Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Osteogenic Induction of Osteoprogenitor Cells
2.2. ELF-PEMF Exposure
2.3. Sulforhodamine B (SRB) Staining
2.4. Calcein-AM Staining
2.5. Alkaline Phosphatase (AP) Activity
2.6. Alizarin Red and Von Kossa Staining
2.7. qRT-PCR
2.8. Measurement of Intracellular Ca2+
2.9. Statistical Analysis
3. Results
3.1. Three 10 Min ELF-PEMF Exposures per Day More Effectively Promoted Maturation of Osteoprogenitor Cells Than a Single 30 Min ELF-PEMF Exposure per Day
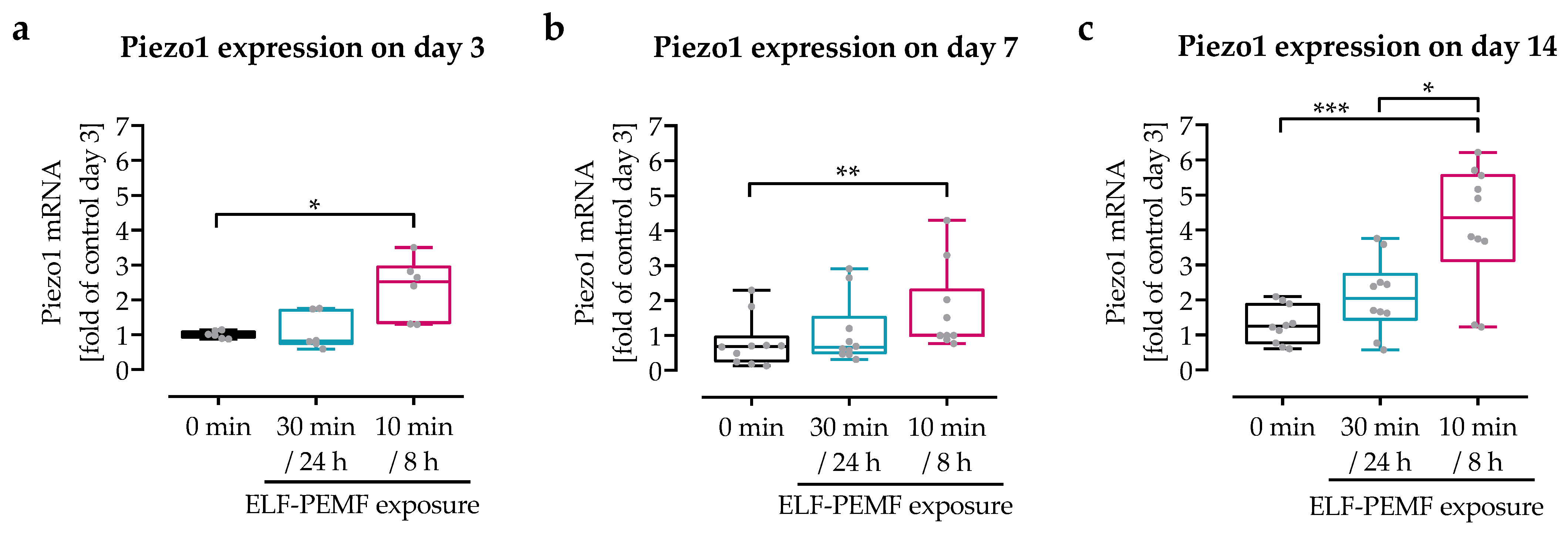
3.2. Intermittent Exposure to 16 Hz ELF-PEMF Induced Expression of Piezo 1 in SCP-1 Cells
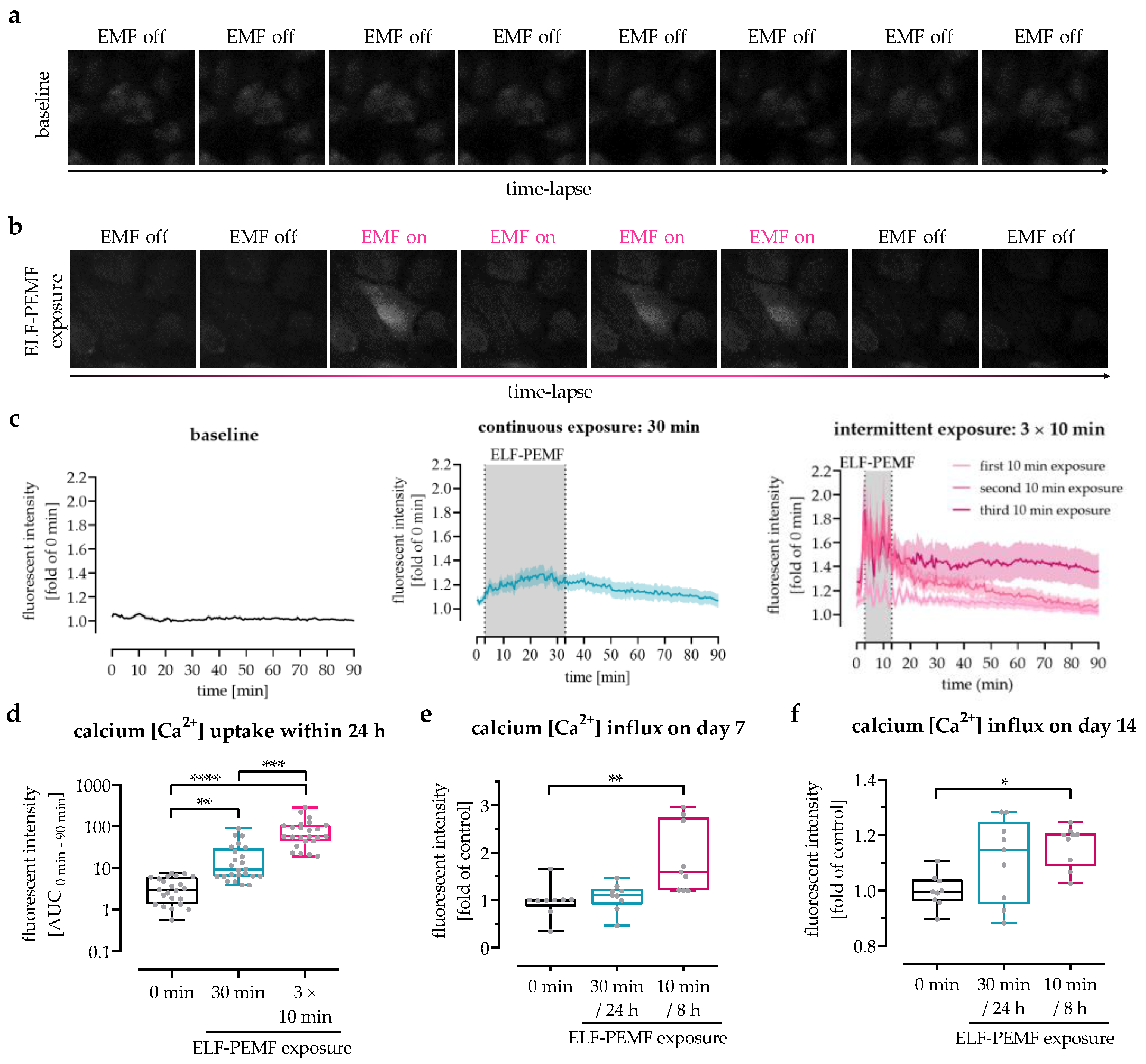
3.3. The 16 Hz ELF-PEMF Exposure Pattern Affected Net-Ca2+ Influx in SCP-1 Cells
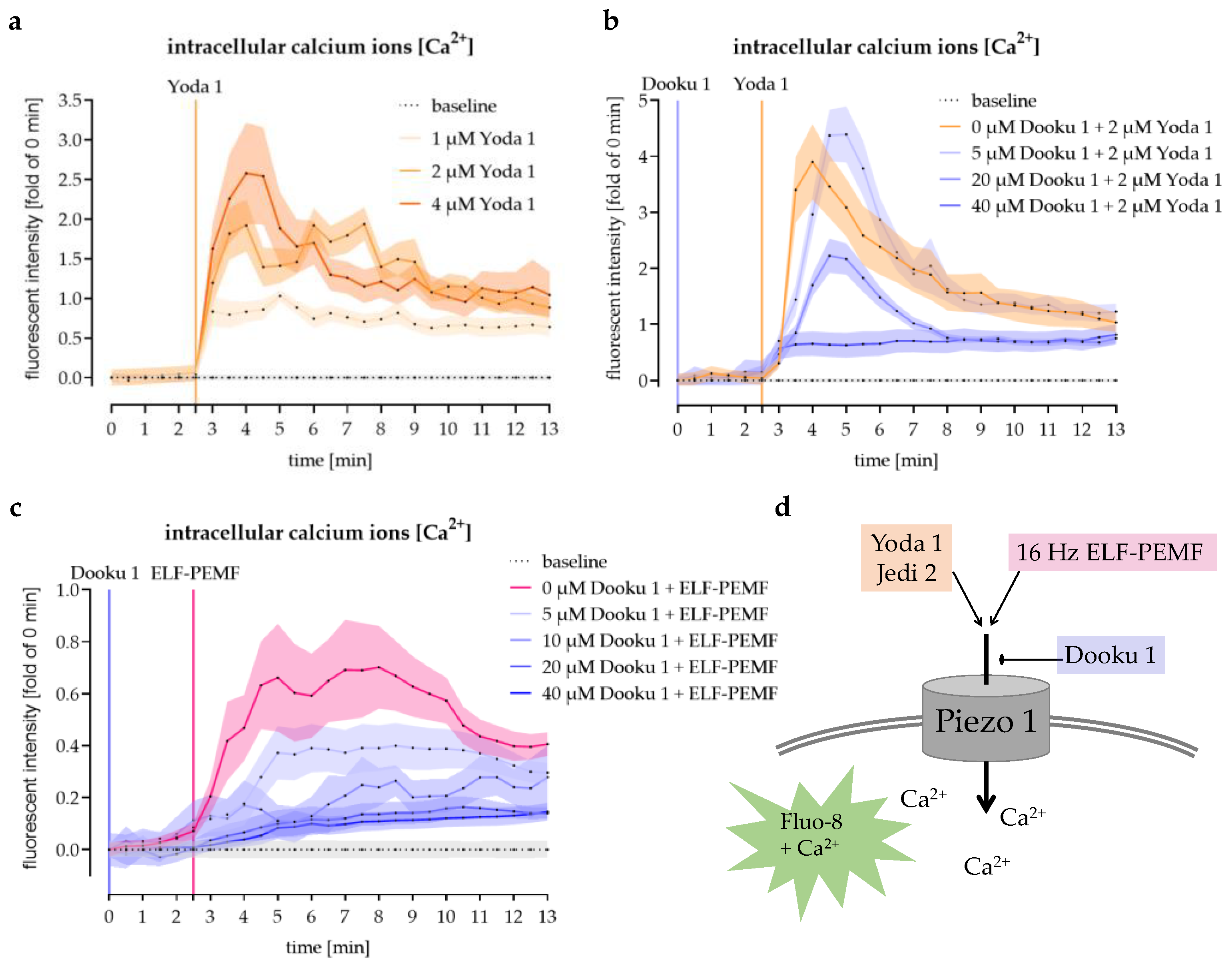
3.4. Modulation of Piezo 1-Mediated Ca2+ Influx into SCP-1 Cells by Small Chemicals
3.5. The 16 Hz ELF-PEMF Exposure-Induced Ca2+ Influx Was Abolished by Antagonizing Piezo 1
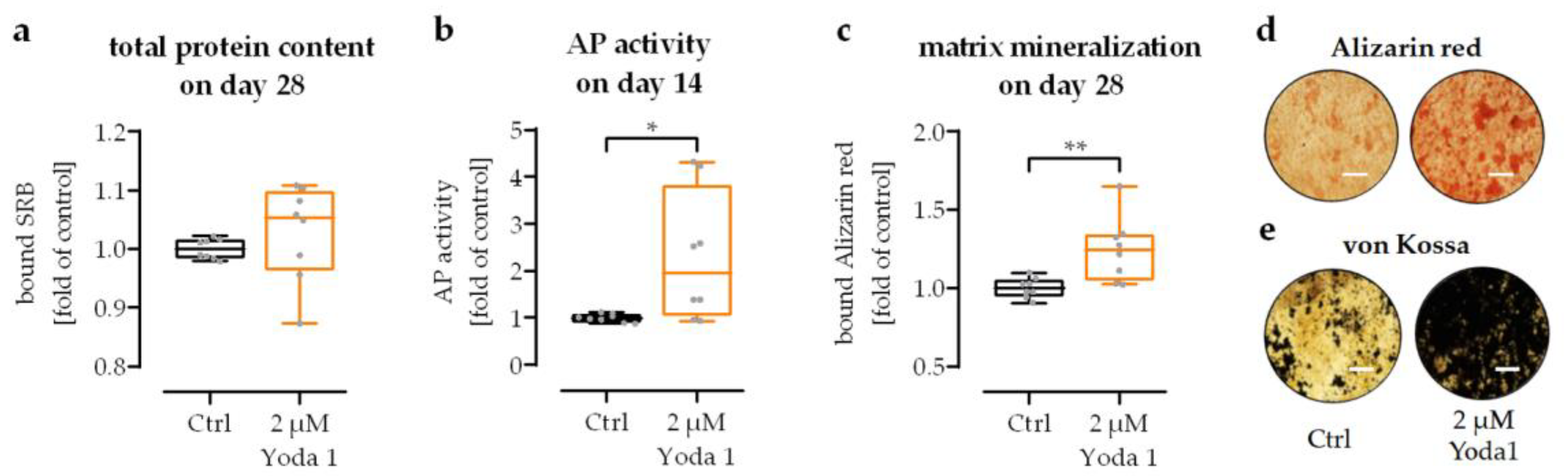
3.6. Pharmacological Activation of Piezo 1 Promoted Osteogenic Differentiation of SCP-1 Cells
3.7. Pharmacological Inhibition of Piezo 1 Partly Abrogated the Positive Effects of 16 Hz ELF-PEMF Exposure on the Maturation of SCP-1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greer, R.B., 3rd. Wolff’s Law. Orthop. Rev. 1993, 22, 1087–1088. [Google Scholar]
- Woo, S.L.; Kuei, S.C.; Amiel, D.; Gomez, M.A.; Hayes, W.C.; White, F.C.; Akeson, W.H. The effect of prolonged physical training on the properties of long bone: A study of Wolff’s Law. J. Bone Joint Surg. Am. 1981, 63, 780–787. [Google Scholar] [CrossRef]
- Chen, Y.; Aspera-Werz, R.H.; Menger, M.M.; Falldorf, K.; Ronniger, M.; Stacke, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-beta Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke. Int. J. Mol. Sci. 2021, 22, 7036. [Google Scholar] [CrossRef]
- Antonsson, E.K.; Mann, R.W. The frequency content of gait. J. Biomech. 1985, 18, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Falldorf, K.; Fentz, A.K.; Ziegler, P.; Schroter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, P.; Nussler, A.K.; Wilbrand, B.; Falldorf, K.; Springer, F.; Fentz, A.K.; Eschenburg, G.; Ziegler, A.; Stockle, U.; Maurer, E.; et al. Pulsed Electromagnetic Field Therapy Improves Osseous Consolidation after High Tibial Osteotomy in Elderly Patients-A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Med. 2019, 8, 2008. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; Schroter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehnert, S.; Fentz, A.K.; Schreiner, A.; Birk, J.; Wilbrand, B.; Ziegler, P.; Reumann, M.K.; Wang, H.; Falldorf, K.; Nussler, A.K. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of *O2(-) and H2O2. Sci. Rep. 2017, 7, 14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivancsits, S.; Diem, E.; Jahn, O.; Rudiger, H.W. Intermittent extremely low frequency electromagnetic fields cause DNA damage in a dose-dependent way. Int. Arch. Occup. Environ. Health 2003, 76, 431–436. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Min Sen Oh, V.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Petecchia, L.; Sbrana, F.; Utzeri, R.; Vercellino, M.; Usai, C.; Visai, L.; Vassalli, M.; Gavazzo, P. Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca(2+)-related mechanisms. Sci. Rep. 2015, 5, 13856. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.C.; Ge, J.L.; Guo, B.; Guo, J.; Hao, M.; Wu, Y.C.; Lin, Y.A.; La, T.; Yao, P.T.; Mei, Y.A.; et al. Extremely Low Frequency Electromagnetic Fields Facilitate Vesicle Endocytosis by Increasing Presynaptic Calcium Channel Expression at a Central Synapse. Sci. Rep. 2016, 6, 21774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. Adv. Exp. Med. Biol. 2020, 1131, 625–648. [Google Scholar]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Tasdogan, A.; Ubellacker, J.M.; Zhang, J.; Nosyreva, E.D.; Du, L.; Murphy, M.M.; Hu, S.; Yi, Y.; Kara, N.; et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 2021, 591, 438–444. [Google Scholar] [CrossRef]
- Wang, L.J.; You, X.L.; Lotinun, S.; Zhang, L.L.; Wu, N.; Zou, W.G. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.J.; Mohieldin, A.M.; Muntean, B.S.; Green, J.A.; Shah, J.V.; Mykytyn, K.; Nauli, S.M. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell. Mol. Life Sci. 2014, 71, 2165–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesantez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Primers 2021, 7, 57. [Google Scholar] [CrossRef]
- Johnson, M.; Williams, W.S. Piezoelectric Effects in Bent Bone. B Am. Phys. Soc. 1977, 22, 43–44. [Google Scholar]
- Ivancsits, S.; Pilger, A.; Diem, E.; Jahn, O.; Rudiger, H.W. Cell type-specific genotoxic effects of intermittent extremely low-frequency electromagnetic fields. Mutat. Res. 2005, 583, 184–188. [Google Scholar] [CrossRef]
- Chen, Y.; Menger, M.M.; Braun, B.J.; Schweizer, S.; Linnemann, C.; Falldorf, K.; Ronniger, M.; Wang, H.; Histing, T.; Nussler, A.K.; et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering 2021, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, J.A.; Bergstrom, W.H. In vivo and in vitro effects of a pulsed electromagnetic field on net calcium flux in rat calvarial bone. Calcif. Tissue Int. 2002, 70, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.J.; Black, M.J.; Soboloff, J.; Gill, D.L.; Dziadek, M.A.; Johnstone, L.S. Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3T3-L1 pre-adipocyte differentiation. Differentiation 2009, 77, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; He, J.; Koh, S.P.; Zhong, Y.; Liu, Z.; Wang, Z.; Zhang, Y.; Li, Z.; Tam, B.T.; Lin, P.; et al. Reprogrammed marrow adipocytes contribute to myeloma-induced bone disease. Sci. Transl. Med. 2019, 11, eaau9087. [Google Scholar] [CrossRef]
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730. [Google Scholar]
- Henriksen, Z.; Hiken, J.F.; Steinberg, T.H.; Jorgensen, N.R. The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium 2006, 39, 435–444. [Google Scholar] [CrossRef]
- Blair, H.C.; Schlesinger, P.H.; Huang, C.L.; Zaidi, M. Calcium signalling and calcium transport in bone disease. Subcell Biochem. 2007, 45, 539–562. [Google Scholar]
- Danciu, T.E.; Adam, R.M.; Naruse, K.; Freeman, M.R.; Hauschka, P.V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003, 536, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, N.; Ma, M.; Sen, B.; Xie, Z.; Gross, T.S.; Rubin, J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J. Biol. Chem. 2008, 283, 29196–29205. [Google Scholar] [CrossRef] [Green Version]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, S.; Dahariya, S.; Sharma, D.S.; Kovuru, N.; Sahu, I.; Gutti, R.K. RUNX1 and TGF-beta signaling cross talk regulates Ca2+ ion channels expression and activity during megakaryocyte development. FEBS J. 2020, 287, 5411–5438. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; Padoan, M.; Franco, L.; Veronesi, V.; Moro, L.; D’Andrea, P. Extracellular NAD(+) induces calcium signaling and apoptosis in human osteoblastic cells. Biochem. Biophys. Res. Commun. 2001, 285, 1226–1231. [Google Scholar] [CrossRef] [PubMed]


| Target | GenID | Primer | Efficiency | Amplicon | TA |
|---|---|---|---|---|---|
| Piezo 1 | NM_001142864.4 | For-ACCAACCTCATCAGCGACTT | 2.14 | 212 bp | 56 °C |
| Rev-AACAGGTATCGGAAGACGGC | |||||
| EF1α | NM_001402.5 | For-CCCCGACACAGTAGCATTTG | 1.90 | 98 bp | 56 °C |
| Rev-TGACTTTCCATCCCTTGAACC | |||||
| RPL13a | NM_012423.3 | For-AAGTACCAGGCAGTGACAG | 2.24 | 100 bp | 56 °C |
| Rev-CCTGTTTCCGTAGCCTCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Braun, B.J.; Menger, M.M.; Ronniger, M.; Falldorf, K.; Histing, T.; Nussler, A.K.; Ehnert, S. Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells. J. Funct. Biomater. 2023, 14, 165. https://doi.org/10.3390/jfb14030165
Chen Y, Braun BJ, Menger MM, Ronniger M, Falldorf K, Histing T, Nussler AK, Ehnert S. Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells. Journal of Functional Biomaterials. 2023; 14(3):165. https://doi.org/10.3390/jfb14030165
Chicago/Turabian StyleChen, Yangmengfan, Benedikt J. Braun, Maximilian M. Menger, Michael Ronniger, Karsten Falldorf, Tina Histing, Andreas K. Nussler, and Sabrina Ehnert. 2023. "Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells" Journal of Functional Biomaterials 14, no. 3: 165. https://doi.org/10.3390/jfb14030165
APA StyleChen, Y., Braun, B. J., Menger, M. M., Ronniger, M., Falldorf, K., Histing, T., Nussler, A. K., & Ehnert, S. (2023). Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells. Journal of Functional Biomaterials, 14(3), 165. https://doi.org/10.3390/jfb14030165








