In Vivo Prevention of Implant-Associated Infections Caused by Antibiotic-Resistant Bacteria through Biofunctionalization of Additively Manufactured Porous Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
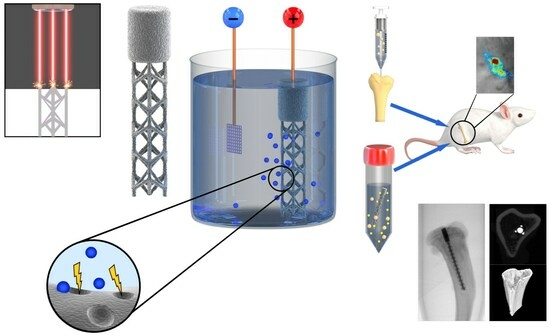
2.2. Implant Design and Additive Manufacturing
2.3. Surface Biofunctionalization
2.4. Characterization of the Surface Morphology and Chemical Composition
2.5. Ion Release Kinetics
2.6. X-ray Diffraction
2.7. Preparation of Bacterial Culture and Implant Inoculation
2.8. Animal Experiment
2.9. Bioluminescence Measurements
2.10. Micro-CT
2.11. Osteomyelitis Score
2.12. CFU Count
2.13. Biofilm Formation
2.14. Histology
2.15. Statistical Analysis
3. Results
3.1. Implant Synthesis and Surface Biofunctionalization
3.2. Biomaterial Characterization
3.3. Antibacterial Properties
3.4. Bone Changes
3.5. Histology
4. Discussion
4.1. In Vivo Implant Infection Models: Prevention vs. Treatment and the Role of the Inoculation Method
4.2. Bioluminescence Imaging
4.3. Surface Biofunctionalization of AM Porous Implants
4.4. Bone Morphology and Immune Response
4.5. Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37, S59–S66. [Google Scholar] [CrossRef]
- Graci, C.; Maccauro, G.; Muratori, F.; Spinelli, M.S.; Rosa, M.A.; Fabbriciani, C. Infection following bone tumor resection and reconstruction with tumoral prostheses. Int. J. Immunopathol. Pharmacol. 2010, 23, 1005–1013. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L., Jr. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Manesh, A.; Varghese, G.M. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe 2021, 2, e419–e420. [Google Scholar] [CrossRef]
- Wafa, H.; Grimer, R.J.; Reddy, K.; Jeys, L.; Abudu, A.; Carter, S.R.; Tillman, R.M. Retrospective evaluation of the incidence of early periprosthetic infection with silvertreated endoprostheses in high-risk patients. Bone Jt. J. 2015, 97-B, 252–257. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Nishida, H.; Yamamoto, N.; Watanabe, K.; Nakase, J.; Terauchi, R.; Arai, Y.; Fujiwara, H.; Kubo, T. Antimicrobial megaprostheses supported with iodine. J. Biomater. Appl. 2014, 29, 617–623. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.T.; Qiu, J.X. The Clinical Application of Porous Tantalum and Its New Development for Bone Tissue Engineering. Materials 2021, 14, 2647. [Google Scholar] [CrossRef]
- Gao, C.; Wang, C.; Jin, H.; Wang, Z.; Li, Z.; Shi, C.; Leng, Y.; Yang, F.; Liu, H.; Wang, J. Additive manufacturing technique-designed metallic porous implants for clinical application in orthopedics. RSC Adv. 2018, 8, 25210–25227. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Park, H.Y.; Dworsky, E.; Zoller, S.D.; Xi, W.; Johansen, D.O.; Loftin, A.H.; Hamad, C.D.; Segura, T.; Bernthal, N.M. The use of a novel antimicrobial implant coating in vivo to prevent spinal implant infection. Spine 2020, 45, E305–E311. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, C.C.; Liao, J.W.; Yen, S.K. Vancomycin-chitosan composite deposited on post porous hydroxyapatite coated Ti6Al4V implant for drug controlled release. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Tan, M.; Attarilar, S.; Li, J.; Uglov, V.V.; Wang, B.; Liu, J.; Lu, L.; Wang, L. An overview of surface modification, a way toward fabrication of nascent biomedical Ti–6Al–4V alloys. J. Mater. Res. Technol. 2023, 24, 5896–5921. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Hardes, J.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Streitbuerger, A. Silver-Coated Megaprosthesis of the Proximal Tibia in Patients With Sarcoma. J. Arthroplast. 2017, 32, 2208–2213. [Google Scholar] [CrossRef]
- Donati, F.; Di Giacomo, G.; D’Adamio, S.; Ziranu, A.; Careri, S.; Rosa, M.; Maccauro, G. Silver-coated hip megaprosthesis in oncological limb savage surgery. Biomed. Res. Int. 2016, 2016, 9079041. [Google Scholar] [CrossRef]
- Wyatt, M.C.; Foxall-Smith, M.; Roberton, A.; Beswick, A.; Kieser, D.C.; Whitehouse, M.R. The use of silver coating in hip megaprostheses: A systematic review. Hip Int. 2019, 29, 7–20. [Google Scholar] [CrossRef]
- Lex, J.R.; Koucheki, R.; Stavropoulos, N.A.; Michele, J.D.; Toor, J.S.; Tsoi, K.; Ferguson, P.C.; Turcotte, R.E.; Papagelopoulos, P.J. Megaprosthesis anti-bacterial coatings: A comprehensive translational review. Acta Biomater. 2022, 140, 136–148. [Google Scholar] [CrossRef]
- Bottagisio, M.; Lovati, A.B.; Galbusera, F.; Drago, L.; Banfi, G. A precautionary approach to guide the use of transition metal-based nanotechnology to prevent orthopedic infections. Materials 2019, 12, 314. [Google Scholar] [CrossRef]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.; Apachitei, I.; Duszczyk, J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef]
- Shin, K.R.; Kim, Y.S.; Kim, G.W.; Yang, H.W.; Ko, Y.G.; Shin, D.H. Effects of concentration of Ag nanoparticles on surface structure and in vitro biological responses of oxide layer on pure titanium via plasma electrolytic oxidation. Appl. Surf. Sci. 2015, 347, 574–582. [Google Scholar] [CrossRef]
- Uhm, S.H.; Kwon, J.S.; Song, D.H.; Lee, E.J.; Jeong, W.S.; Oh, S.; Kim, K.N.; Choi, E.H.; Kim, K.M. Long-term antibacterial performance and bioactivity of plasma-engineered Ag-NPs/TiO(2). J. Biomed. Nanotechnol. 2016, 12, 1890–1906. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mohedano, M.; Martínez-Corriá, R.; Ramos, V.; Arrabal, R.; Matykina, E. In vitro and in vivo evaluation of PEO-modified titanium for bone implant applications. Surf. Coat. Technol. 2018, 347, 358–368. [Google Scholar] [CrossRef]
- He, J.; Feng, W.; Zhao, B.H.; Zhang, W.; Lin, Z. In vivo effect of titanium implants with porous zinc-containing coatings prepared by plasma electrolytic oxidation method on osseointegration in rabbits. Int. J. Oral. Maxillofac. Implant. 2018, 33, 298–310. [Google Scholar] [CrossRef]
- Park, T.-E.; Choe, H.-C.; Brantley, W.A. Bioactivity evaluation of porous TiO2 surface formed on titanium in mixed electrolyte by spark anodization. Surf. Coat. Technol. 2013, 235, 706–713. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Niska, J.A.; Meganck, J.A.; Pribaz, J.R.; Shahbazian, J.H.; Lim, E.; Zhang, N.; Rice, B.W.; Akin, A.; Ramos, R.I.; Bernthal, N.M.; et al. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and muCT imaging in an orthopaedic implant infection in mice. PLoS ONE 2012, 7, e47397. [Google Scholar] [CrossRef]
- Funao, H.; Ishii, K.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Aizawa, M.; Okada, Y.; Chiba, K.; Koyasu, S.; Toyama, Y.; et al. Establishment of a real-time, quantitative, and reproducible mouse model of Staphylococcus osteomyelitis using bioluminescence imaging. Infect. Immun. 2012, 80, 733–741. [Google Scholar] [CrossRef]
- Miller, R.J.; Thompson, J.M.; Zheng, J.; Marchitto, M.C.; Archer, N.K.; Pinsker, B.L.; Ortines, R.V.; Jiang, X.; Martin, R.A.; Brown, I.D.; et al. In Vivo bioluminescence imaging in a rabbit model of orthopaedic implant-associated infection to monitor efficacy of an antibiotic-releasing coating. J. Bone Jt. Surg. Am. 2019, 101, e12. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Crosby, H.A.; Schilcher, K.; Wang, Y.; Ortines, R.V.; Mazhar, M.; Dikeman, D.A.; Pinsker, B.L.; Brown, I.D.; Joyce, D.P.; et al. Development of a Staphylococcus aureus reporter strain with click beetle red luciferase for enhanced in vivo imaging of experimental bacteremia and mixed infections. Sci. Rep. 2019, 9, 16663. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Nagay, B.E.; Dini, C.; Borges, M.H.R.; Miranda, L.F.B.; Cordeiro, J.M.; Souza, J.G.S.; Sukotjo, C.; Cruz, N.C.; Barão, V.A.R. The race for the optimal antimicrobial surface: Perspectives and challenges related to plasma electrolytic oxidation coating for titanium-based implants. Adv. Colloid. Interface Sci. 2023, 311, 102805. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr. Infect. Dis. Rep. 2008, 10, 394–403. [Google Scholar] [CrossRef]
- Saleh, K.; Sonesson, A.; Persson, B.; Riesbeck, K.; Schmidtchen, A. A descriptive study of bacterial load of full-thickness surgical wounds in dermatologic surgery. Dermatol. Surg. 2011, 37, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Friberg, S.; Burman, L.G. Inconsistent correlation between aerobic bacterial surface and air counts in operating rooms with ultra clean laminar air flows: Proposal of a new bacteriological standard for surface contamination. J. Hosp. Infect. 1999, 42, 287–293. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial titanium implants biofunctionalized by plasma electrolytic oxidation with silver, zinc, and copper: A systematic review. Int. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef]
- Schomig, F.; Perka, C.; Pumberger, M.; Ascherl, R. Implant contamination as a cause of surgical site infection in spinal surgery: Are single-use implants a reasonable solution?—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 634. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Riool, M.; de Boer, L.; Jaspers, V.; van der Loos, C.M.; van Wamel, W.J.B.; Wu, G.; Kwakman, P.H.S.; Zaat, S.A.J. Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 2014, 10, 5202–5212. [Google Scholar] [CrossRef]
- Sheehan, E.; McKenna, J.; Mulhall, K.J.; Marks, P.; McCormack, D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J. Orthop. Res. 2004, 22, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Waldvogel, F.A.; Vaudaux, P.; Nydegger, U.E. Pathogenesis of foreign body infection description and characteristics of an animal model. J. Infect. Dis. 1982, 146, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur. Cell. Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Animal models of orthopedic implant infection. J. Investig. Surg. 1998, 11, 139–146. [Google Scholar] [CrossRef]
- Nowakowska, J.; Landmann, R.; Khanna, N. Foreign body infection models to study host-pathogen response and antimicrobial tolerance of bacterial biofilm. Antibiotics 2014, 3, 378–397. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS ONE 2010, 5, e12580. [Google Scholar] [CrossRef]
- Merritt, K.; Shafer, J.W.; Brown, S.A. Implant site infection rates with porous and dense materials.pdf. J. Biomed. Mater. Res. 1979, 13, 101–108. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horiz. 2018, 5, 28–35. [Google Scholar] [CrossRef]
- Hong, J.Y.; Ko, S.Y.; Lee, W.; Chang, Y.Y.; Kim, S.H.; Yun, J.H. Enhancement of bone ingrowth into a porous titanium structure to improve osseointegration of dental implants: A pilot study in the canine model. Materials 2020, 13, 3061. [Google Scholar] [CrossRef]
- Reis de Vasconcellos, L.M.; Leite, D.O.; Nascimento de Oliveira, F.; Carvalho, Y.R.; Cairo, C.A.A. Evaluation of bone ingrowth into porous titanium implant: Histomorphometric analysis in rabbits. Braz. Oral. Res. 2010, 24, 399–405. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; van Wijnen, A.J. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng. Part. B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-packed additively manufactured porous titanium implants. Mater. Today Bio. 2020, 7, 100060. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Gonzalez Tenorio, R.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of antibacterial and osteogenic properties of Ti6Al4V by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Thukkaram, M.; Coryn, R.; Asadian, M.; Esbah Tabaei, P.S.; Rigole, P.; Rajendhran, N.; Nikiforov, A.; Sukumaran, J.; Coenye, T.; Van Der Voort, P.; et al. Fabrication of microporous coatings on titanium implants with improved mechanical, antibacterial, and cell-interactive properties. ACS Appl. Mater. Interfaces 2020, 12, 30155–30169. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, I.A.J.; Tierolf, M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2019, 8, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Su, R.T.; Chu, H.J.; Chen, H.T.; Tsou, H.K.; He, J.L. Plasma electrolytic oxidation of titanium and improvement in osseointegration. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1023–1030. [Google Scholar] [CrossRef]
- Kuehl, R.; Brunetto, P.S.; Woischnig, A.K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terracciano, L.; Fromm, K.M.; Khanna, N. Preventing implant-associated infections by silver coating. Antimicrob. Agents Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef]
- Cavanaugh, D.L.; Tan, Z.G.; Norris, J.P.t.; Hardee, A.; Weinhold, P.S.; Dahners, L.E.; Orndorff, P.E.; Shirwaiker, R.A. Evaluation of silver-titanium implants activated by low intensity direct current for orthopedic infection control: An in vitro and in vivo study. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1023–1031. [Google Scholar] [CrossRef]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Julke, H.; Zehe, M.; Lemarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial Silver Multilayer Coating for Prevention of Bacterial Colonization of Orthopedic Implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lee, P.; Lui, V.C.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.; Wong, K.K. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine 2015, 11, 1949–1959. [Google Scholar] [CrossRef]
- Shivaram, A.; Bose, S.; Bandyopadhyay, A. Understanding long-term silver release from surface modified porous titanium implants. Acta Biomater. 2017, 58, 550–560. [Google Scholar] [CrossRef]
- Akiyama, T.; Miyamoto, H.; Yonekura, Y.; Tsukamoto, M.; Ando, Y.; Noda, I.; Sonohata, M.; Mawatari, M. Silver oxide-containing hydroxyapatite coating has in vivo antibacterial activity in the rat tibia. J. Orthop. Res. 2013, 31, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, V.A.; Potapova, I.; Camenisch, K.; Nehrbass, D.; Richards, R.G.; Moriarty, T.F. In vivo microCT monitoring of osteomyelitis in a rat model. Biomed. Res. Int. 2015, 2015, 587857. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Bedigrew, K.M.; Wenke, J.C. Development of a hematogenous implant-related infection in a rat model. BMC Musculoskelet. Disord. 2015, 16, 255. [Google Scholar] [CrossRef]
- Odekerken, J.C.E.; Arts, J.J.C.; Surtel, D.A.M.; Walenkanmp, G.H.I.M.; Welting, T.J.M. A rabbit osteomyelitis model for the longitudinal assessment of early post-operative implant infections. J. Orthop. Surg. Res. 2013, 8, 38. [Google Scholar] [CrossRef]
- Pineda, C.; Espinosa, R.; Pena, A. Radiographic imaging in osteomyelitis: The role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin. Plast. Surg. 2009, 23, 80–89. [Google Scholar] [CrossRef]
- Croes, M.; Kruyt, M.C.; Boot, W.; Pouran, B.; Braham, M.V.; Pakpahan, S.A.; Weinans, H.; Vogely, H.C.; Fluit, A.C.; Dhert, W.J.; et al. The role of bacterial stimuli in inflammation-driven bone formation. Eur. Cell. Mater. 2019, 37, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Krisher, T.; Bar-Shavit, Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J. Cell. Biochem. 2014, 115, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Reikeras, O.; Wang, J.E.; Foster, S.J.; Utvag, S.E. Staphylococcus aureus peptidoglycan impairs fracture healing: An experimental study in rats. J. Orthop. Res. 2007, 25, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Hammer, N.D.; Campbell, J.P.; Benson, M.A.; Perrien, D.S.; Mrak, L.N.; Smeltzer, M.S.; Torres, V.J.; Skaar, E.P. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 2013, 13, 759–772. [Google Scholar] [CrossRef]
- Croes, M.; van der Wal, B.C.H.; Vogely, H.C. Impact of bacterial infections on osteogenesis: Evidence from in vivo studies. J. Orthop. Res. 2019, 37, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Seebach, E.; Kubatzky, K.F. Chronic implant-related bone infections-can immune modulation be a therapeutic strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef]






| Bacterial Inoculation Method | Bacterial Infection | Implant | PEO Treatment (PT) | Ag NPs | Label |
|---|---|---|---|---|---|
| In vivo injection of bacteria into intramedullary cavity | Yes | Yes | - | - | inject-NT (no treatment) |
| Yes | Yes | Yes | Yes | inject-PT-Ag | |
| Yes | - | - | - | inject-no-implant | |
| In vitro inoculation of implant prior to implantation | Yes | Yes | - | - | ino-NT |
| Yes | Yes | Yes | - | ino-PT | |
| Yes | Yes | Yes | Yes | ino-PT-Ag | |
| No (PBS) | Yes | - | - | ino-NT-no-inf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hengel, I.A.J.; van Dijk, B.; Modaresifar, K.; Hooning van Duyvenbode, J.F.F.; Nurmohamed, F.R.H.A.; Leeflang, M.A.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Weinans, H.; et al. In Vivo Prevention of Implant-Associated Infections Caused by Antibiotic-Resistant Bacteria through Biofunctionalization of Additively Manufactured Porous Titanium. J. Funct. Biomater. 2023, 14, 520. https://doi.org/10.3390/jfb14100520
van Hengel IAJ, van Dijk B, Modaresifar K, Hooning van Duyvenbode JFF, Nurmohamed FRHA, Leeflang MA, Fluit AC, Fratila-Apachitei LE, Apachitei I, Weinans H, et al. In Vivo Prevention of Implant-Associated Infections Caused by Antibiotic-Resistant Bacteria through Biofunctionalization of Additively Manufactured Porous Titanium. Journal of Functional Biomaterials. 2023; 14(10):520. https://doi.org/10.3390/jfb14100520
Chicago/Turabian Stylevan Hengel, Ingmar Aeneas Jan, Bruce van Dijk, Khashayar Modaresifar, Johan Frederik Felix Hooning van Duyvenbode, Faisal Ruben Hamzah Aziz Nurmohamed, Marius Alexander Leeflang, Adriaan Camille Fluit, Lidy Elena Fratila-Apachitei, Iulian Apachitei, Harrie Weinans, and et al. 2023. "In Vivo Prevention of Implant-Associated Infections Caused by Antibiotic-Resistant Bacteria through Biofunctionalization of Additively Manufactured Porous Titanium" Journal of Functional Biomaterials 14, no. 10: 520. https://doi.org/10.3390/jfb14100520