New 3D Printed Scaffolds Based on Walstromite Synthesized by Sol–Gel Method
Abstract
:1. Introduction
2. Materials and Methods
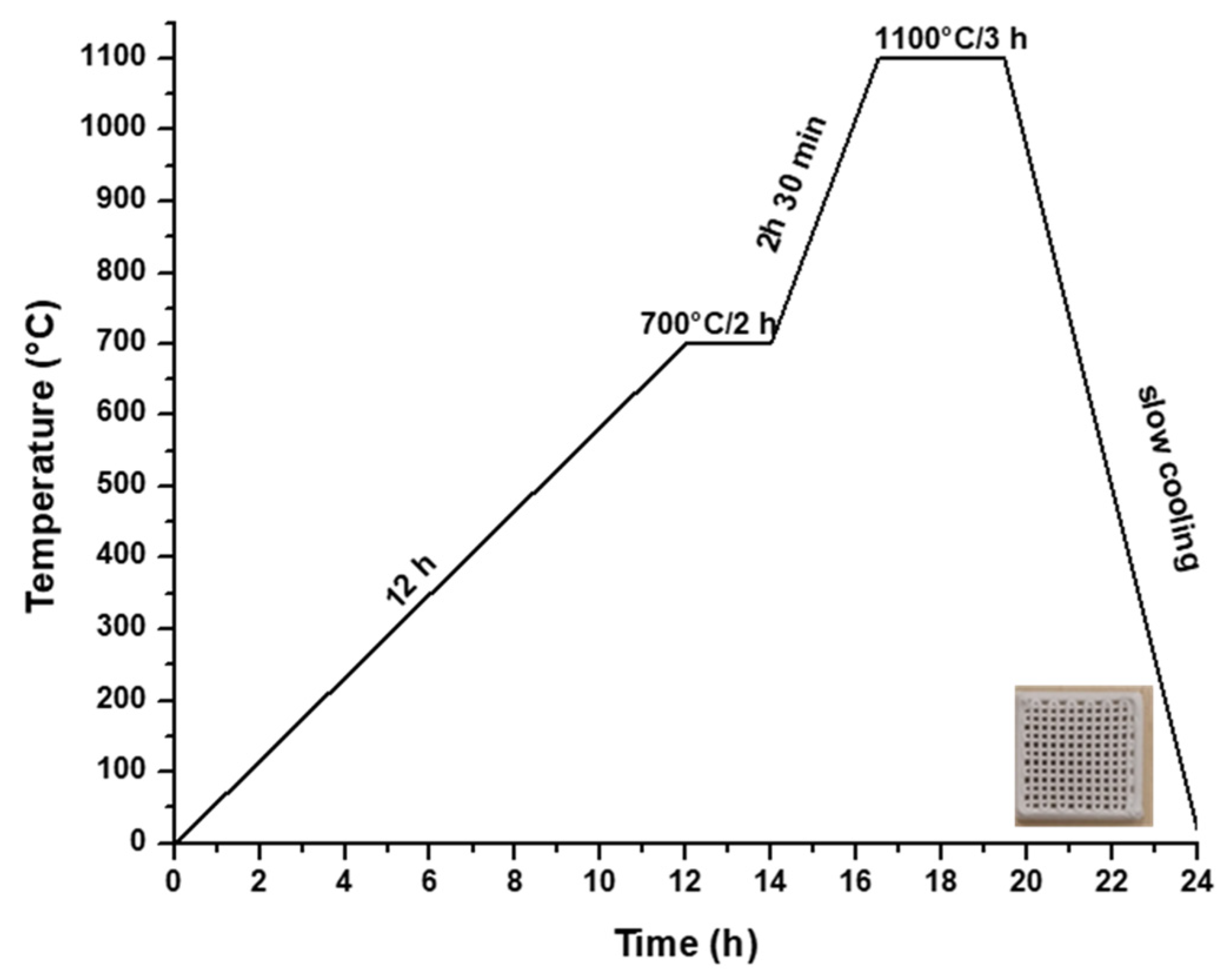
2.1. Scaffold Synthesis
2.2. Materials Characterization
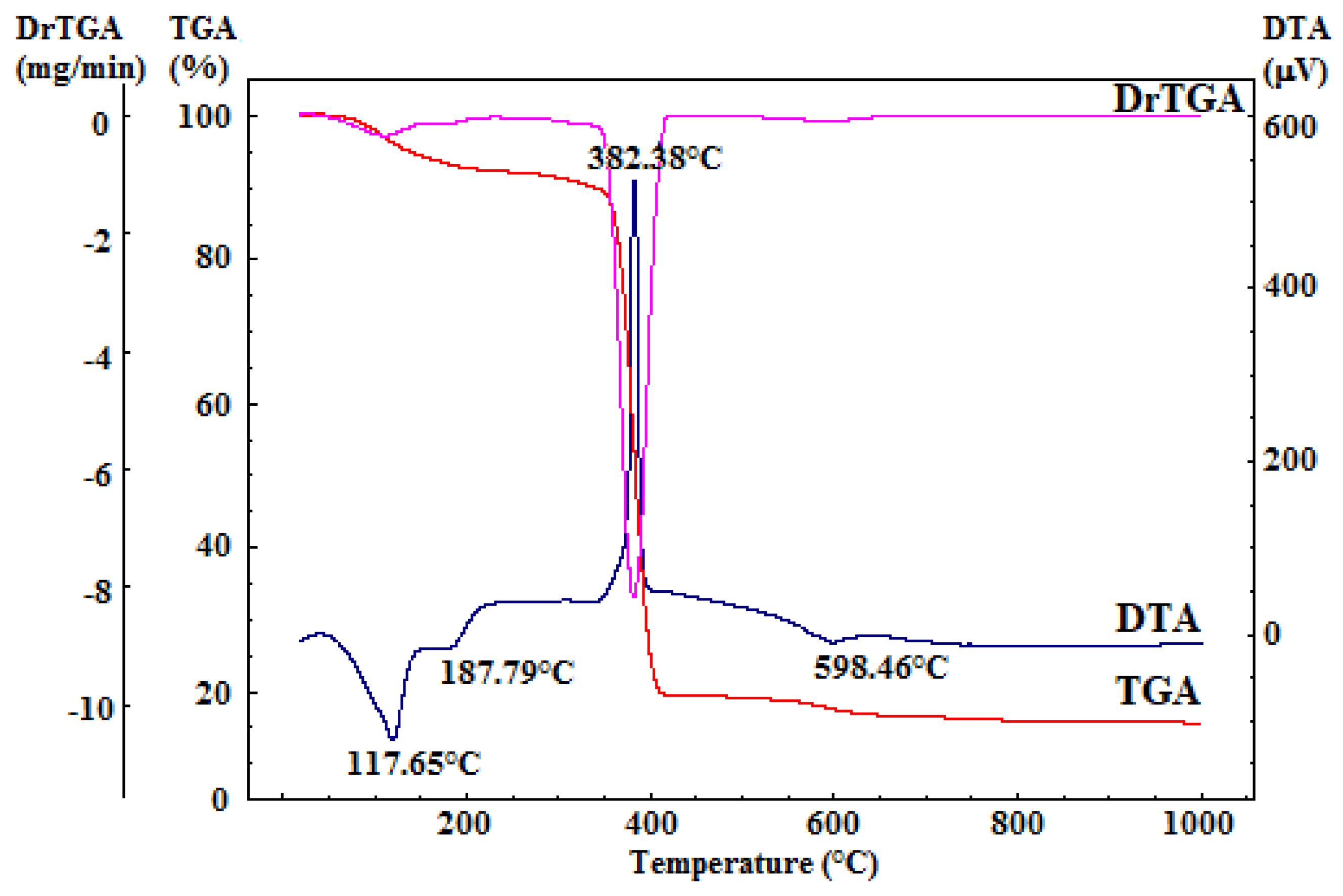
2.2.1. Thermal Analysis
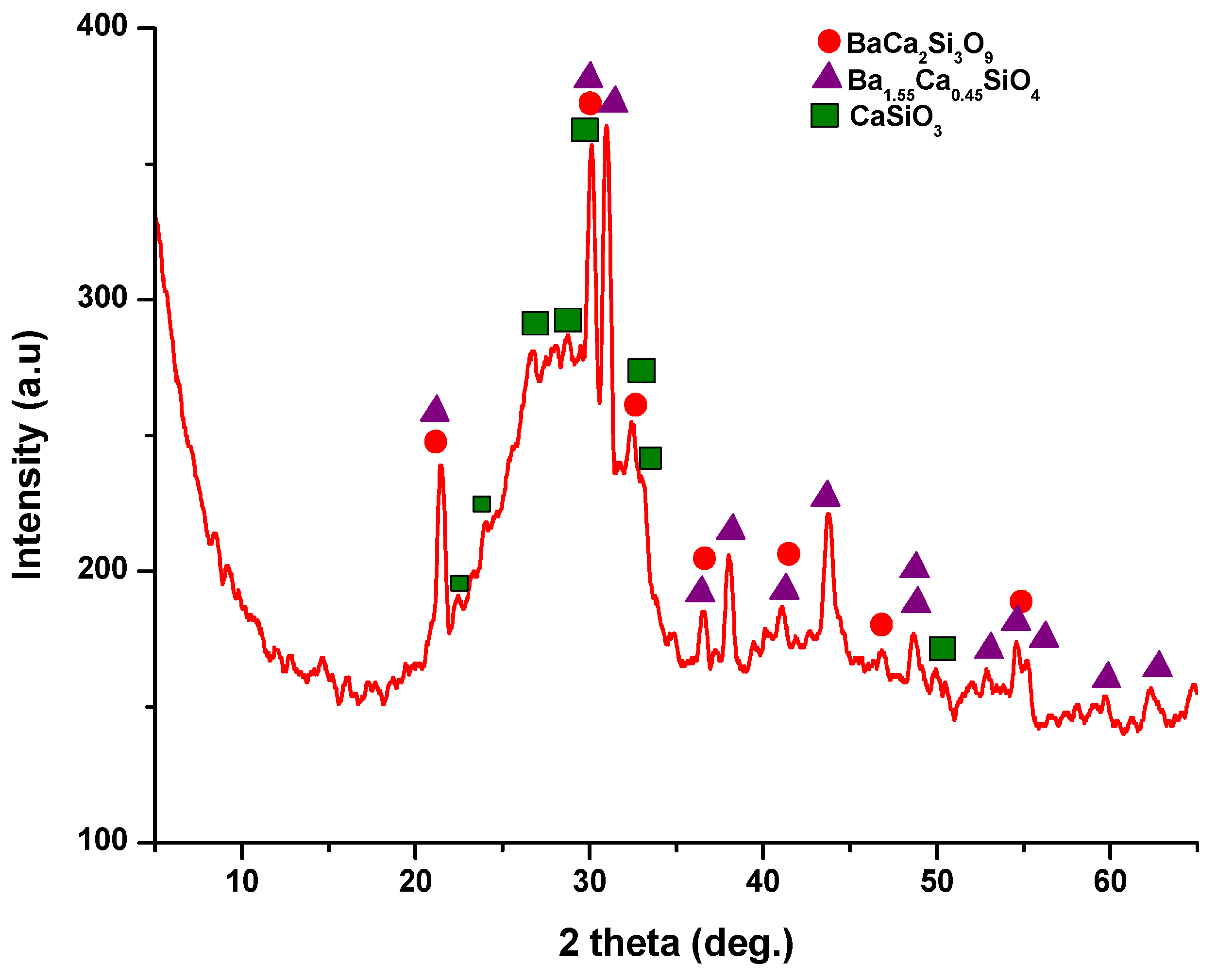
2.2.2. XRD Analysis
2.2.3. Ceramic Properties
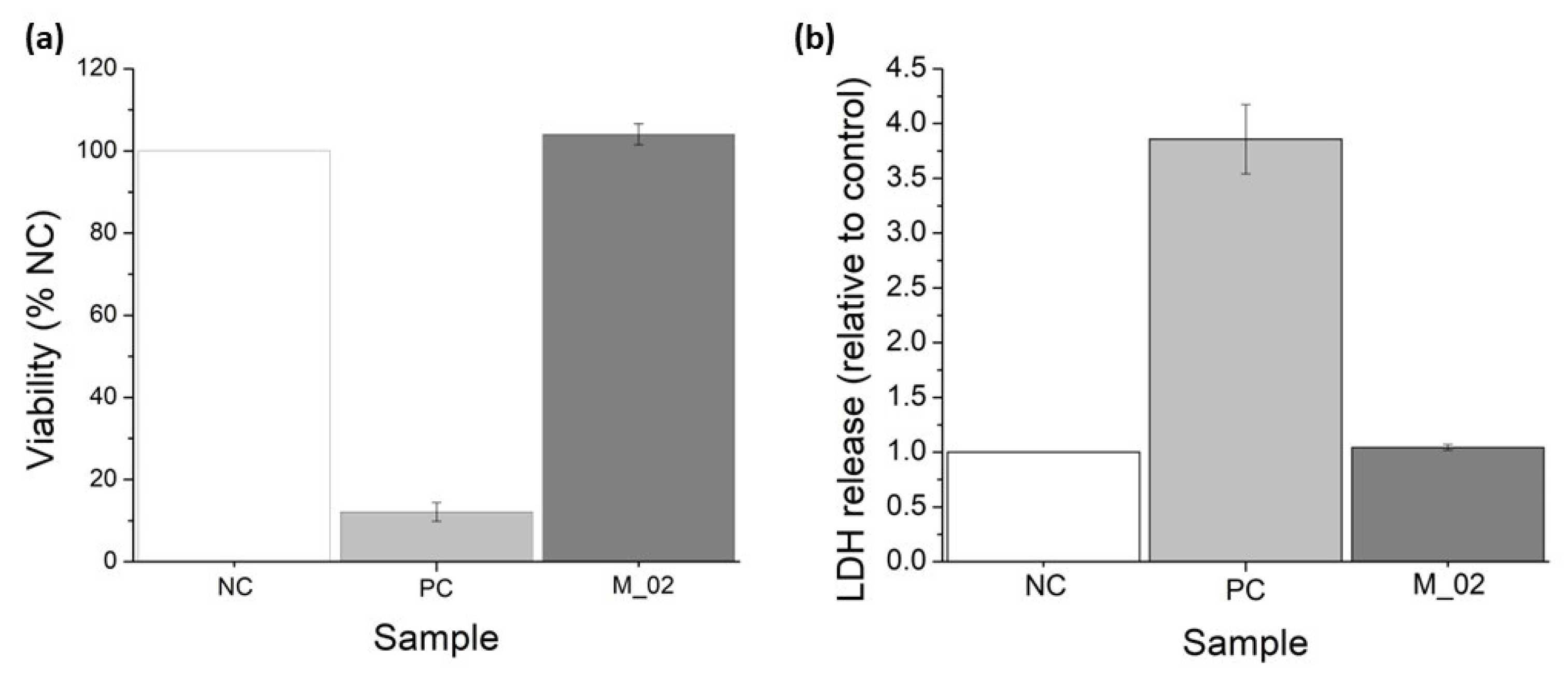
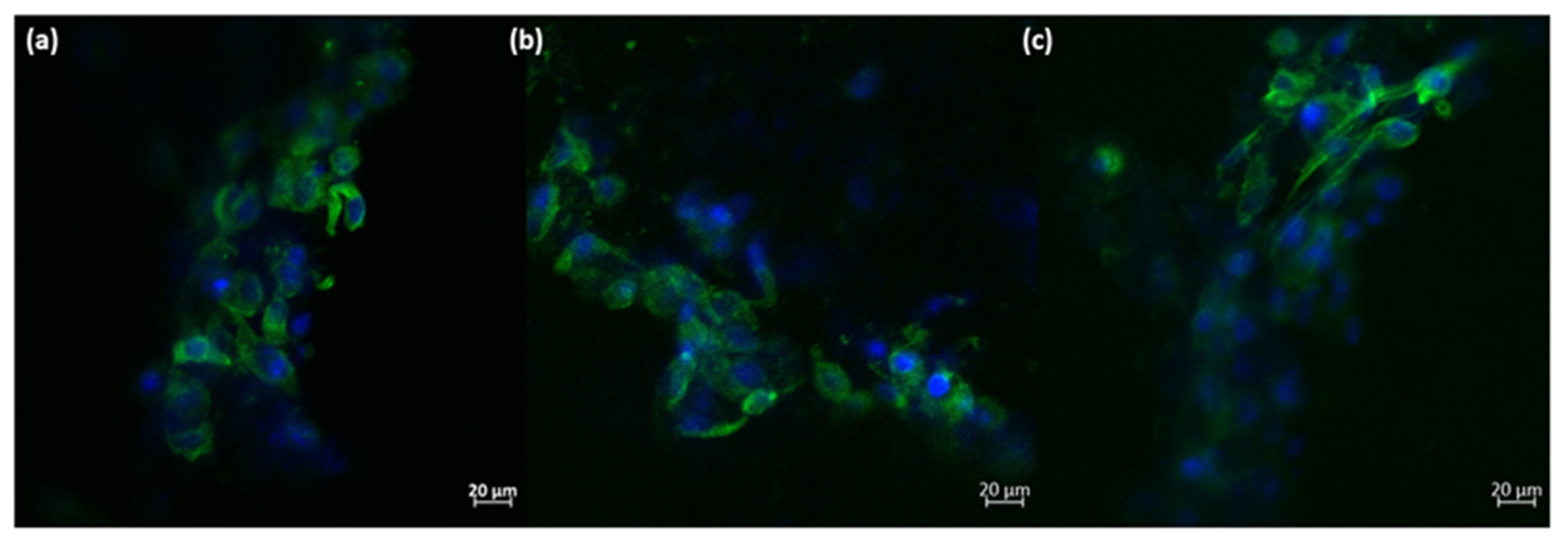
2.2.4. Biological Evaluation
3. Results and Discussion
3.1. Gel and Powder Investigation
3.2. Walstromit Scaffold—Obtainment and Characterization
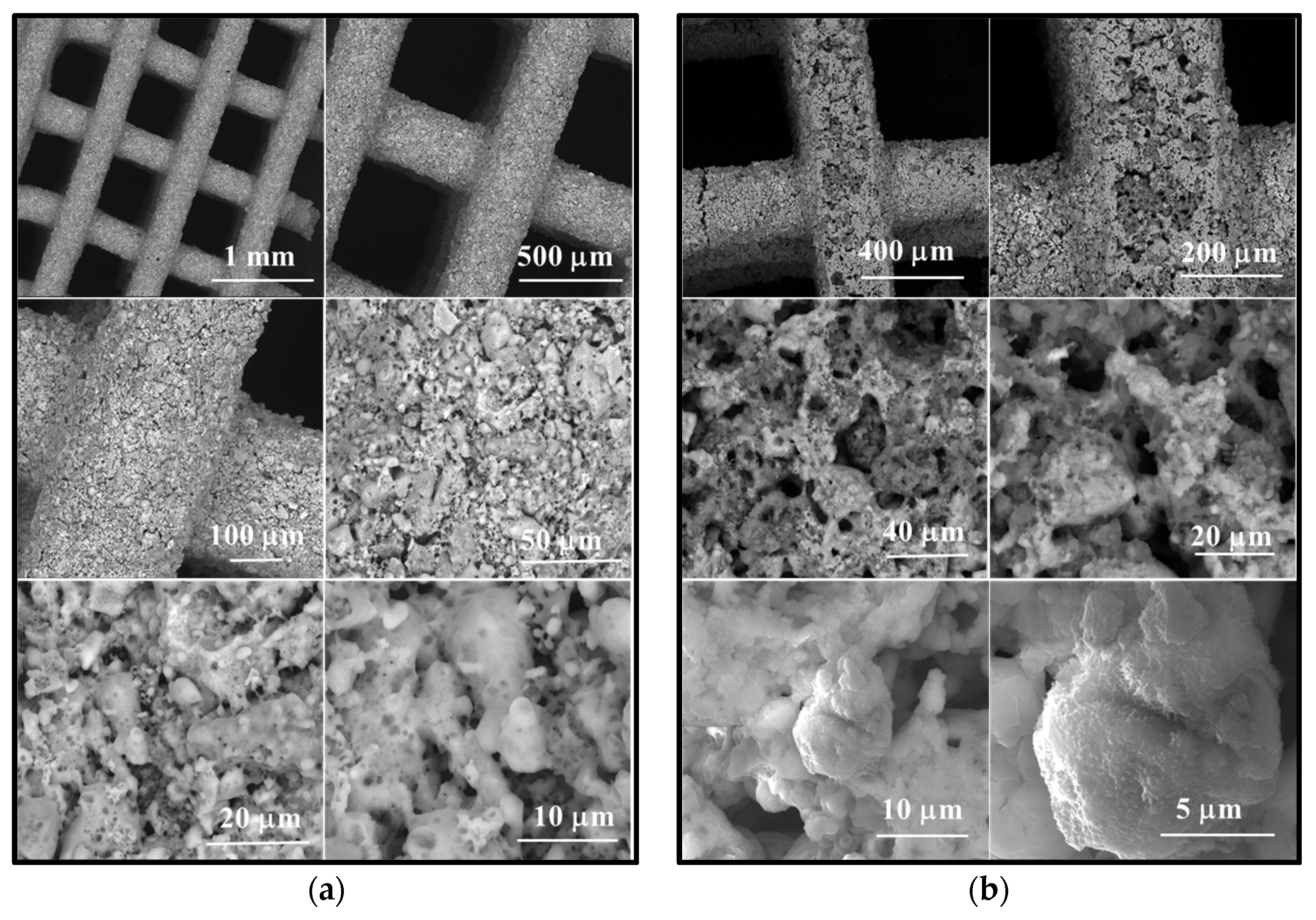
- In the case of the unimmersed ceramic scaffold (M_02), the filaments appear continuously, exhibit uniform thickness, and display open porosity. This information is consistent with the data in Table 1. Additionally, both intra- and inter-granular porosity is observed, suggesting enhanced circulation of physiological fluids and growth factors within the material at the implantation site.
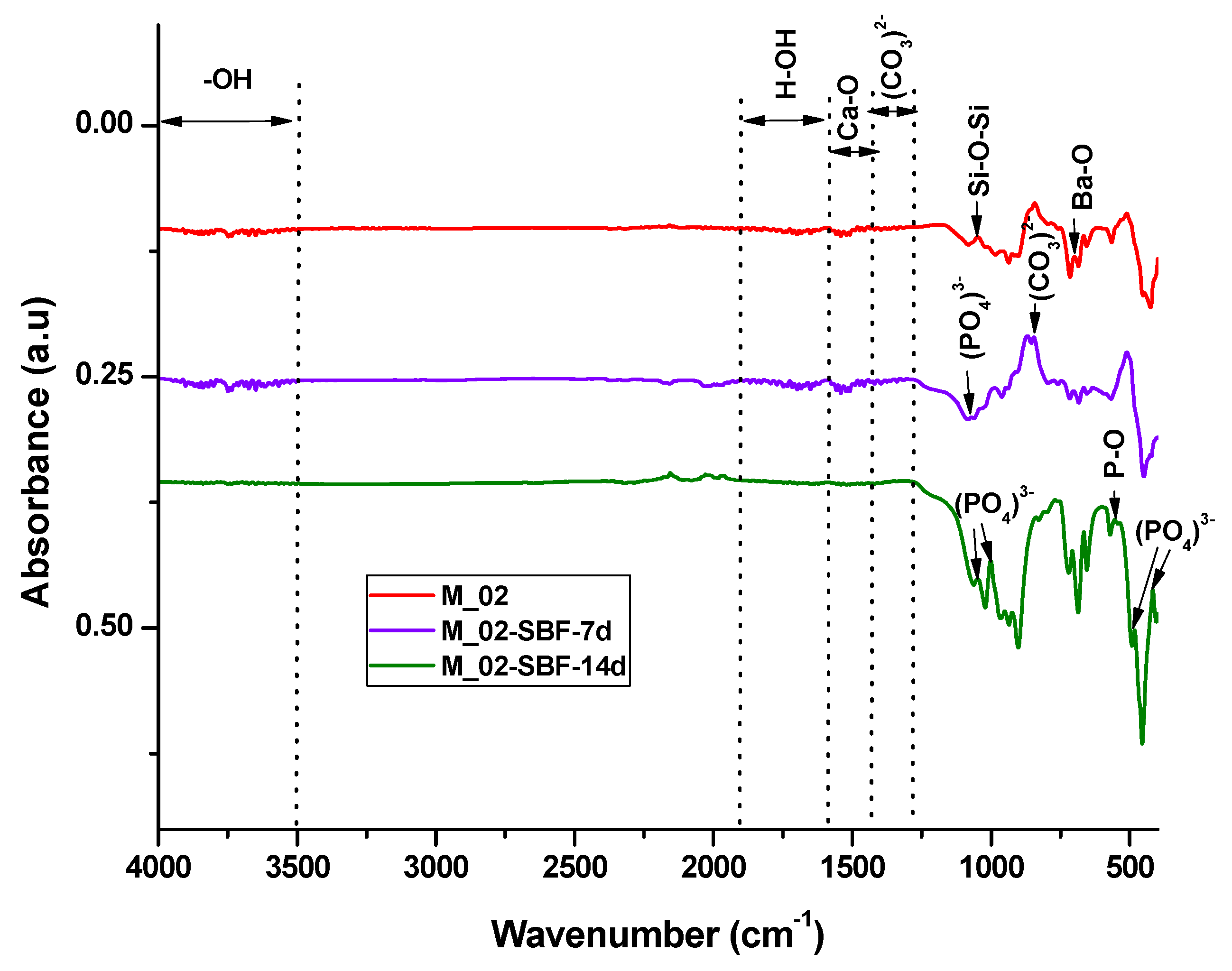
- For the sample immersed for 14 days, SEM images clearly illustrate alterations in the morpho-structural and surface characteristics of the scaffold filaments. Notably, the surface roughness of the filaments increases, indicating an interaction between the scaffold and simulated body fluid (SBF). At higher magnifications, quasi-spherical particles composed of very fine plates can be observed. These particles are attributed to the partially carbonate apatite phase formed during surface mineralization, which aligns with the FTIR spectroscopy findings mentioned earlier.
- Furthermore, the presence of phosphorus in the EDX analysis, as shown in Figure 9, confirms the interaction between SBF and the material. It is worth noting that the SBF solution, in which the scaffold was immersed for 14 days, has a slightly basic pH value of 8.5.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maksoud, F.J.; Velázquez de la Paz, M.F.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous Biomaterials for Tissue Engineering: A Review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Tag El-deen, D.M.; Taha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2023, 15, 2493–2505. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable Magnesium Phosphates in Biomedical Applications. J. Mater. Chem. B 2022, 10, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized Scaffolds to Enhance Tissue Regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- John, C.; Middleton, A.J.T. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar]
- Yu, Q.; Chang, J.; Wu, C. Silicate Bioceramics: From Soft Tissue Regeneration to Tumor Therapy. J. Mater. Chem. B 2019, 7, 5449–5460. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A Review of Bioactive Silicate Ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Sepantafar, M.; Ostadrahimi, A. The Role of Bioinorganics in Improving the Mechanical Properties of Silicate Ceramics as Bone Regenerative Materials. J. Ceram. Sci. Technol. 2015, 6, 1–8. [Google Scholar]
- Nicoara, A.I.; Alecu, A.E.; Balaceanu, G.-C.; Puscasu, E.M.; Vasile, B.S.; Trusca, R. Fabrication and Characterization of Porous Diopside/Akermanite Ceramics with Prospective Tissue Engineering Applications. Materials 2023, 16, 5548. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced Osteoporotic Bone Regeneration by Strontium-Substituted Calcium Silicate Bioactive Ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Deng, L.; Huang, L.; Pan, H.; Zhang, Q.; Que, Y.; Fan, C.; Chang, J.; Ni, S.; Yang, C. 3D Printed Strontium–Zinc-Phosphate Bioceramic Scaffolds with Multiple Biological Functions for Bone Tissue Regeneration. J. Mater. Chem. B 2023, 11, 5469–5482. [Google Scholar] [CrossRef]
- Shao, H.; Liu, A.; Ke, X.; Sun, M.; He, Y.; Yang, X.; Fu, J.; Zhang, L.; Yang, G.; Liu, Y.; et al. 3D Robocasting Magnesium-Doped Wollastonite/TCP Bioceramic Scaffolds with Improved Bone Regeneration Capacity in Critical Sized Calvarial Defects. J. Mater. Chem. B 2017, 5, 2941–2951. [Google Scholar] [CrossRef]
- Ghițulică, C.-D.; Cucuruz, A.; Voicu, G.; Cucuruz, A.T.; Dinescu, S.; Selaru, A.; Costache, M. Ceramics Based on Calcium Phosphates Substituted with Magnesium Ions for Bone Regeneration. Int. J. Appl. Ceram. Technol. 2019, 17, 342–353. [Google Scholar] [CrossRef]
- He, F.; Yuan, X.; Lu, T.; Wang, Y.; Feng, S.; Shi, X.; Wang, L.; Ye, J.; Yang, H. Preparation and Characterization of Novel Lithium Magnesium Phosphate Bioceramic Scaffolds Facilitating Bone Generation. J. Mater. Chem. B 2022, 10, 4040–4047. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The Roles of Ions on Bone Regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The Synergistic Effects of Sr and Si Bioactive Ions on Osteogenesis, Osteoclastogenesis and Angiogenesis for Osteoporotic Bone Regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Wang, M.; Backman, L.J.; Chen, J. Effects of Zinc, Magnesium, and Iron Ions on Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Kovrlija, I.; Locs, J.; Loca, D. Incorporation of Barium Ions into Biomaterials: Dangerous Liaison or Potential Revolution? Materials 2021, 14, 5772. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, V.; Vermani, Y.K.; Al-Buriahi, M.S.; Alzahrani, J.S.; Singh, T. Fabrication and Characterization of Barium Based Bioactive Glasses in Terms of Physical, Structural, Mechanical and Radiation Shielding Properties. Ceram. Int. 2021, 47, 21730–21743. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Tripathi, H.; Vyas, V.K.; Jain, S.; Suman, S.K.; Pyare, R.; Singh, S.P. Influence of Barium Substitution on Bioactivity, Thermal and Physico-Mechanical Properties of Bioactive Glass. Mater. Sci. Eng. C 2015, 49, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Ibrahim Fouad, G.; Beherei, H.H.; Das, D.B. Barium Oxide Doped Magnesium Silicate Nanopowders for Bone Fracture Healing: Preparation, Characterization, Antibacterial and In Vivo Animal Studies. Pharmaceutics 2022, 14, 1582. [Google Scholar] [CrossRef]
- Myat-Htun, M.; Mohd Noor, A.-F.; Kawashita, M.; Baba Ismail, Y.M. Enhanced Sinterability and in Vitro Bioactivity of Barium-Doped Akermanite Ceramic. Ceram. Int. 2020, 46, 19062–19068. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Moztarzadeh, F. Synthesis and Characterization of Barium–Iron Containing Magnetic Bioactive Glasses: The Effect of Magnetic Component on Structure and in Vitro Bioactivity. Colloids Surf. B Biointerfaces 2019, 176, 27–37. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Lin, Y.-H.; Chen, Y.-W.; Kuo, T.-Y.; Shie, M.-Y. Additive Manufacturing of Barium-Doped Calcium Silicate/Poly-ε-Caprolactone Scaffolds to Activate CaSR and AKT Signalling and Osteogenic Differentiation of Mesenchymal Stem Cells. J. Mater. Chem. B 2023, 11, 4666–4676. [Google Scholar] [CrossRef]
- Wisniewski, W.; Thieme, C.; Müller, R.; Reinsch, S.; Groß-Barsnick, S.-M.; Rüssel, C. Oriented Surface Nucleation and Crystal Growth in a 18BaO·22CaO·60SiO2 Mol% Glass Used for SOFC Seals. CrystEngComm 2018, 20, 787–795. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Ao, L.; Wu, S.; Zhu, X.; Zhong, Q.; Xu, Y.; Fang, Z.; Tang, X.; Tang, B. Structure and Microwave Dielectric Properties of Novel Walstromite-Type MCa2Si3O9 (M = Ba, Sr) Ceramics. Ceram. Int. 2023, 49, 27147–27153. [Google Scholar] [CrossRef]
- Müller, M.; Jüstel, T. Energy Transfer and Unusual Decay Behaviour of BaCa2Si3O9: Eu2+, Mn2+ Phosphor. Dalton Trans. 2015, 44, 10368–10376. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Hernández, V.H.; Fang, Q.; Babelot, C.; Lohoff, R.; Blum, L. An Experimental Investigation of Fracture Processes in Glass-Ceramic Sealant by Means of Acoustic Emission. Int. J. Hydrogen Energy 2020, 45, 27539–27550. [Google Scholar] [CrossRef]
- Raut, S.K.; Dhoble, N.S.; Park, K.; Dhoble, S.J. Precipitation Based Synthesis and Luminescence of Ln3+ (Eu, Ce, Dy, Sm, Tb) Activated BaCa2Si3O9-Walstromite Cyclosilicate Phosphors. Mater. Chem. Phys. 2014, 147, 594–603. [Google Scholar] [CrossRef]
- Barkley, M.C.; Downs, R.T.; Yang, H. Structure of Walstromite, BaCa2Si3O9, and Its Relationship to CaSiO3-Walstromite and Wollastonite-II. Am. Mineral. 2011, 96, 797–801. [Google Scholar] [CrossRef]
- Monfared, M.H.; Nemati, A.; Loghman, F.; Ghasemian, M.; Farzin, A.; Beheshtizadeh, N.; Azami, M. A Deep Insight into the Preparation of Ceramic Bone Scaffolds Utilizing Robocasting Technique. Ceram. Int. 2022, 48, 5939–5954. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]
- Qu, H. Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Today Commun. 2020, 24, 101024. [Google Scholar] [CrossRef]
- Dobriţa, C.-I.; Bădănoiu, A.-I.; Voicu, G.; Nicoară, A.-I.; Dumitru, S.-M.; Puşcaşu, M.-E.; Chiriac, Ș.; Ene, R.; Iordache, F. Porous Bioceramic Scaffolds Based on Akermanite Obtained by 3D Printing for Bone Tissue Engineering. Ceram. Int. 2023, 49, 35898–35906. [Google Scholar] [CrossRef]
- ISO10545-3:1995; Ceramic Tiles–Part 3. Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density. International Organization for Standardization (ISO): Geneva, Switzerland, 1995.
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel Bioactive Materials with Different Mechanical Properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Xiong, B.; Han, Y.; Tong, H. Thermal Decomposition Paths of Calcium Nitrate Tetrahydrate and Calcium Nitrite. Thermochim. Acta 2022, 714, 179264. [Google Scholar] [CrossRef]
- Krzątała, A.; Krüger, B.; Galuskina, I.; Vapnik, Y.; Galuskin, E. Walstromite, BaCa2(Si3O9), from Rankinite Paralava within Gehlenite Hornfels of the Hatrurim Basin, Negev Desert, Israel. Minerals 2020, 10, 407. [Google Scholar] [CrossRef]
- Ansari, M.A.; Jahan, N. Structural and Optical Properties of BaO Nanoparticles Synthesized by Facile Co-Precipitation Method. Mater. Highlights 2021, 2, 23. [Google Scholar] [CrossRef]
- Thamma, U.; Kowal, T.J.; Falk, M.M.; Jain, H. Nanostructure of Bioactive Glass Affects Bone Cell Attachment via Protein Restructuring upon Adsorption. Sci. Rep. 2021, 11, 5763. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility Pathways and Mechanisms for Bioactive Materials: The Bioactivity Zone. Bioact. Mater. 2022, 10, 306–322. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.-J.; Davydov, A.V.; Frukhtbeyen, S.; Seppala, J.E.; Takagi, S.; Chow, L.; Alimperti, S. Biofabrication of 3D Printed Hydroxyapatite Composite Scaffolds for Bone Regeneration. Biomed. Mater. 2021, 16, 045002. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304, 1800698. [Google Scholar] [CrossRef]
- Dai, K.; Yang, Z.; Ding, L.; Yang, Z.; Hang, F.; Cao, X.; Chen, D.; Zhao, F.; Chen, X. 3D-Printed Strontium-Doped BG-CaSiO3-HA Composite Scaffolds Promote Critical Bone Defect Repair by Improving Mechanical Strength and Increasing Osteogenic Activity. Ceram. Int. 2023, 49, 19773–19785. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-Printed Bioactive Ca3SiO5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef]




| Sample | ρa (g/cm3) | A (%) | Pd (%) | Rc (MPa) |
|---|---|---|---|---|
| M_02 | 2.67 ± 0.03 | 12.05 ± 0.03 | 41.34 ± 0.03 | 2.56 ± 0.03 |
| Unimmersed in SBF | Immersed in SBF: | ||||
|---|---|---|---|---|---|
| 7 Days | 14 Days | ||||
| Wavenumber (cm−1) | Functional Group | Wavenumber (cm−1) | Functional Group | Wavenumber (cm−1) | Functional Group |
| 700 | Ba-O | 855 | C-O din CO32− | 480/414 | (PO4)3− |
| 1043 | Si-O-Si | 1073 | PO43− | 551 | P-O from (PO4)3− |
| 923–1100 | [SiO4]4− | 1338–1400 | CO32− | 855 | C-O from CO32− |
| 1460 | Ca-O | 1460 | Ca-O | 1052/1000 | PO43− |
| 1600–1900 | atmosphere water | ||||
| 3500–4000 | –OH from hydroxyapatite | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, Ş.; Popescu, R.-C.; Pele, M.-M.; Ghiţulică, C.-D.; Cucuruz, A.; Geanaliu-Nicolae, R.-E.; Stancu, I.-C.; Voicu, G.; Ciocan, L.-T. New 3D Printed Scaffolds Based on Walstromite Synthesized by Sol–Gel Method. J. Funct. Biomater. 2024, 15, 19. https://doi.org/10.3390/jfb15010019
Chiriac Ş, Popescu R-C, Pele M-M, Ghiţulică C-D, Cucuruz A, Geanaliu-Nicolae R-E, Stancu I-C, Voicu G, Ciocan L-T. New 3D Printed Scaffolds Based on Walstromite Synthesized by Sol–Gel Method. Journal of Functional Biomaterials. 2024; 15(1):19. https://doi.org/10.3390/jfb15010019
Chicago/Turabian StyleChiriac, Ştefania, Roxana-Cristina Popescu, Mihnea-Mihăiță Pele, Cristina-Daniela Ghiţulică, Andreia Cucuruz, Ruxandra-Elena Geanaliu-Nicolae, Izabela-Cristina Stancu, Georgeta Voicu, and Lucian-Toma Ciocan. 2024. "New 3D Printed Scaffolds Based on Walstromite Synthesized by Sol–Gel Method" Journal of Functional Biomaterials 15, no. 1: 19. https://doi.org/10.3390/jfb15010019