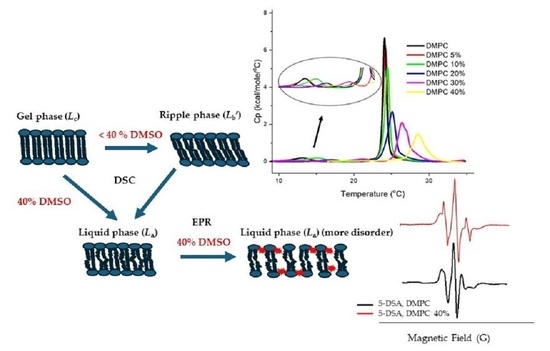
Effect of DMSO on Structural Properties of DMPC and DPPC Liposome Suspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposomes
2.3. Differential Scanning Calorimetry (DSC)
- (a)
- Large unilamellar vesicles, LUVs, were prepared in HEPES buffer, and the mixture to be analyzed by DSC was prepared by adding the desired amount of DMSO at room temperature to the LUVs suspension previously prepared in buffer, just prior to the DSC experiment (“DMSO added after preparation”).
- (b)
- Multilamellar vesicles, MLVs, prepared using the same procedure as described above for LUVs (“DMSO added after preparation”).
- (c)
- Multilamellar vesicles, MLVs, prepared by hydration of the lipid film with HEPES buffer already containing DMSO at the desired molar fraction, (“DMSO added on preparation, incorporation method”).
2.4. Electron Paramagnetic Resonance (EPR)
3. Results and Discussion
3.1. Effect of DMSO on DMPC and DPPC Liposomes—DSC Results
3.1.1. LUVs, “DMSO Added after Preparation”
3.1.2. MLVS with “DMSO Added to already Formed Liposomes”
3.1.3. MLVS with “DMSO Added on Liposome Preparation, Incorporation Method”
3.2. Effect of DMSO on DMPC and DPPC Liposomes—EPR Results
LUVs, “DMSO Added after Preparation”
3.3. SDS and Hexylmpp Partition to DMPC Liposomes at Different DMSO Contents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucio, M.; Lima, J.L.F.C.; Reis, S. Drug-membrane interactions: Significance for medicinal chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef]
- Claro, B.; González-Freire, E.; Granja, J.R.; Garcia-Fandiño, R.; Gallová, J.; Uhríková, D.; Fedorov, A.; Coutinho, A.; Bastos, M. Partition of antimicrobial D,L-α-Cyclic peptides into bacterial model membranes. BBA—Biomembranes 2022, 1864, 183729. [Google Scholar] [CrossRef]
- Boafo, G.F.; Magar, T.K.; Ekpo, M.D.; Qian, W.; Tan, S.; Chen, C. The role of cryoprotective agents in liposome stabilization and preservation. Int. J. Mol. Sci. 2022, 23, 12487. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.; Schrooders, M.L.Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; Gotta, S.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef]
- Hanslick, J.L.; Lau, K.; Noguchi, K.K.; Olney, J.W.; Zorumski, C.F.; Mennerick, S.; Farber, N.B. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. 2009, 34, 1–10. [Google Scholar] [CrossRef]
- Rangel, M.; Amorim, J.; Nunes, A.; Leite, A.; Pereira, E.; de Castro, B.; Sousa, C.; Yoshikawa, Y.; Sakurai, H. Novel 3-hydroxy-4-pyridinonato oxidovanadium(IV) complexes to investigate structure/activity relationships. J. Inorg. Chem. 2009, 103, 496–502. [Google Scholar] [CrossRef]
- McLaren, C.W.F. An accurate and convenient organic phosphorous assay. Anal. Biochem. 1971, 39, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Claro, B.; González-Freire, E.; Calvelo, M.; Bessa, L.J.; Goormaghtigh, E.; Amorín, M.; Granja, J.R.; Garcia-Fandiño, R.; Bastos, M. Membrane targeting antimicrobial cyclic peptide nanotubes—An experimental and computational study. Colloids Surf. B 2020, 196, 111349. [Google Scholar] [CrossRef]
- Torricella, F.; Pierro, A.; Mileo, E.; Belle, V.; Bonucci, A. Nitroxide spin labels and EPR spectroscopy: A powerful association for protein dynamics studies. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140653. [Google Scholar] [CrossRef]
- Martini, G.; Ciani, L. Electron spin resonance spectroscopy in drug delivery. Phys. Chem. Chem. Phys. 2009, 11, 211–254. [Google Scholar] [CrossRef]
- Marsh, D. Handbook of Lipid Bilayers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Yu, Z.-W.; Quinn, P.J. Phase stability of phosphatidylcholines in dimethylsulfoxide solutions. Biophys. J. 1995, 69, 1456–1463. [Google Scholar] [CrossRef]
- Ricci, M.; Oliva, R.; Del Vecchio, P.; Paolantoni, M.; Morresi, A.; Sassi, P. DMSO-induced perturbation of thermotropic properties of cholesterol-containing DPPC liposomes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Dea, P.K. Insights into the dynamics of DMSO in phosphatidylcholine bilayers. Biophys. Chem. 2001, 94, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, M.A.; Lesieur, P.; Kisselev, A.M.; Grabielle-Madelmond, C.; Ollivon, M. DMSO-induced dehydration of DPPC membranes studied by X-ray diffraction, small-angle neutron scattering, and calorimetry. J. Alloys Compd. 1999, 286, 195–202. [Google Scholar] [CrossRef]
- Yu, Z.-W.; Quinn, P.J. Solvation effects of dimethyl sulfoxide on the structure of phospholipid bilayers. Biophys. Chem. 1998, 70, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Gordeliy, V.I.; Kiselev, M.A.; Lesieur, P.; Pole, A.V.; Teixeira, J. Lipid membrane structure and interactions in dimethyl sulfoxide/water mixtures. Biophys. J. 1998, 75, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Song, J.; Pas, J.; Meijer, L.H.H.; Han, S. DMSO induces dehydration near lipid membrane surface. Biophys. J. 2015, 109, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, S.N.; Kiselev, M.A.; Tioutiounnikov, S.N.; Kiselev, A.M.; Lesieur, P. The study of DMSO/water and DPPC/DMSO/water system by means of the X-ray, neutron small-angle scattering, calorimetry and IR spectroscopy. Phys. B: Condens. Matter 1999, 271, 184–191. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Anwar, J. Modulating the structure and properties of cell membranes: The molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. molecular basis for dimethylsulfoxide (dmso) action on lipid membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef] [PubMed]
- Hughes, Z.E.; Mark, A.E.; Mancera, R.L. Molecular dynamics simulations of the interactions of DMSO with DPPC and DOPC phospholipid membranes. J. Phys. Chem. B 2012, 116, 11911–11923. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Ziegler, A.; Steinbauer, B.; Seelig, J. Thermodynamics of sodium dodecyl sulfate partitioning into lipid membranes. Biophys. J. 2002, 83, 1547–1556. [Google Scholar] [CrossRef]
- Saghaie, L.; Hider, R.; Mostafavi, S.A. Comparison of automated continuous flow method with shake- flask method in determining partition coefficients of bidentate hydroxypyridinone ligands. DARU J. Pharm. Sci. 2003, 11, 38–46. [Google Scholar]







| XDMSO | % DMSO (v/v) | Tm /°C | ΔT1/2/°C | ΔH kJ·mol−1 | Tm/°C | ΔT1/2/°C | ΔH kJ·mol−1 |
|---|---|---|---|---|---|---|---|
| DMPC | DPPC | ||||||
| 0 | 0 | 24.5 | 0.8 | 18 | 41.3 | 1.6 | 33 |
| 0.0018 | 0.7 | 24.5 | 0.8 | 19 | 41.4 | 1.6 | 33 |
| 0.0026 | 1.0 | 24.5 | 0.8 | 19 | 41.3 | 1.7 | 32 |
| 0.0051 | 2.0 | 24.6 | 0.8 | 19 | 41.3 | 1.7 | 32 |
| 0.078 | 3.0 | 24.6 | 0.8 | 19 | 41.4 | 1.7 | 31 |
| 0.010 | 4.0 | 24.7 | 0.9 | 18 | 41.4 | 1.8 | 31 |
| 0.013 | 5.0 | 24.7 | 0.8 | 18 | 41.5 | 1.8 | 32 |
| 0.019 | 7.0 | 24.7 | 0.9 | 17 | 41.5 | 1.8 | 31 |
| 0.027 | 10 | 24.8 | 1.0 | 17 | 41.6 | 1.9 | 32 |
| 0.060 | 20 | 25.2 | 1.7 | 17 | 41.6 | 2.6 | 30 |
| 0.098 | 30 | 26.2 | 3.5 | 17 | 42.4 | 2.9 | 30 |
| 0.14 | 40 | 27.9 | 5.2 | 17 | 42.8 | 3.2 | 27 |
| XDMSO | % DMSO (v/v) | Tp/°C | ΔT1/2/°C | ΔHp kJ·mol−1 | Tm/°C | ΔT1/2/°C | ΔH kJ·mol−1 |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 13.0 | 2.2 | 2 | 23.9 | 0.6 | 21 |
| 0.013 | 5.0 | 13.2 | 2.7 | 3 | 24.2 | 0.6 | 21 |
| 0.027 | 10 | 15.2 | 3.3 | 3 | 24.4 | 0.7 | 20 |
| 0.060 | 20 | 17.9 | 3.8 | 2 | 25.2 | 1.2 | 17 |
| 0.098 | 30 | 21.5 | 3.8 | 2 | 26.1 | 1.3 | 16 |
| 0.14 | 40 | --- | --- | --- | 28.2 | 2.0 | 15 |
| XDMSO | % DMSO (v/v) | Tp/°C | ΔT1/2/°C | ΔHp kJ·mol−1 | Tm /°C | ΔT1/2 /°C | ΔH kJ·mol−1 |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 13.1 | 2.3 | 2 | 23.9 | 0.6 | 21 |
| 0.027 | 10 | 15.8 | 2.2 | 3 | 24.6 | 0.6 | 22 |
| 0.060 | 20 | 18.2 | 1.6 | 2 | 25.5 | 0.6 | 22 |
| 0.098 | 30 | 24.2 | 1.3 | 2 | 27.5 | 0.6 | 18 |
| 0.14 | 40 | 26.0 | 1.0 | 2 | 28.3 | 0.6 | 16 |
| XDMSO | %DMSO (v/v) | Tm/°C | ΔT1/2/°C | ΔH kJ·mol−1 | Tm/°C | ΔT1/2/°C | ΔH kJ·mol−1 |
|---|---|---|---|---|---|---|---|
| SDS 200 µM | SDS 500 µM | ||||||
| DMPC | 24.5 | 1.0 | 18 | 24.5 | 1.0 | 18 | |
| 0 | 0 | 23.7 | 1.0 | 18 | 23.0 | 1.0 | 19 |
| 0.0078 | 3.0 | 23.7 | 1.0 | 18 | 23.0 | 1.0 | 19 |
| 0.013 | 5.0 | 23.7 | 1.0 | 18 | 23.1 | 1.0 | 19 |
| 0.019 | 7.0 | 23.8 | 1.0 | 18 | 23.2 | 1.0 | 18 |
| 0.027 | 10 | 23.9 | 1.0 | 18 | 23.5 | 1.0 | 17 |
| XDMSO | % DMSO (v/v) | Tm/°C | ΔT1/2/°C | ΔH kJ·mol−1 |
|---|---|---|---|---|
| Hexyl 5 mM | ||||
| DMPC | 24.5 | 0.8 | 19 | |
| 0 | 0 | 22.6 | 0.8 | 21 |
| 0.0078 | 3.0 | 22.7 | 0.8 | 21 |
| 0.013 | 5.0 | 22.7 | 0.8 | 20 |
| 0.019 | 7.0 | 23.0 | 0.8 | 22 |
| 0.027 | 10 | 23.5 | 1.0 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, L.M.P.F.; Rangel, M.; Bastos, M. Effect of DMSO on Structural Properties of DMPC and DPPC Liposome Suspensions. J. Funct. Biomater. 2024, 15, 67. https://doi.org/10.3390/jfb15030067
Amaral LMPF, Rangel M, Bastos M. Effect of DMSO on Structural Properties of DMPC and DPPC Liposome Suspensions. Journal of Functional Biomaterials. 2024; 15(3):67. https://doi.org/10.3390/jfb15030067
Chicago/Turabian StyleAmaral, Luísa M. P. F., Maria Rangel, and Margarida Bastos. 2024. "Effect of DMSO on Structural Properties of DMPC and DPPC Liposome Suspensions" Journal of Functional Biomaterials 15, no. 3: 67. https://doi.org/10.3390/jfb15030067