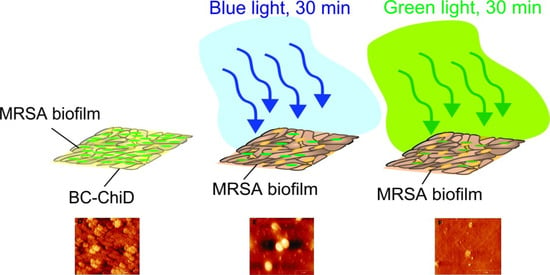
Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanochitosan Dots (ChiDs), Bacterial Cellulose (BC) and Preparing Photoactive Composites BC-ChiD
2.2. Characterization Methods
2.2.1. Scanning Electron Microscopy (SEM) Imaging
2.2.2. Fourier Transform Infrared (FTIR) Absorption Spectroscopy
2.2.3. X-ray Diffraction (XRD)
2.2.4. Atomic Force Microscopy (AFM) Imaging
2.2.5. Porosity of BC-ChiD_Control, BC-ChiD_Blue and BC-ChiD_Green Composite Hydrogels
2.2.6. Electron Paramagnetic Resonance (EPR) Measurements of BC-ChiD_Blue and BC-ChiD_Green Composites
2.2.7. In Vitro Release of ChiD from BC-ChiD_Blue and BC-ChiD_Green Composites
2.2.8. Photo-Induced Antibiofilm In Vitro Test
- Biofilm formation and plate preparation
- Antibiofilm test
3. Results and Discussion
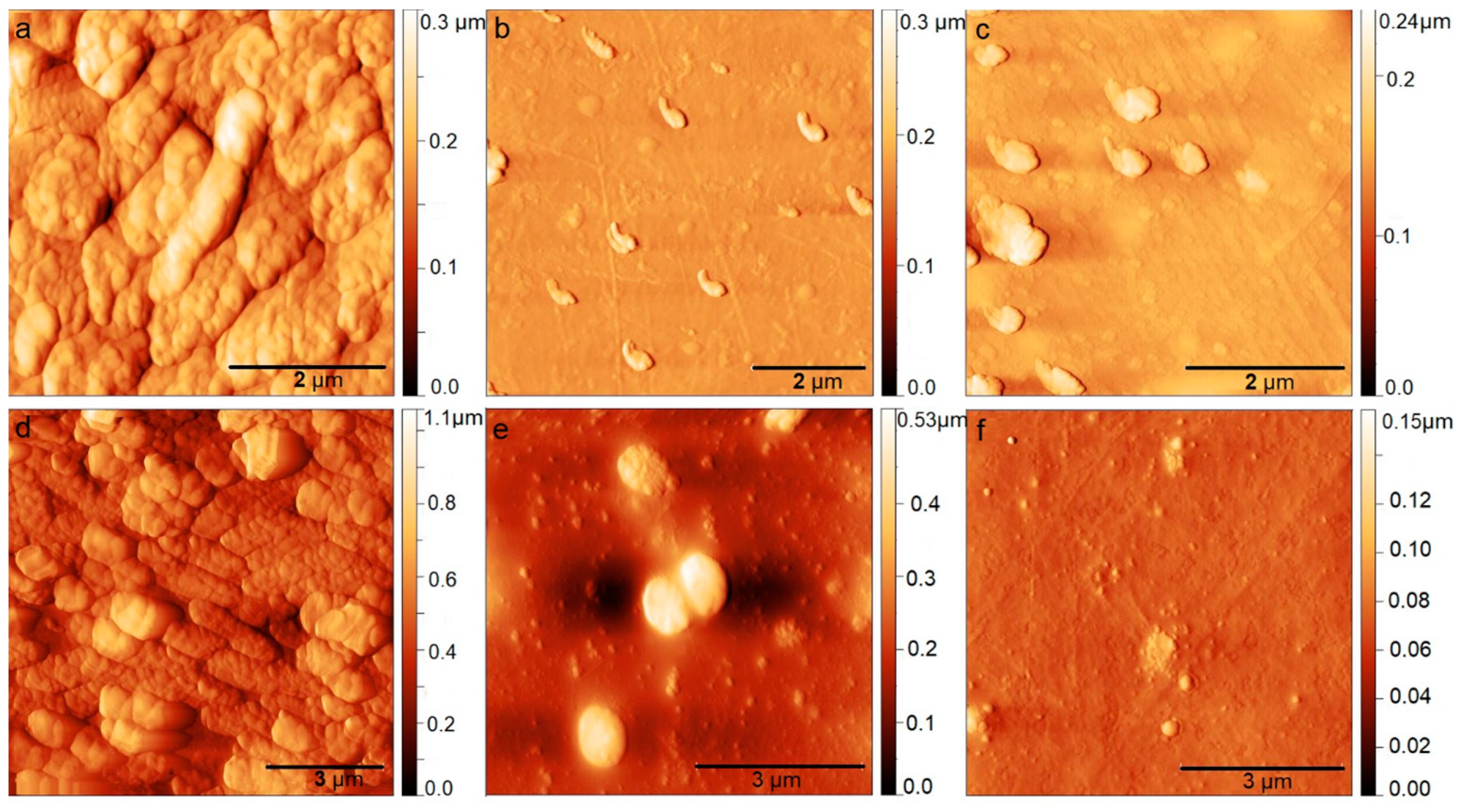
3.1. Surface Morphology of BC-ChiD_Blue and BC-ChiD_Green Composites
3.2. Chemical Composition
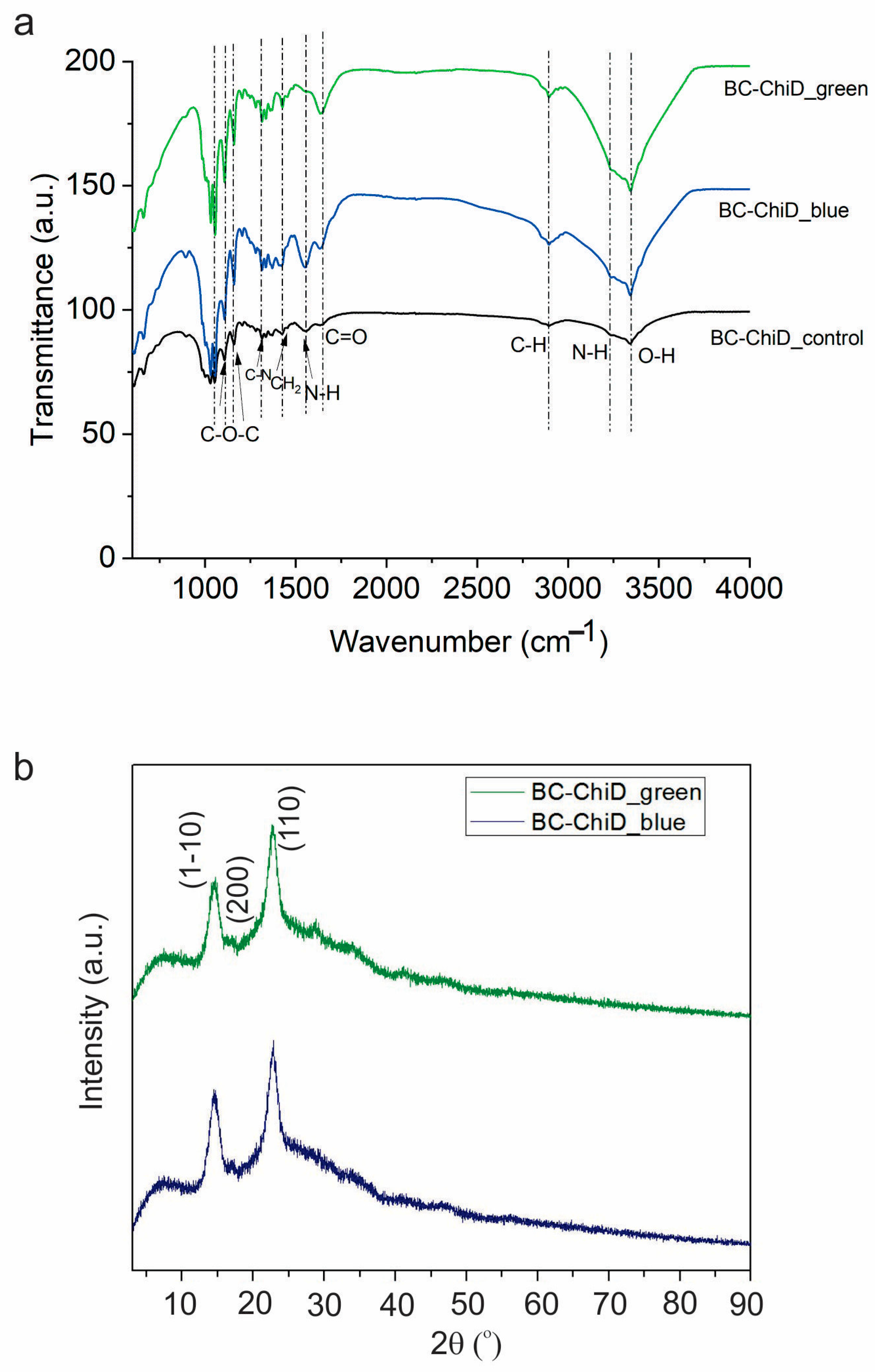
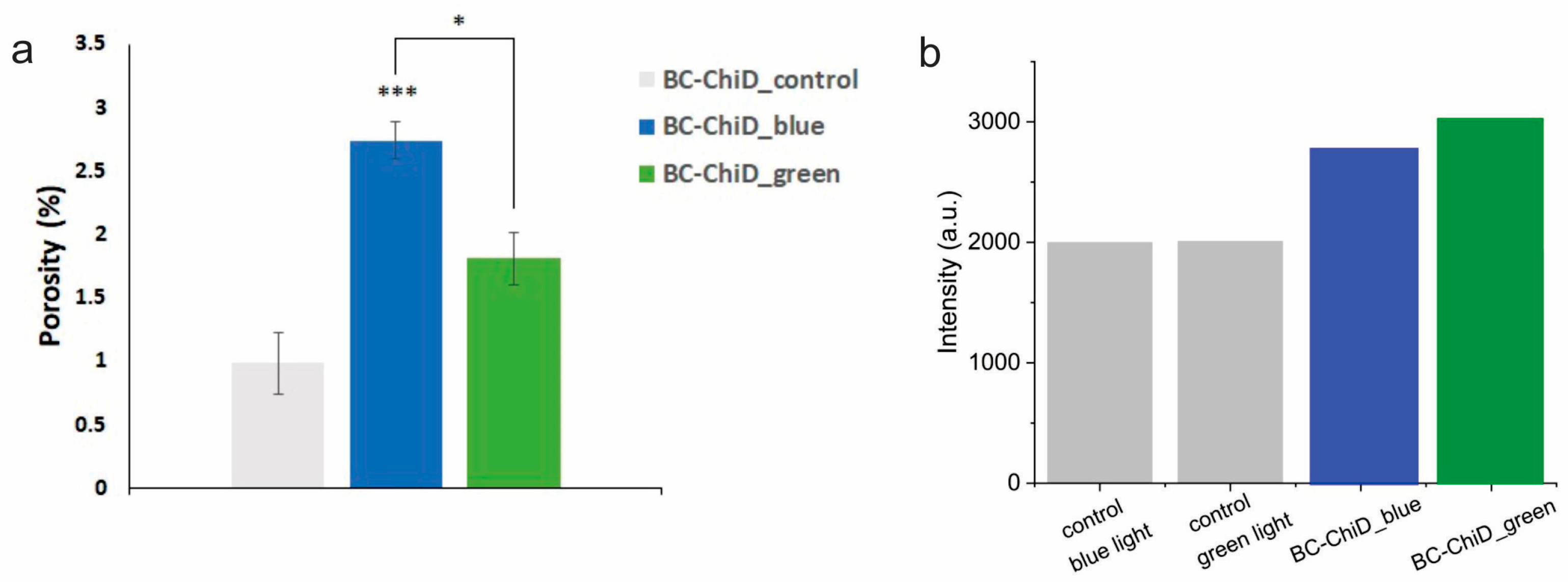
3.3. Porosity of BC-ChiD_Control, BC-ChiD_Blue and BC-ChiD_Green Composite Hydrogels
3.4. EPR Measurement of Singlet Oxygen Formation
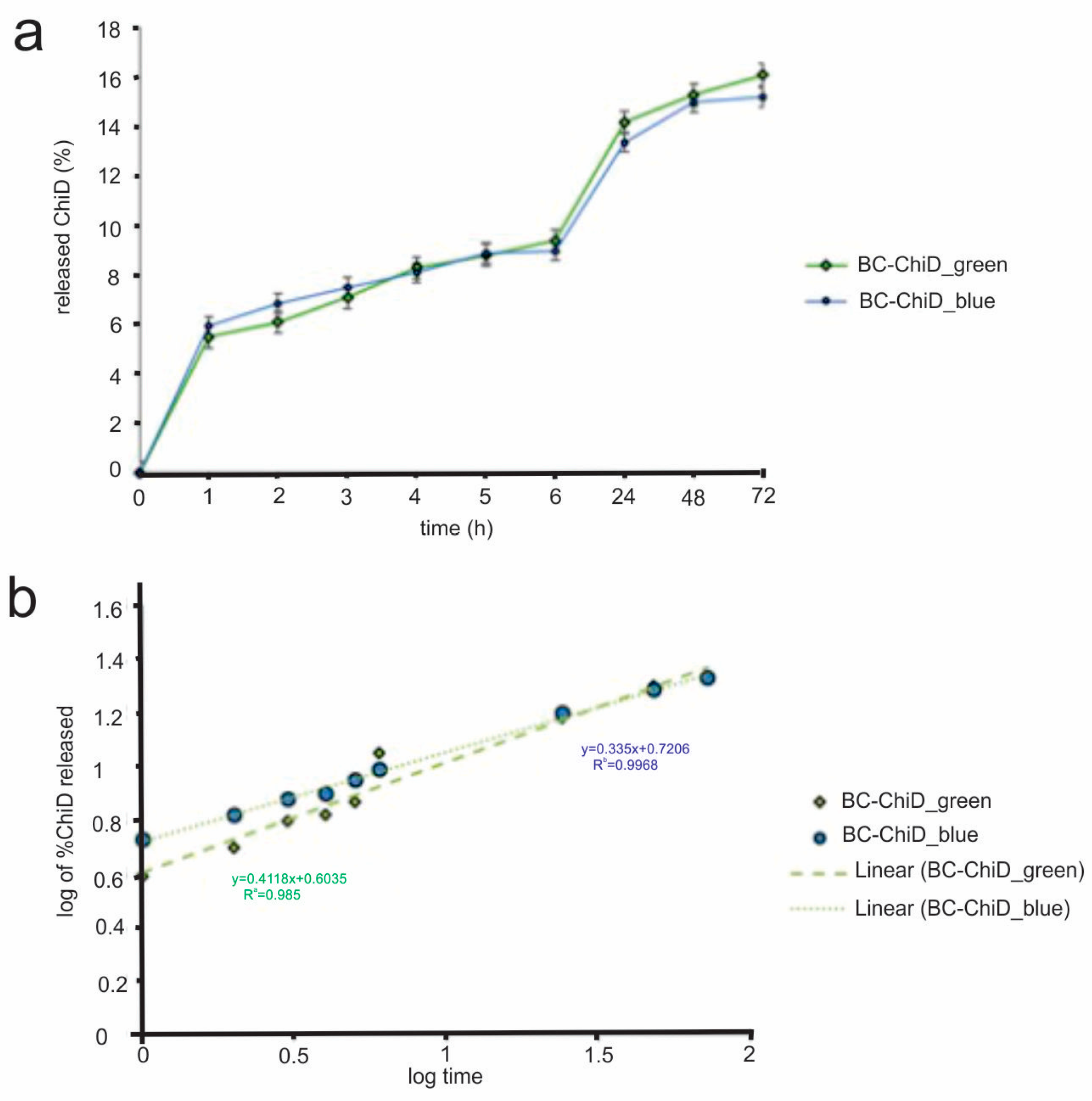
3.5. In Vitro Release of ChiD from the BC-ChiD Composite Hydrogels Exposed to Blue and Green Light
3.6. Antibiofilm Activity of BC-ChiD_Blue and BC-ChiD_Green Composite Samples and AFM Imaging of Pathogenic Biofilms after the Application of BC-ChiD_Blue and BC-ChiD_Green Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Wang, M.; Chen, Z.; Luo, Y.; Xia, X.S.; Song, Y.; Sun, Y.; Zhang, A.M. Cathelicidin-DM is an Antimicrobial Peptide from Duttaphrynus melanostictus and Has Wound-Healing Therapeutic Potential. ACS Omega 2020, 5, 9301–9310. [Google Scholar] [CrossRef]
- Karam, G.; Chastre, J.; Wilcox, M.H.; Vincent, J.L. Antibiotic strategies in the era of multidrug resistance. Crit. Care 2016, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Durand, B.; Pouget, C.; Magnan, C.; Molle, V.; Lavigne, J.P.; Dunyach-Remy, C. Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review. Microorganisms 2022, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.R.; Brennan, M.B. The role of the microbiome in nonhealing diabetic wounds. Ann. N. Y. Acad. Sci. 2019, 1435, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Gronseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1922. [Google Scholar] [CrossRef] [PubMed]
- Hota, B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004, 39, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Magaela, N.B.; Makola, L.C.; Managa, M.; Nyokong, T. Photodynamic activity of novel cationic porphyrins conjugated to graphene quantum dots against Staphylococcus aureus. J. Porphyr. Phthalocyanines 2022, 26, 392–402. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontology 2000 1997, 14, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Nechifor, M.T.; Neagu, T.M. Reactive oxygen species, cancer and anti-cancer therapies. Curr. Chem. Biol. 2009, 3, 342–366. [Google Scholar] [CrossRef]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef]
- Schastak, S.; Ziganshyna, S.; Gitter, B.; Wiedemann, P.; Claudepierre, T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using thpts, a cationic photosensitizer excited by infrared wavelength. PLoS ONE 2010, 5, e11674. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Zdravkovic, N.M.; Trisic, D.D.; Budimir, M.D.; Markovic, Z.M.; Kozyrovska, N.O.; Todorovic Markovic, B.M. Chronic wound dressings—Pathogenic bacteria anti-biofilm treatment with bacterial cellulose-chitosan polymer or bacterial cellulose-chitosan dots composite hydrogels. Int. J. Biol. Macromol. 2021, 191, 315–323. [Google Scholar] [CrossRef]
- Koyani, R.D.; Vazquez-Duhalt, R. Laccase encapsulation in chitosan nanoparticles enhances the protein stability against microbial degradation. Environ. Sci. Pollut. Res. Int. 2016, 23, 18850–18857. [Google Scholar] [CrossRef] [PubMed]
- Chavez de Paz, L.E.; Resin, A.; Howard, K.A.; Sutherland, D.S.; Wejse, P.L. Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms. Appl. Environ. Microbiol. 2011, 77, 3892–3895. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Markovic, Z.M.; Zdravkovic, N.M.; Trisic, D.D.; Budimir, M.D.; Kuzman, S.B.; Kozyrovska, N.O.; Orlovska, I.V.; Bugarova, N.; Petrovic, D.Z.; et al. Bactericidal and antioxidant bacterial cellulose hydrogels doped with chitosan as potential urinary tract infection biomedical agent. RSC Adv. 2021, 11, 8559–8568. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Markovic, Z.M.; Budimir, M.D.; Zdravkovic, N.M.; Trisic, D.D.; Bugarova, N.; Danko, M.; Kozyrovska, N.O.; Spitalsky, Z.; Kleinova, A.; et al. Photoactive and antioxidant nanochitosan dots/biocellulose hydrogels for wound healing treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111925. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Pervaiz, F.; Ashames, A.; Buabeid, M.; Fahelelbom, K.; Shoukat, H.; Maqbool, I.; Murtaza, G. Optimization of Novel Naproxen-Loaded Chitosan/Carrageenan Nanocarrier-Based Gel for Topical Delivery: Ex Vivo, Histopathological, and In Vivo Evaluation. Pharmaceuticals 2021, 14, 557. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Veiga, M.; Tavaria, F.K.; Pintado, M.M. A review of chitosan’s effect on oral biofilms: Perspectives from the tube to the mouth. J. Oral Biosci. 2017, 59, 205–210. [Google Scholar] [CrossRef]
- Bacela, J.; Labowska, M.B.; Detyna, J.; Ziety, A.; Michalak, I. Functional Coatings for Orthodontic Archwires-A Review. Materials 2020, 13, 3257. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Julian-Belmonte, E.; Chiva-Garcia, F.; Martinez-Beneyto, Y. Bactericidal Efficacy of Photodynamic Therapy and Chitosan in Root Canals Experimentally Infected with Enterococcus faecalis: An In Vitro Study. Photomed. Laser Surg. 2017, 35, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; de Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-Based Drug Delivery Systems for Optimization of Photodynamic Therapy: A Review. AAPS PharmSciTech 2019, 20, 253. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 574–584. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojevic, D.; Orlovska, I.; Kozyrovska, N.; Sokovic, M.; Glamoclija, J.; Dmitrovic, S.; Matovic, B.; Tasic, N.; Maksimovic, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Markovic, Z.M.; Mitic, D.D.; Zdravkovic, N.M.; Kozyrovska, N.O.; Bugarova, N.; Todorovic Markovic, B.M. Antibacterial composite hydrogels of graphene quantum dots and bacterial cellulose accelerate wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1796–1805. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Improving bacterial cellulose films by ex-situ and in-situ modifications: A review. Food Hydrocoll. 2021, 113, 106514. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Kozlowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Ren, Z.; Han, J.; Zhang, X.; Zhu, B.; Yan, Z.; Xiao, H.; Wei, Q. Design of biodegradable polyurethanes and post-modification with long alkyl chains via inhibiting biofilm formation and killing drug-resistant bacteria for the treatment of wound bacterial infection. Biomater. Sci. 2023, 12, 176–186. [Google Scholar] [CrossRef]
- Focher, B.; Palma, M.T.; Canetti, M.; Torri, G.; Cosentino, C.; Gastaldi, G. Structural differences between non-wood plant celluloses: Evidence from solid state NMR, vibrational spectroscopy and X-ray diffractometry. Ind. Crops Prod. 2001, 13, 193–208. [Google Scholar] [CrossRef]
- Surman, S.B.; Walker, J.T.; Goddard, D.T.; Morton, L.H.G.; Keevil, C.W.; Weaver, W.; Skinner, A.; Hanson, K.; Caldwell, D.; Kurtz, J. Comparison of microscope techniques for the examination of biofilms. J. Microbiol. Methods 1996, 25, 57–70. [Google Scholar] [CrossRef]
- Beech, I.B.; Smith, J.R.; Steele, A.A.; Penegar, I.; Campbell, S.A. The use of atomic force microscopy for studying interactions of bacterial biofilms with surfaces. Colloids Surf. B Biointerfaces 2002, 23, 231–247. [Google Scholar] [CrossRef]
- Bremer, P.J.; Geese, G.G.; Drake, B. Atomic force microscopy examination of the topography of a hydrated bacterial biofilm on a copper surface. Curr. Microbiol. 1992, 24, 223–230. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Goins, A.; Ramaswamy, V.; Dirr, E.; Dulany, K.; Irby, S.; Webb, A.; Allen, J. Development of poly (1,8 octanediol-co-citrate) and poly (acrylic acid) nanofibrous scaffolds for wound healing applications. Biomed. Mater. 2017, 13, 015002. [Google Scholar] [CrossRef]
- ISO 20776-1:2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization: Geneva, Switzerland, 2006.
- Chusri, S.; Sompetch, K.; Mukdee, S.; Jansrisewangwong, S.; Srichai, T.; Maneenoon, K.; Limsuwan, S.; Voravuthikunchai, S.P. Inhibition of Staphylococcus epidermidis Biofilm Formation by Traditional Thai Herbal Recipes Used for Wound Treatment. Evid. Based Complement. Alternat. Med. 2012, 2012, 159797. [Google Scholar] [CrossRef]
- Sigma Aldrich. IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/RS/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 1 September 2022).
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Morsy, M.; Mostafa, K.; Amyn, H.; El-Ebissy, A.; Salah, A.; Youssef, M. Synthesis and Characterization of Freeze Dryer Chitosan Nano particles as Multi functional Eco-Friendly Finish for Fabricating Easy Care and Antibacterial Cotton Textiles. Egypt. J. Chem. 2019, 62, 1277–1293. [Google Scholar] [CrossRef]
- Lin, S.-P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, C.M.; Kafle, K. Characterization of crystalline cellulose in biomass: Basic principles, applications, and limitations of XRD, NMR, IR, Raman, and SFG. Korean J. Chem. Eng. 2013, 30, 2127–2141. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Preparation of antibacterial ZnO NP-containing schizophyllan/bacterial cellulose nanocomposite for wound dressing. Cellulose 2021, 28, 9269–9282. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; Tambasco de Oliveira, P.; Minarelli Gaspar, A.M.; Lima Ribeiro, S.J.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Xiong, C. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 2009, 16, 65. [Google Scholar] [CrossRef]
- Bianco, G.V.; Sacchetti, A.; Grande, M.; D’Orazio, A.; Milella, A.; Bruno, G. Effective hole conductivity in nitrogen-doped CVD-graphene by singlet oxygen treatment under photoactivation conditions. Sci. Rep. 2022, 12, 8703. [Google Scholar] [CrossRef]
- Fahimipour, A.K.; Hartmann, E.M.; Siemens, A.; Kline, J.; Levin, D.A.; Wilson, H.; Betancourt-Roman, C.M.; Brown, G.Z.; Fretz, M.; Northcutt, D.; et al. Daylight exposure modulates bacterial communities associated with household dust. Microbiome 2018, 6, 175. [Google Scholar] [CrossRef]
- Rehman, N.N.M.A.; Dixit, P.P. Influence of light wavelengths, light intensity, temperature, and pH on biosynthesis of extracellular and intracellular pigment and biomass of Pseudomonas aeruginosa NR1. J. King Saud Univ.—Sci. 2020, 32, 745–752. [Google Scholar] [CrossRef]
- Kamel, F.H.; Saeed, C.H.; Hassan, N.I. Comparative effect of different visible light energy on bacterial growth. Int. J. Adv. Res. 2016, 4, 263–270. [Google Scholar]
- Perlova, T.; Gruebele, M.; Chemla, Y.R. Blue Light Is a Universal Signal for Escherichia coli Chemoreceptors. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C.; Viana, M.S.; Meira, G.A. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers Med. Sci. 2016, 31, 27–32. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Photoinactivation of bacteria attached to glass and acrylic surfaces by 405 nm light: Potential application for biofilm decontamination. Photochem. Photobiol. 2013, 89, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef]
- Tarsi, R.; Corbin, B.; Pruzzo, C.; Muzzarelli, R.A. Effect of low-molecular-weight chitosans on the adhesive properties of oral streptococci. Oral Microbiol. Immunol. 1998, 13, 217–224. [Google Scholar] [CrossRef]
- Busscher, H.J.; Engels, E.; Dijkstra, R.J.; van der Mei, H.C. Influence of a chitosan on oral bacterial adhesion and growth in vitro. Eur. J. Oral Sci. 2008, 116, 493–495. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar] [CrossRef]
- Agrawal, N.; Bhagel, D.; Mishra, P.; Prasad, D.; Kohli, E. Post-synthetic modification of graphene quantum dots bestows enhanced biosensing and antibiofilm ability: Efficiency facet. RSC Adv. 2022, 12, 12310–12320. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.M.; Baumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef]

| Sample | Crystallite Size (nm) | Crystallinity Index (%) |
|---|---|---|
| BC-ChiD_blue | 4.7 | 30.7 |
| BC-ChiD_green | 4.3 | 32 |
| BC-ChiD_control [25] | 2.2 | 74 |
| Bacteria | BC-ChiD_Blue | BC-ChiD_Green | ||
|---|---|---|---|---|
| 0.2 | 2 | 0.2 | 2 | |
| S. aureus | 48 | 52 | 81 | 80 |
| MRSA | 57 | 56 | 80 | 85 |
| E. coli | 51 | 51 | 78 | 79 |
| K. pneumoniae | 47 | 57 | 78 | 79 |
| P. mirabilis | 54 | 45 | 80 | 70 |
| P. aeruginisa | 54 | 52 | 80 | 70 |
| Bacteria | BC-ChiD_Control | BC-ChiD_Blue | BC-ChiD_Green |
|---|---|---|---|
| Average surface roughness—RMS (nm) | |||
| E. coli | 93.46 ± 22.66 | 16.66 ± 4.46 | 71.6 ± 16.29 |
| MRSA | 138.33 ± 24.96 | 58.7 ± 8.93 | 14.43 ± 3.47 |
| Surface area [µm2] | |||
| E. coli | 144.07 ± 11.95 | 107.32 ± 1.97 | 110.41 ± 3.92 |
| MRSA | 162.06 ± 6.35 | 113.08 ± 2.18 | 104.27 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zmejkoski, D.Z.; Zdravković, N.M.; Budimir Filimonović, M.D.; Pavlović, V.B.; Butulija, S.V.; Milivojević, D.D.; Marković, Z.M.; Todorović Marković, B.M. Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light. J. Funct. Biomater. 2024, 15, 72. https://doi.org/10.3390/jfb15030072
Zmejkoski DZ, Zdravković NM, Budimir Filimonović MD, Pavlović VB, Butulija SV, Milivojević DD, Marković ZM, Todorović Marković BM. Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light. Journal of Functional Biomaterials. 2024; 15(3):72. https://doi.org/10.3390/jfb15030072
Chicago/Turabian StyleZmejkoski, Danica Z., Nemanja M. Zdravković, Milica D. Budimir Filimonović, Vladimir B. Pavlović, Svetlana V. Butulija, Dušan D. Milivojević, Zoran M. Marković, and Biljana M. Todorović Marković. 2024. "Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light" Journal of Functional Biomaterials 15, no. 3: 72. https://doi.org/10.3390/jfb15030072