. Journal of Functional Biomaterials. 2023; 14(10):518. https://doi.org/10.3390/jfb14100518
Chicago/Turabian StyleAllu, Ishita, Ajay Kumar Sahi, Meghana Koppadi, Shravanya Gundu, and Alina Sionkowska. 2023. "Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration" Journal of Functional Biomaterials 14, no. 10: 518. https://doi.org/10.3390/jfb14100518