A Comprehensive Study on Folate-Targeted Mesoporous Silica Nanoparticles Loaded with 5-Fluorouracil for the Enhanced Treatment of Gynecological Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Aminofunctionalized Silica Nanoparticles (MSN-NH2)
2.3. Estimation of Amine Content by CHN and TGA Analysis
2.3.1. Carbon, Hydrogen, and Nitrogen Elemental Analysis (CHN Analysis)
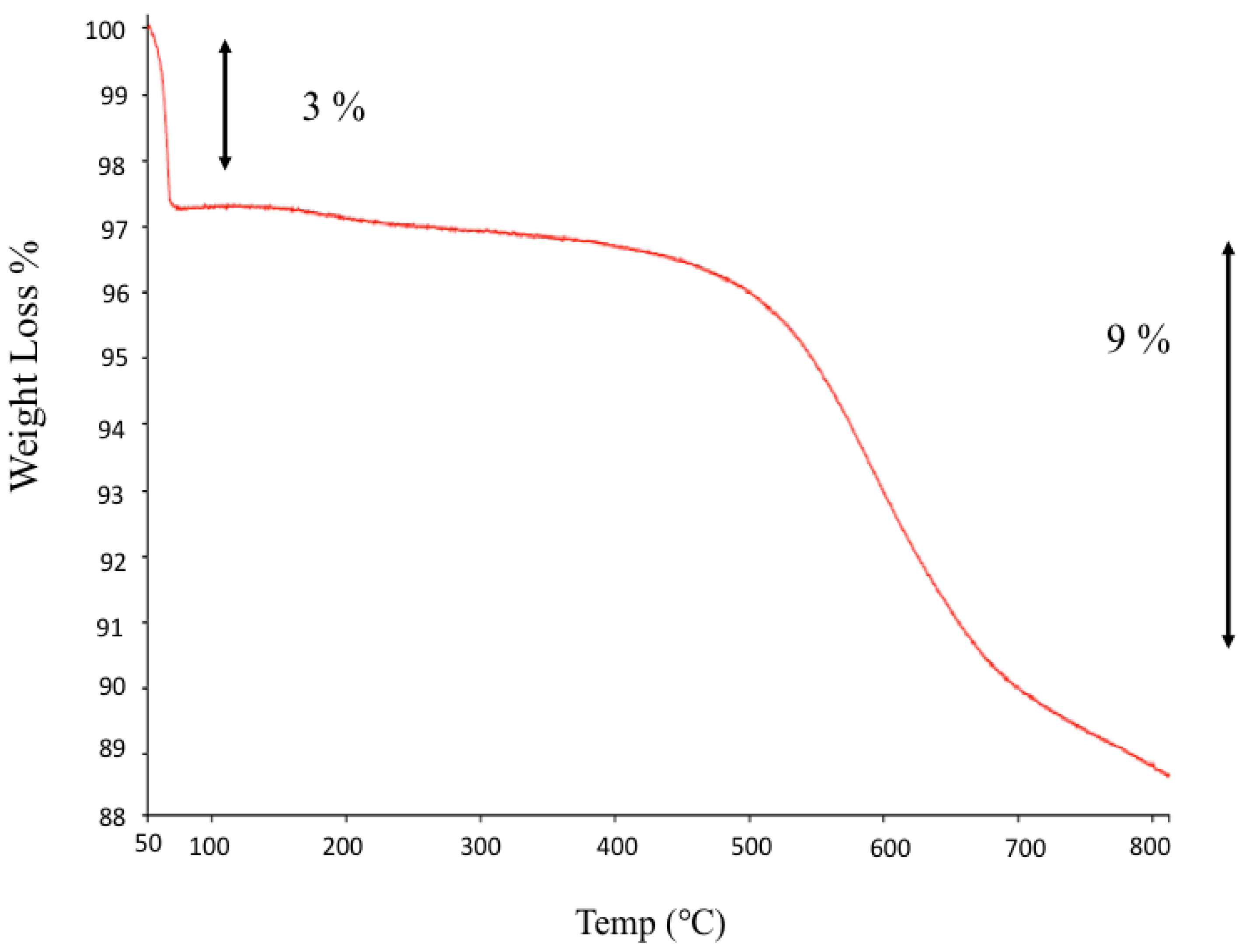
2.3.2. Thermogravimetric Analyses (TGA)
2.4. Drug Loading and Preparation of MSN-NH2 Loaded 5-FU (MSN-NH2-5FU)
2.5. Functionalization of 5-FU-Loaded MSN-NH2 with Folate (MSN-NH2-5FU-FA)
2.6. Particle Size, Particle Size Distribution, and Zeta-Potential Analysis
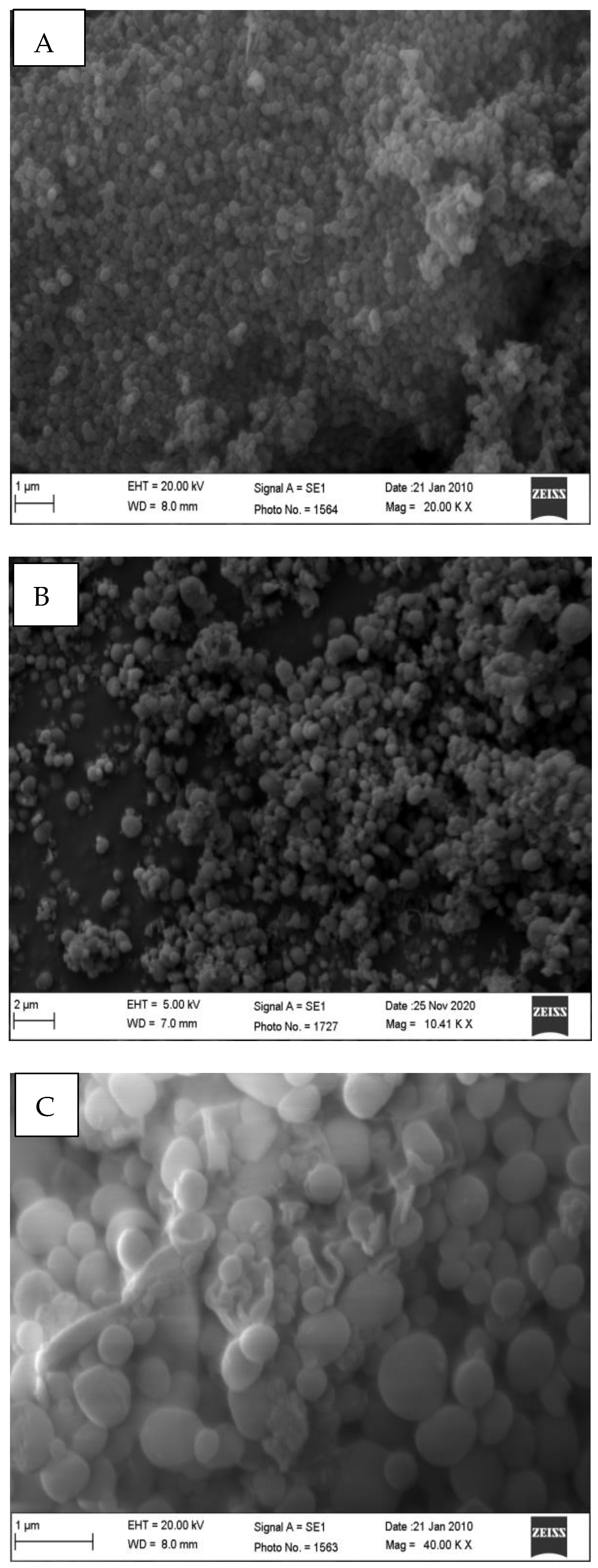
2.7. Particle Morphology by Scanning Electron Microscopy (SEM) and Transmission Electron Microscope (TEM)
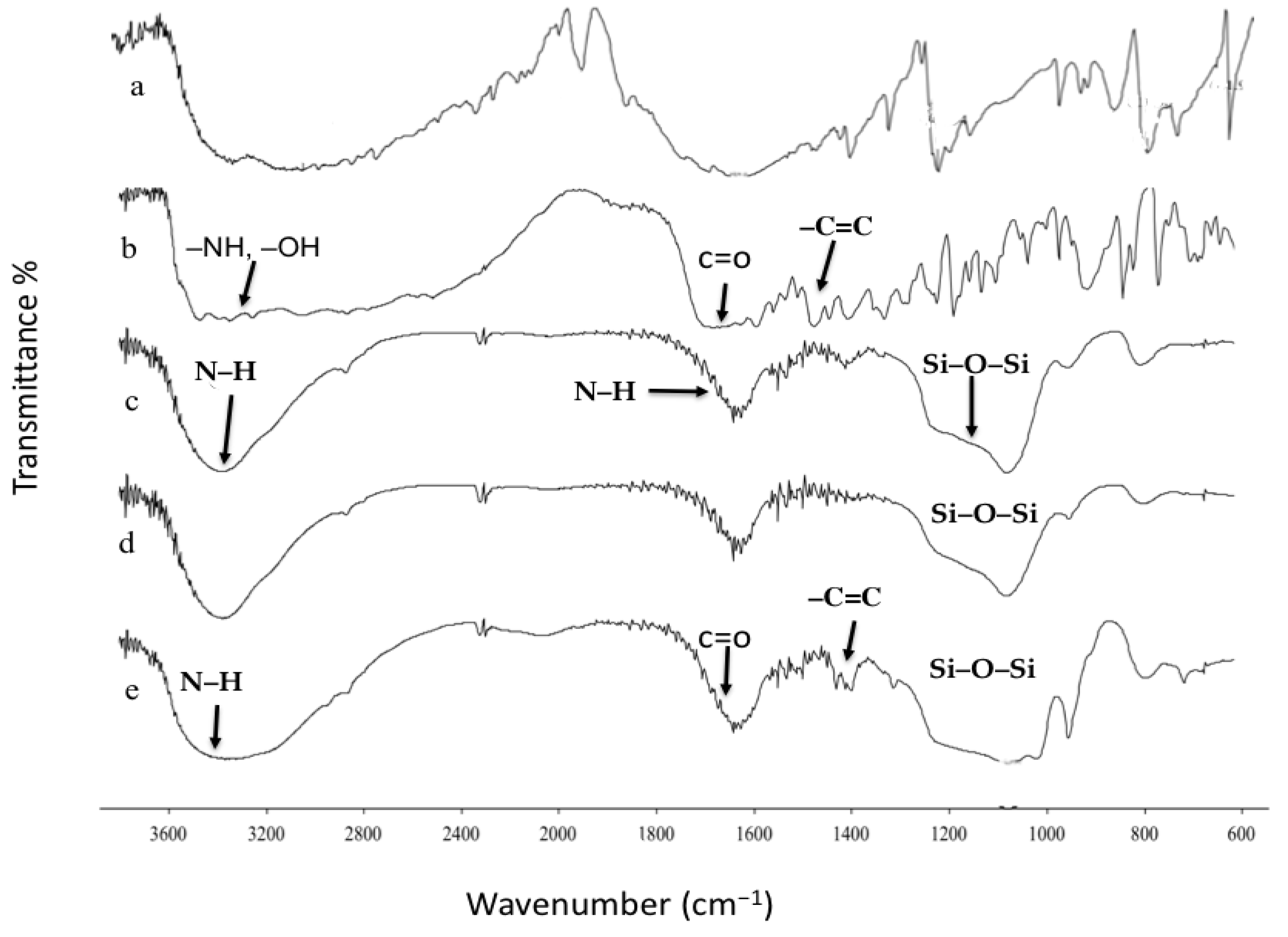
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
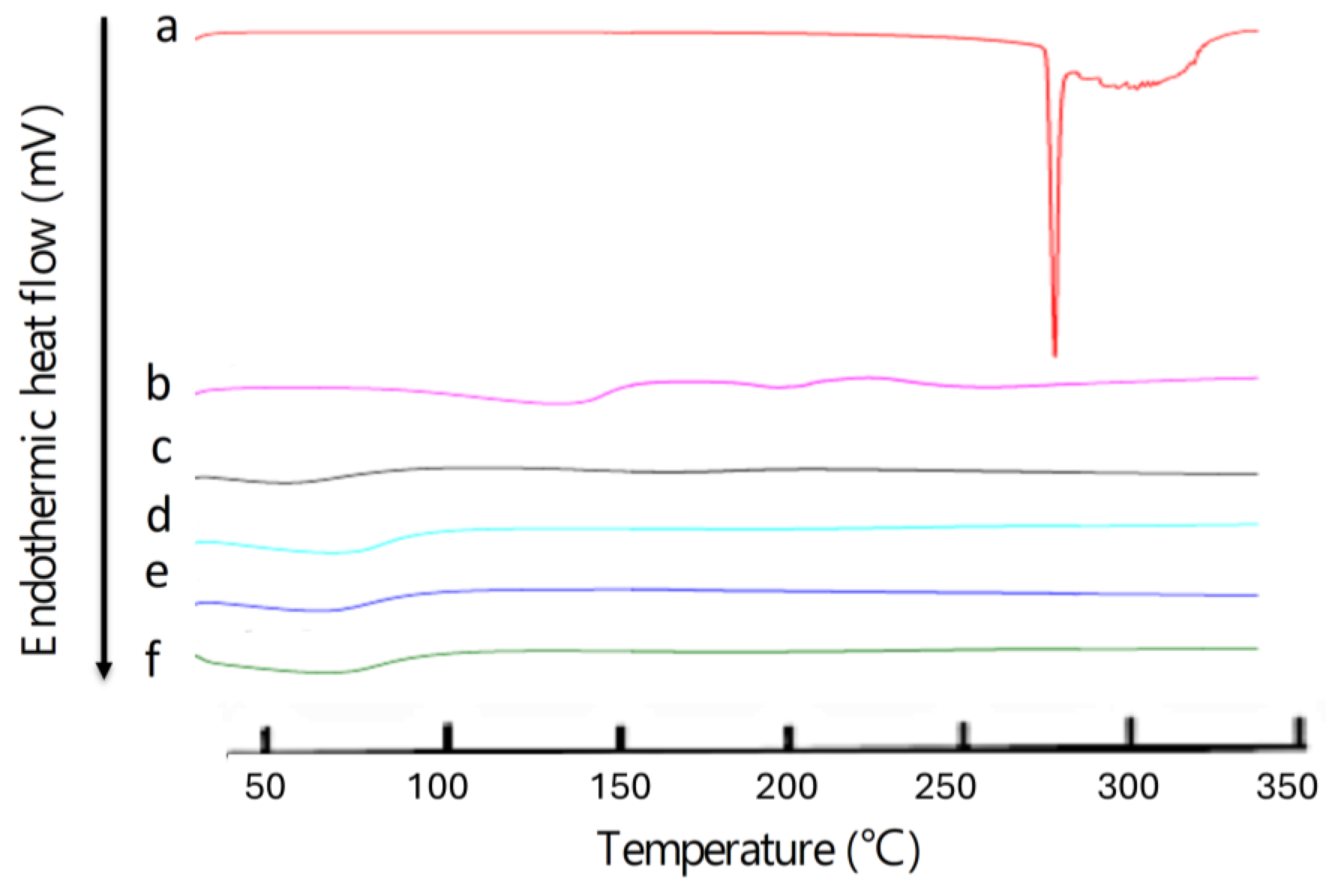
2.9. Differential Scanning Calorimetry (DCS)
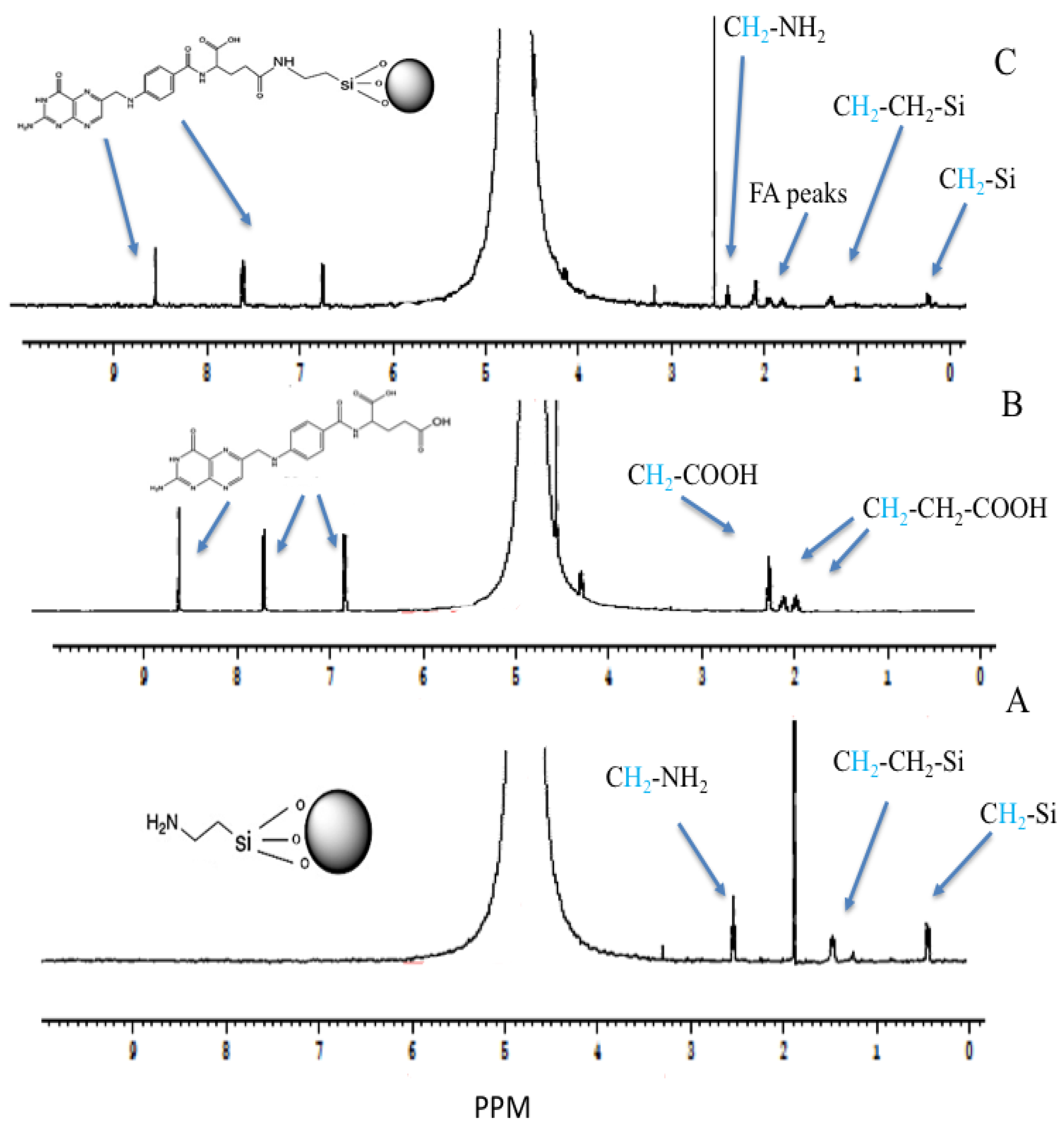
2.10. The Proton Nuclear Magnetic Resonance (1H-NMR)
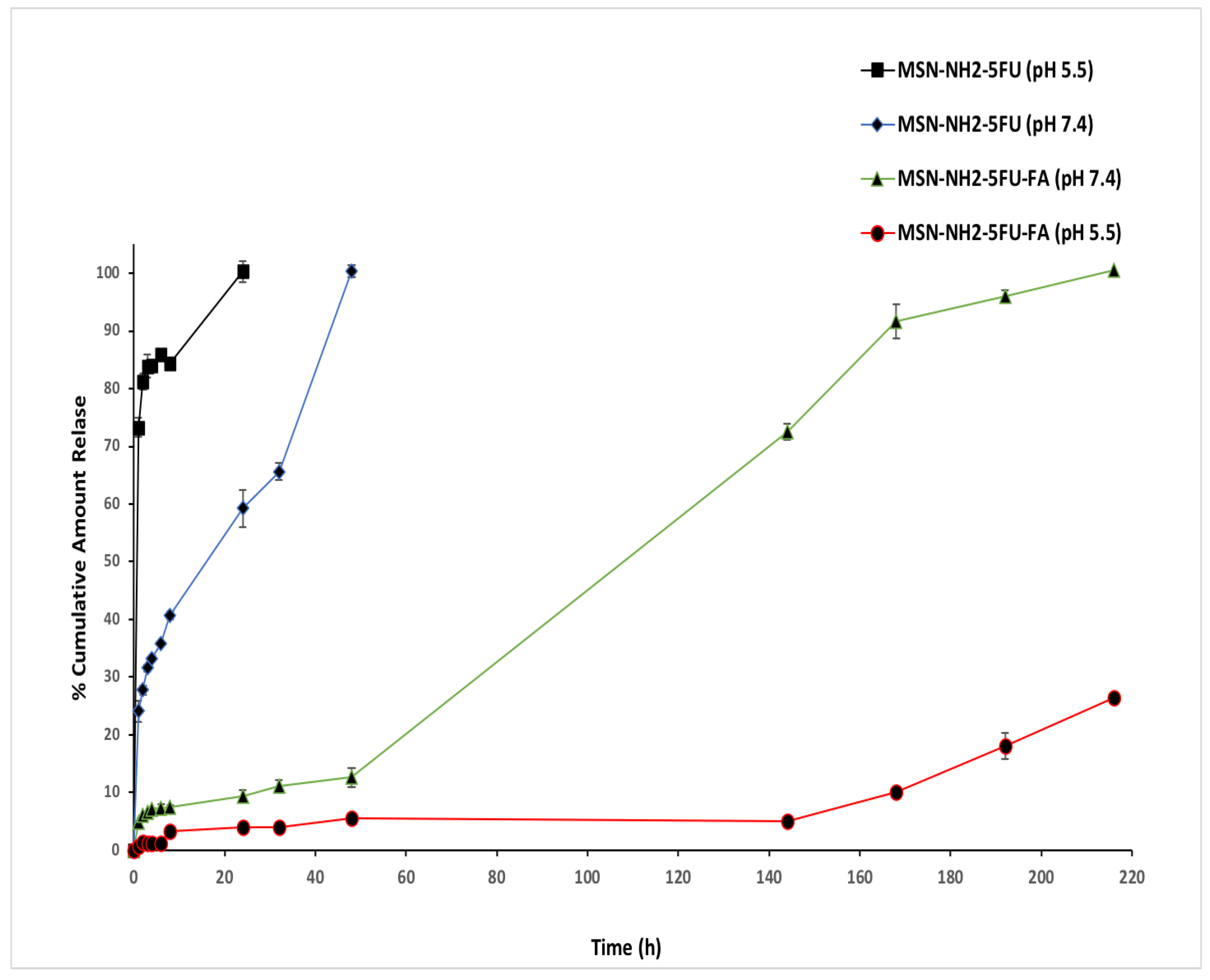
2.11. In Vitro Drug Release
2.12. Ex Vivo Intestinal Permeation
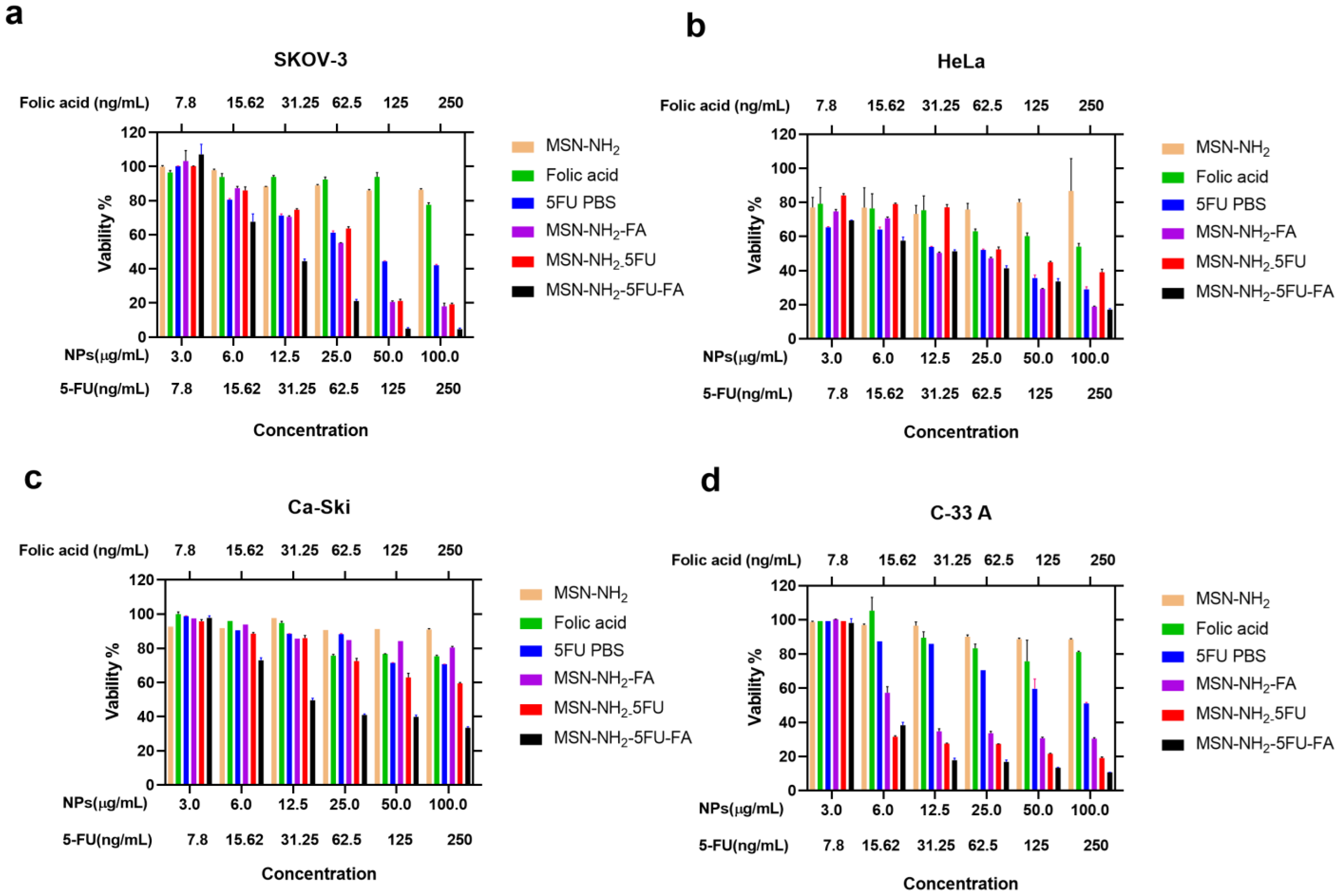
2.13. In Vitro Cytotoxicity Assay
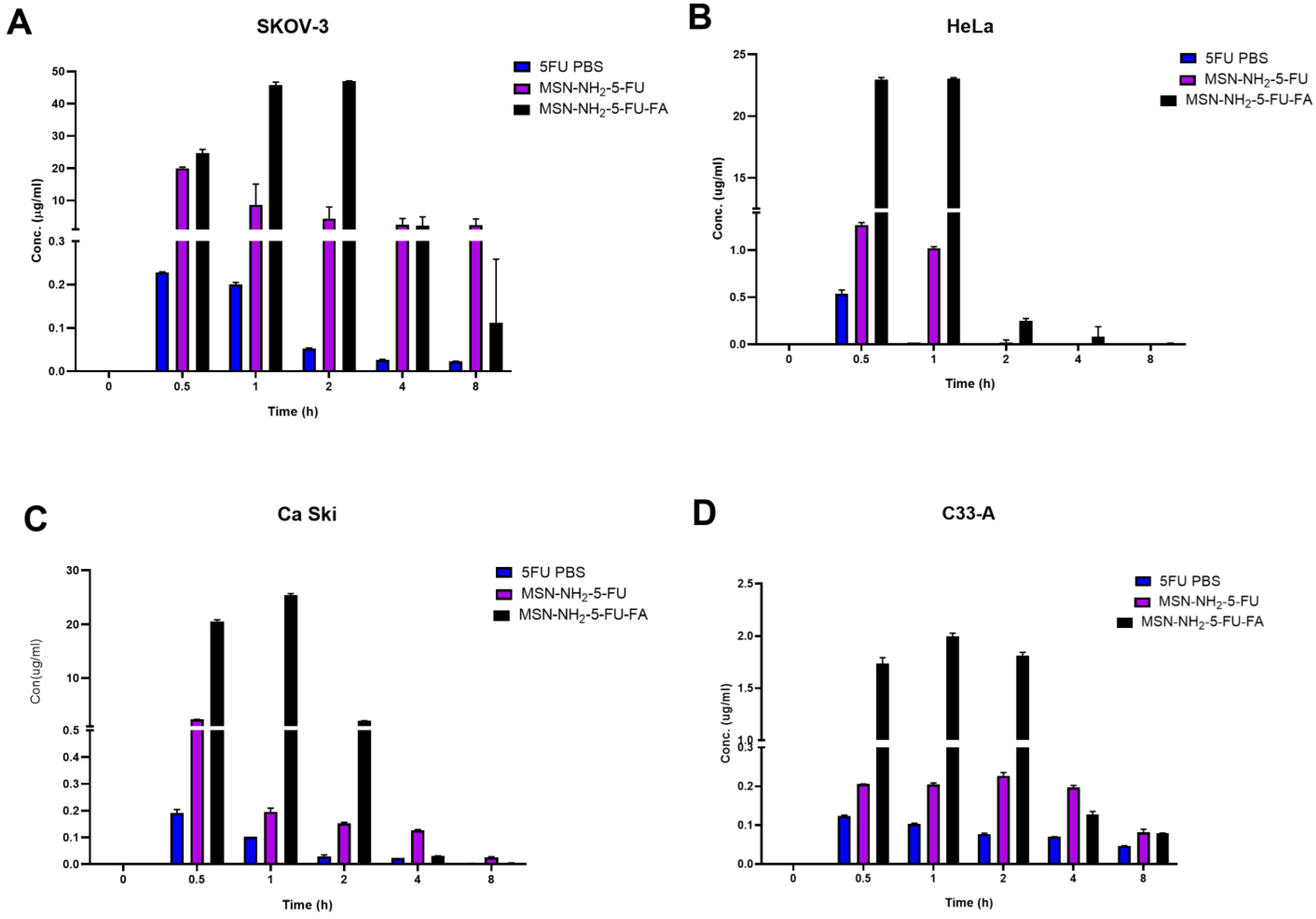
2.14. Intracellular Uptake
2.15. Cellular Uptake Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.16. Statistical Analysis
3. Results and Discussion
3.1. Estimation of Amine Content by CHN and TGA Analysis
3.1.1. CHN Analysis
3.1.2. From TGA Analyses
3.2. Nanoparticles DL%, EE% and Characterizations
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Differential Scanning Calorimetry (DCS)
3.5. The Proton Nuclear Magnetic Resonance (1H-NMR)
3.6. In Vitro Drug Release
3.7. Ex Vivo Drug Permeation Experiment
3.8. Cell Viability Study in Ovarian and Cervical Cancer Cells
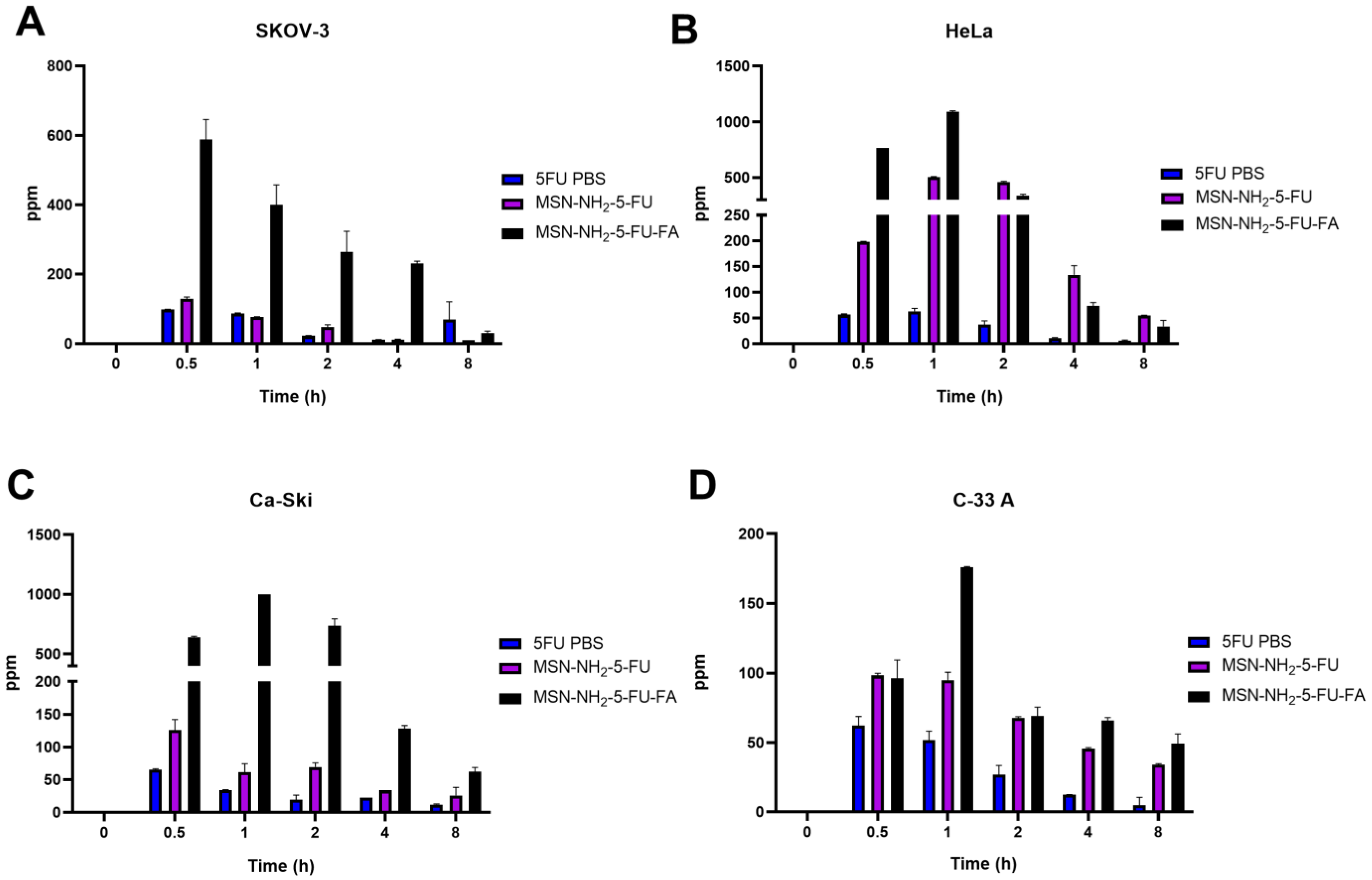
3.9. Cellular Uptake Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Maani, E.V.; Dunton, C.J.; Jack, B.W. Cervical Cancer; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mayadev, J.S.; Ke, G.; Mahantshetty, U.; Pereira, M.D.; Tarnawski, R.; Toita, T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.A. Ovarian cancer. Survival and treatment differences by age. Cancer 1993, 71, 524–529. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 1 August 2023).
- Survival Rates for Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 7 July 2023).
- Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®)—Health Professional Version. Available online: https://www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdq (accessed on 7 July 2023).
- Maneo, A.; Landoni, F.; Cormio, G.; Colombo, A.; Placa, F.; Pellegrino, A.; Mangioni, C. Concurrent carboplatin/5-fluorouracil and radiotherapy for recurrent cervical carcinoma. Ann. Oncol. 1999, 10, 803–807. [Google Scholar] [CrossRef]
- Choi, I.J.; Cha, M.S.; Park, E.S.; Han, M.S.; Choi, Y.; Je, G.H.; Kim, H.H. The efficacy of concurrent cisplatin and 5-flurouracil chemotherapy and radiation therapy for locally advanced cancer of the uterine cervix. J. Gynecol. Oncol. 2008, 19, 129–134. [Google Scholar] [CrossRef]
- Cohen, S.; Schwartz, M.; Dottino, P.; Beddoe, A.M. Use of a multi-drug regimen gemcitabine, 5-fluorouracil, irinotecan, cisplatin, bevacizumab, docetaxel, and cyclophosphamide (GFIP/BDC) for heavily pretreated relapsed epithelial ovarian, fallopian tube and primary peritoneal cancer. J. Ovarian Res. 2019, 12, 36. [Google Scholar] [CrossRef]
- Jewell, C.M.; Lynn, D.M. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 979–999. [Google Scholar] [CrossRef]
- Elumalai, R.; Patil, S.; Maliyakkal, N.; Rangarajan, A.; Kondaiah, P.; Raichur, A.M. Protamine-carboxymethyl cellulose magnetic nanocapsules for enhanced delivery of anticancer drugs against drug resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 969–981. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of hollow mesoporous silica nanoparticles for improved 5-FU loading. J. Nanomater. 2015, 2015, 872035. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Wang, F.; Li, X.; Liu, X.; Pang, J.; Pan, W. Galactosylated chitosan-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. R. Soc. Open Sci. 2018, 5, 181027. [Google Scholar] [CrossRef] [PubMed]
- Daryasari, M.P.; Akhgar, M.R.; Mamashli, F.; Bigdeli, B.; Khoobi, M. Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. RSC Adv. 2016, 6, 105578–105588. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ritter, C.A.; von Woedtke, T.; Bekeschus, S.; Wende, K. Package delivered: Folate receptor-mediated transporters in cancer therapy and diagnosis. Chem. Sci. 2024, 15, 1966–2006. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Polymeric mesoporous silica nanoparticles for enhanced delivery of 5-fluorouracil in vitro. Pharmaceutics 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Hillery, A.M.; Lloyd, A.W.; Swarbrick, J. Drug Delivery and Targeting: For Pharmacists and Pharmaceutical Scientists; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Khosravian, P.; Ardestani, M.S.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Javar, H.A.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. OncoTargets Ther. 2016, 9, 7315. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Patil, Y.; Panyam, J. Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. Int. J. Pharm. 2006, 320, 150–156. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Prasad, R.; Agawane, S.B.; Chauhan, D.S.; Srivastava, R.; Selvaraj, K. In vivo examination of folic acid-conjugated gold-silica nanohybrids as contrast agents for localized tumor diagnosis and biodistribution. Bioconjugate Chem. 2018, 29, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kaneko, M.; Maitani, Y. Functional coating of liposomes using a folate–polymer conjugate to target folate receptors. Int. J. Nanomed. 2012, 7, 3679. [Google Scholar]
- Liu, C.; Ding, L.; Bai, L.; Chen, X.; Kang, H.; Hou, L.; Wang, J. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem. Biophys. Res. Commun. 2017, 491, 1083–1091. [Google Scholar] [CrossRef]
- Almomen, A.; El-Toni, A.M.; Badran, M.; Alhowyan, A.; Abul Kalam, M.; Alshamsan, A.; Alkholief, M. The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery. Pharmaceutics 2020, 12, 1035. [Google Scholar] [CrossRef]
- Alhowyan, A.A.; Kalam, M.A.; Iqbal, M.; Raish, M.; El-Toni, A.M.; Alkholief, M.; Almomen, A.A.; Alshamsan, A. Mesoporous Silica Nanoparticles Coated with Carboxymethyl Chitosan for 5-Fluorouracil Ocular Delivery: Characterization, In Vitro and In Vivo Studies. Molecules 2023, 28, 1260. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.E.B.; Anwer, M.K.; Mowafy, H.A.; El-Bagory, I.M.; Bayomi, M.A.; Alsarra, I.A. Optimization of 5-flurouracil solid-lipid nanoparticles: A preliminary study to treat colon cancer. Int. J. Med. Sci. 2010, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Aydin, R.S.T.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 42. [Google Scholar]
- Ps, S.; Joshi, V. Preparation and characterisation of 5-fluorouracil loaded PLGA nanoparticles for colorectal cancer therapy. Unique J. Pharm. Biol. Sci. 2013, 1, 52–58. [Google Scholar]
- Tawfik, E.; Ahamed, M.; Almalik, A.; Alfaqeeh, M.; Alshamsan, A. Prolonged exposure of colon cancer cells to 5-fluorouracil nanoparticles improves its anticancer activity. Saudi Pharm. J. 2017, 25, 206–213. [Google Scholar] [CrossRef]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.-q.; Wang, S.-j.; Liu, P.; Qin, Y.-h.; Ma, Y.; Li, X.-C.; Huo, Z.-J. Hyaluronic acid-tagged silica nanoparticles in colon cancer therapy: Therapeutic efficacy evaluation. Int. J. Nanomed. 2015, 10, 6445. [Google Scholar]
- Anirudhan, T.S.; Vasantha, C.S.; Sasidharan, A.V. Layer-by-layer assembly of hyaluronic acid/carboxymethylchitosan polyelectrolytes on the surface of aminated mesoporous silica for the oral delivery of 5-fluorouracil. Eur. Polym. J. 2017, 93, 572–589. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kumar, R.; Pandit, J. Chitosan coated sodium alginate–chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. Eur. J. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef]
- Saczko, J.; Kamińska, I.; Kotulska, M.; Bar, J.; Choromańska, A.; Rembiałkowska, N.; Bieżuńska-Kusiak, K.; Rossowska, J.; Nowakowska, D.; Kulbacka, J. Combination of therapy with 5-fluorouracil and cisplatin with electroporation in human ovarian carcinoma model in vitro. Biomed. Pharmacother. 2014, 68, 573–580. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Chen, L.; Gao, W.; Zeng, F.; Kong, L.X. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv. 2015, 22, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Siemiaszko, G.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Misztalewska-Turkowicz, I.; Dudź, E.; Milewska, S.; Misiak, P.; Kurowska, I.; Sadowska, A.; Car, H. Synergistic effect of folate-conjugated polymers and 5-fluorouracil in the treatment of colon cancer. Cancer Nanotechnol. 2021, 12, 31. [Google Scholar] [CrossRef]
- Kazi, J.; Mukhopadhyay, R.; Sen, R.; Jha, T.; Ganguly, S.; Debnath, M.C. Design of 5-fluorouracil (5-FU) loaded, folate conjugated peptide linked nanoparticles, a potential new drug carrier for selective targeting of tumor cells. Medchemcomm 2019, 10, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.U.; Khattak, S.; Khan, M.I.; Saddozai, U.A.K.; Ali, N.; AlAsmari, A.F.; Zaheer, M.; Badar, M. A pH-responsive bi-MIL-88B MOF coated with folic acid-conjugated chitosan as a promising nanocarrier for targeted drug delivery of 5-Fluorouracil. Front. Pharmacol. 2023, 14, 1265440. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Q.; Liu, Y.; Yang, L.; Liu, Y.; Luo, Y.; Chen, T.; Li, N.; Wu, M. Co-encapsulation of paclitaxel and 5-fluorouracil in folic acid-modified, lipid-encapsulated hollow mesoporous silica nanoparticles for synergistic breast cancer treatment. RSC Adv. 2022, 12, 32534–32551. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Che, S. Amino and quaternary ammonium group functionalized mesoporous silica: An efficient ion-exchange method to remove anionic surfactant from AMS. Microporous Mesoporous Mater. 2008, 116, 299–307. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Eldeen Yassin, A.; El-Badry, M.; Mowafy, H.A.; Alsarra, I.A. Validated high-performance liquid chromatographic technique for determination of 5-fluorouracil: Applications to stability studies and simulated colonic media. J. Chromatogr. Sci. 2009, 47, 558–563. [Google Scholar] [CrossRef]
- Wang, X.; Yao, S.; Ahn, H.-Y.; Zhang, Y.; Bondar, M.V.; Torres, J.A.; Belfield, K.D. Folate receptor targeting silica nanoparticle probe for two-photon fluorescence bioimaging. Biomed. Opt. Express 2010, 1, 453–462. [Google Scholar] [CrossRef]
- Peretti, E.; Miletto, I.; Stella, B.; Rocco, F.; Berlier, G.; Arpicco, S. Strategies to obtain encapsulation and controlled release of pentamidine in mesoporous silica nanoparticles. Pharmaceutics 2018, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ke, L.; Chen, H.; Zhuo, M.; Yang, X.; Zhao, D.; Zeng, S.; Xiao, X. Natural material-decorated mesoporous silica nanoparticle container for multifunctional membrane-controlled targeted drug delivery. Int. J. Nanomed. 2017, 12, 8411. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Peng, Z.; She, F.; Kong, L. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Peng, Z.; She, M.F.; Kong, L. Physichemical property and morphology of 5-fluorouracil loaded chitosan nanoparticles. In Proceedings of the 2010 International Conference on Nanoscience and Nanotechnology, Sydney, Australia, 22–26 February 2010; pp. 248–250. [Google Scholar]
- Sahoo, B.; Devi, K.S.P.; Sahu, S.K.; Nayak, S.; Maiti, T.K.; Dhara, D.; Pramanik, P. Facile preparation of multifunctional hollow silica nanoparticles and their cancer specific targeting effect. Biomater. Sci. 2013, 1, 647–657. [Google Scholar] [CrossRef]
- Šuleková, M.; Váhovská, L.; Hudák, A.; Žid, L.; Zeleňák, V. A Study of 5-fluorouracil desorption from mesoporous silica by RP-UHPLC. Molecules 2019, 24, 1317. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Lavasanifar, A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346. [Google Scholar] [CrossRef]
- Almomen, A.; Cho, S.; Yang, C.H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janat-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Cionti, C.; Taroni, T.; Sabatini, V.; Meroni, D. Nanostructured oxide-based systems for the pH-triggered release of cinnamaldehyde. Materials 2021, 14, 1536. [Google Scholar] [CrossRef] [PubMed]
- Markova, N.; Enchev, V.; Ivanova, G. Tautomeric equilibria of 5-fluorouracil anionic species in water. J. Phys. Chem. A 2010, 114, 13154–13162. [Google Scholar] [CrossRef] [PubMed]
- Hofsäss, M.A.; de Souza, J.; Silva-Barcellos, N.M.; Bellavinha, K.R.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.; Parr, A.; Langguth, P.; Polli, J.E. Biowaiver monographs for immediate-release solid oral dosage forms: Folic acid. J. Pharm. Sci. 2017, 106, 3421–3430. [Google Scholar] [CrossRef]
- Parın, F.N.; Ullah, S.; Yıldırım, K.; Hashmi, M.; Kim, I.-S. Fabrication and characterization of electrospun folic acid/hybrid fibers: In vitro controlled release study and cytocompatibility assays. Polymers 2021, 13, 3594. [Google Scholar] [CrossRef]
- Jalilian, A.; Hosseini-Salekdeh, S.; Mahmoudi, M.; Yousefnia, H.; Majdabadi, A.; Pouladian, M. Preparation and biological evaluation of radiolabeled-folate embedded superparamagnetic nanoparticles in wild-type rats. J. Radioanal. Nucl. Chem. 2011, 287, 119–127. [Google Scholar] [CrossRef]
- Varuna Kumara, J.; Ravikumara, N.; Madhusudhan, B. Evaluation of Surfactants-Assisted Folic Acid-Loaded Pectin Submicrospheres: Characterization and Hemocompatibility Assay. Indian J. Clin. Biochem. 2016, 31, 390–401. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azaïs, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of folic acid under several parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Lee, B.Y.; Choi, J.W.; Chun, H.J.; Kim, H.S.; Yang, D.H. Injectable glycol chitosan hydrogel containing folic acid-functionalized cyclodextrin-paclitaxel complex for breast cancer therapy. Nanomaterials 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Kunc, F.; Balhara, V.; Brinkmann, A.; Sun, Y.; Leek, D.M.; Johnston, L.J. Quantification and stability determination of surface amine groups on silica nanoparticles using solution NMR. Anal. Chem. 2018, 90, 13322–13330. [Google Scholar] [CrossRef] [PubMed]
- Tataurova, Y.N. Proton NMR Studies of Functionalized Nanoparticles in Aqueous Environments. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2014. [Google Scholar]
- Hughes, S.; Dasary, S.S.; Begum, S.; Williams, N.; Yu, H. Meisenheimer complex between 2,4,6-trinitrotoluene and 3-aminopropyltriethoxysilane and its use for a paper-based sensor. Sens. Bio-Sens. Res. 2015, 5, 37–41. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Zhang, Y.; Wang, L.; Zhang, X. Autocatalytic synthesis of molecular-bridged silica aerogels with excellent absorption and super elasticity. RSC Adv. 2015, 5, 91407–91413. [Google Scholar] [CrossRef]
- Riehle, N.; Götz, T.; Kandelbauer, A.; Tovar, G.E.; Lorenz, G. Data on the synthesis and mechanical characterization of polysiloxane-based urea-elastomers prepared from amino-terminated polydimethylsiloxanes and polydimethyl-methyl-phenyl-siloxane-copolymers. Data Brief 2018, 18, 1784–1794. [Google Scholar] [CrossRef]
- el-Moneum, A.; Mohamed Abdel-Bar, H.; Sadder El-Leithy, E. Synthesis, Optimization and Characterization of Folate-Chitosan Polymer Conjugate for Possible Oral Delivery of Macromolecular Drugs. J. Pharm. 2017, 7, 30–38. [Google Scholar]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi Aliabadi, H.; Nayebsadrian, M.; Banitalebi, M.; Rostami, M. Synthesis and characterization of folate-targeted dextran/retinoic acid micelles for doxorubicin delivery in acute leukemia. BioMed Res. Int. 2014, 2014, 525684. [Google Scholar] [CrossRef]
- Salar, R.K.; Kumar, N. Synthesis and characterization of vincristine loaded folic acid–chitosan conjugated nanoparticles. Resour.-Effic. Technol. 2016, 2, 199–214. [Google Scholar]
- Shi, X.; He, D.; Tang, G.; Tang, Q.; Xiong, R.; Ouyang, H.; Yu, C.-Y. Fabrication and characterization of a folic acid-bound 5-fluorouracil loaded quantum dot system for hepatocellular carcinoma targeted therapy. RSC Adv. 2018, 8, 19868–19878. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in deposition and permeation of 5-fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef]
- Musso, G.; Bottinelli, E.; Celi, L.; Magnacca, G.; Berlier, G. Influence of surface functionalization on the hydrophilic character of mesoporous silica nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 13882–13894. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, R.B.; Singh, J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int. J. Pharm. 2005, 298, 98–107. [Google Scholar] [CrossRef]
- Rahman, Z.; Kohli, K.; Zhang, S.Q.; Khar, R.K.; Ali, M.; Charoo, N.A.; Tauseef, M.; Shamsher, A.A.; Mohammed, N.N.; Repka, M.A. In-vivo evaluation in rats of colon-specific microspheres containing 5-fluorouracil. J. Pharm. Pharmacol. 2008, 60, 615–623. [Google Scholar] [CrossRef]
- Siwowska, K.; Schmid, R.M.; Cohrs, S.; Schibli, R.; Müller, C. Folate receptor-positive gynecological cancer cells: In vitro and in vivo characterization. Pharmaceuticals 2017, 10, 72. [Google Scholar] [CrossRef]
- Chandran, S.P.; Natarajan, S.B.; Chandraseharan, S.; Shahimi, M.S.B.M. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J. Cancer Res. Pract. 2017, 4, 45–48. [Google Scholar] [CrossRef]
- Cai, S.; Alhowyan, A.A.B.; Yang, Q.; Forrest, W.M.; Shnayder, Y.; Forrest, M.L. Cellular uptake and internalization of hyaluronan-based doxorubicin and cisplatin conjugates. J. Drug Target. 2014, 22, 648–657. [Google Scholar] [CrossRef]
- Pattni, B.S.; Torchilin, V.P. Targeted drug delivery systems: Strategies and challenges. In Targeted Drug Delivery: Concepts and Design; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–38. [Google Scholar]
- Ou, J.; Peng, Y.; Yang, W.; Zhang, Y.; Hao, J.; Li, F.; Chen, Y.; Zhao, Y.; Xie, X.; Wu, S. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat. Commun. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Lee, B.-Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef]
- Verma, U.; Naik, J. Inclusion of Aceclofenac in Mesoporous Silica Nanoparticles: Drug Release Study and Statistical Optimization of Encapsulation Efficiency by Response Surface Methodology. Mater. Technol. 2019, 34, 751–763. [Google Scholar] [CrossRef]
- Bronk, J.; Lister, N.; Lynch, S. Absorption of 5-fluorouracil and related pyrimidines in rat small intestine. Clin. Sci. 1987, 72, 705–716. [Google Scholar] [CrossRef]
- Hirata, K.; Horie, T. Changes in intestinal absorption of 5-fluorouracil-treated rats. Pharmacol. Toxicol. 1999, 85, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Silva, C.; Chaves, L. Tissue-based in vitro and ex vivo models for intestinal permeability studies. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–236. [Google Scholar]
- Sakai-Kato, K.; Hidaka, M.; Un, K.; Kawanishi, T.; Okuda, H. Physicochemical properties and in vitro intestinal permeability properties and intestinal cell toxicity of silica particles, performed in simulated gastrointestinal fluids. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 1171–1180. [Google Scholar] [CrossRef]
- Morris, A.S.; Adamcakova-Dodd, A.; Lehman, S.E.; Wongrakpanich, A.; Thorne, P.S.; Larsen, S.C.; Salem, A.K. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol. Lett. 2016, 241, 207–215. [Google Scholar] [CrossRef]
- Ding, L.; Ma, J.; Zhou, Q.; Wang, J. Effect of folate on the proliferation of human cervical cancer cell and relationship with HPV16. Wei Sheng Yan Jiu 2013, 42, 748–753. [Google Scholar]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Ge, M.; Zhou, G.; Sun, W.; Liu, D.; Liang, X.-J.; Zhang, J. A distinct endocytic mechanism of functionalized-silica nanoparticles in breast cancer stem cells. Sci. Rep. 2017, 7, 16236. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Yang, J.C.; Liang, J.X.; Wang, S.L. Pharmacokinetic study and clinical evaluation of a slow-release 5-fluorouracil implant in pancreatic cancer patients. Anti-Cancer Drugs 2016, 27, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Fanciullino, R.; Giacometti, S.; Mercier, C.; Aubert, C.; Blanquicett, C.; Piccerelle, P.; Ciccolini, J. In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. Br. J. Cancer 2007, 97, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 79. [Google Scholar] [CrossRef] [PubMed]
- Dulin, W. Oral targeted drug delivery systems: Enteric coating. In Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 205–223. [Google Scholar]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2017, 150, 352–361. [Google Scholar] [CrossRef]



| System | Cancer | Advantages | Reference |
|---|---|---|---|
| Solid lipid nanoparticles | Colorectal | Enhanced anticancer activity | [32] |
| Chitosan nanoparticles | Colorectal | Better targeting efficiency and localized drug in cancer cells | [33] |
| PLGA nanoparticles | Colorectal | Maximum cell-lysis effect and better targeting efficiency | [34,35] |
| Citrus pectin nanoparticles coated with Eudragit S100 | Colorectal | Prolonged drug release and enhance selectivity | [36] |
| Silica nanoparticles conjugated to hyalurocin acid | Colon | Enhanced cellular uptake and improved cytotoxicity | [37] |
| Aminofunctionalized MSN | Colorectal | Improved cytotoxicity and pH-responsive controlled drug release system | [38] |
| Alginate–chitosan nanoparticles | Ocular application | Enhanced ocular absorption and pharmacokinetics | [39] |
| Galactosylated chitosan functionalized MSN | Colon cancer | Enhanced anti-cancer activity and targeting | [16] |
| Chitosan and PEG coated MSNs | Breast and cervical cancer | Enhanced anti-cancer activity with the loading of two anticancer drugs | [19] |
| MSN | Melanoma | Enhanced anti-cancer activity and skin permeation | [30] |
| Carboxymethyl chitosan-coated MSN | Ocular application | Enhance ocular absorption and pharmacokinetic | [31] |
| Combination of 5-FU and cisplatin with electroporation | Ovarian | Enhanced anti-cancer activity | [40] |
| Folic acid and PLGA conjugates | Colorectal | Enhanced anticancer activity | [41] |
| Folate-conjugated polymers | Colon | Enhanced anti-cancer activity and targeting | [42] |
| PLGA folate-conjugated peptide nanoparticles | Melanoma | Enhanced cytotoxicity and targeting | [43] |
| Bi-MIL-88B MOF nanoparticles coated with chitosan–folic acid conjugate | Colon | Enhanced anti-cancer activity and targeting | [44] |
| MSN nanoparticles coated with folic acid-modified lipid | Breast cancer | Enhanced cytotoxicity and targeting with the loading of two anticancer drugs | [45] |
| MSN-NH2 | MSN-NH2-5FU | MSN-NH2-5FU-FA | |
|---|---|---|---|
| Particle size (nm) | 169.3 ± 4.2 | 193.9 ± 8.7 | 907.6 ± 10.21 |
| Polydispersity index (PDI) | 0.057 ± 0.002 | 0.219 | 0.405 ± 0.001 |
| Zeta potential (mV) in pH 5.5 | 27.1 ± 0.75 | 18 ± 2.9 | 18.5 ± 0.5 |
| Zeta potential (mV) in PBS | 6.36 ± 1.49 | 30.4 ± 5.71 | 8.57 ± 3.48 |
| EE% | - | 18.01 ± 3.7 | 13.05 ± 0.73 |
| LC% | - | 15.26 ± 3.13 | 5.89 ± 0.32 |
| Formulation | pH | Zero-Order (R2) | First Order (R2) | Higuchi (R2) | Korsmyer–Peppas (n) | Hixen–Crowell (R2) | |
|---|---|---|---|---|---|---|---|
| MSN-NH2-5FU | 7.4 | 0.904 | 0.77 | 0.95 | 0.98 | 0.3 | 0.79 |
| MSN-NH2-5FU-FA | 7.4 | 0.98 | 0.91 | 0.91 | 0.85 | 0.413 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomen, A.; Alhowyan, A. A Comprehensive Study on Folate-Targeted Mesoporous Silica Nanoparticles Loaded with 5-Fluorouracil for the Enhanced Treatment of Gynecological Cancers. J. Funct. Biomater. 2024, 15, 74. https://doi.org/10.3390/jfb15030074
Almomen A, Alhowyan A. A Comprehensive Study on Folate-Targeted Mesoporous Silica Nanoparticles Loaded with 5-Fluorouracil for the Enhanced Treatment of Gynecological Cancers. Journal of Functional Biomaterials. 2024; 15(3):74. https://doi.org/10.3390/jfb15030074
Chicago/Turabian StyleAlmomen, Aliyah, and Adel Alhowyan. 2024. "A Comprehensive Study on Folate-Targeted Mesoporous Silica Nanoparticles Loaded with 5-Fluorouracil for the Enhanced Treatment of Gynecological Cancers" Journal of Functional Biomaterials 15, no. 3: 74. https://doi.org/10.3390/jfb15030074





