The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Properties of Cardiovascular Tissues and Main Proteins
2.1.1. Characterization in the Hydrated State

2.1.2. Characterization in the Freeze-Dried State

2.2. First Application: Classification of Chemical Treatments for the Stabilization of Collagen in Bovine Pericardium

| Td (°C) | Hd (J/g) | |
|---|---|---|
| \PBS | 214.8 ± 0.8 | 12.3 ± 2.4 |
| Glu\Oct | 230.0 ± 0.6 | 7.8 ± 0.7 |
2.3. Second Application: Effect of Low Temperature Plasma Jet on Thermal Stability of Type I Collagen
2.3.1. Dehydrated State

| Sample | ΔHd (J/g) | Td (°C) |
|---|---|---|
| Control | 7.05 | 225 |
| 5 min | 13.7 | 215–223 |
| 10 min | 8.42 | 215–220–230 |
| 60 min | 3.7 | 217–220–231 |
2.3.2. Hydrated State

| Sample | ΔHd (J/g dry collagen) | Td (°C) |
|---|---|---|
| Control | 47.8 | 78.3 |
| 10 min | 66.4 | 75.1–79.9 |
| 20 min | 48.8 | 76.5 |
| 45 min | 70.6 | 75.2–79.1 |
| 90 min | 126.2 | 78.0–85.1 |
2.4. Third Application: Comparison of Detergent Treatments for Cardiac Valves Bioprostheses
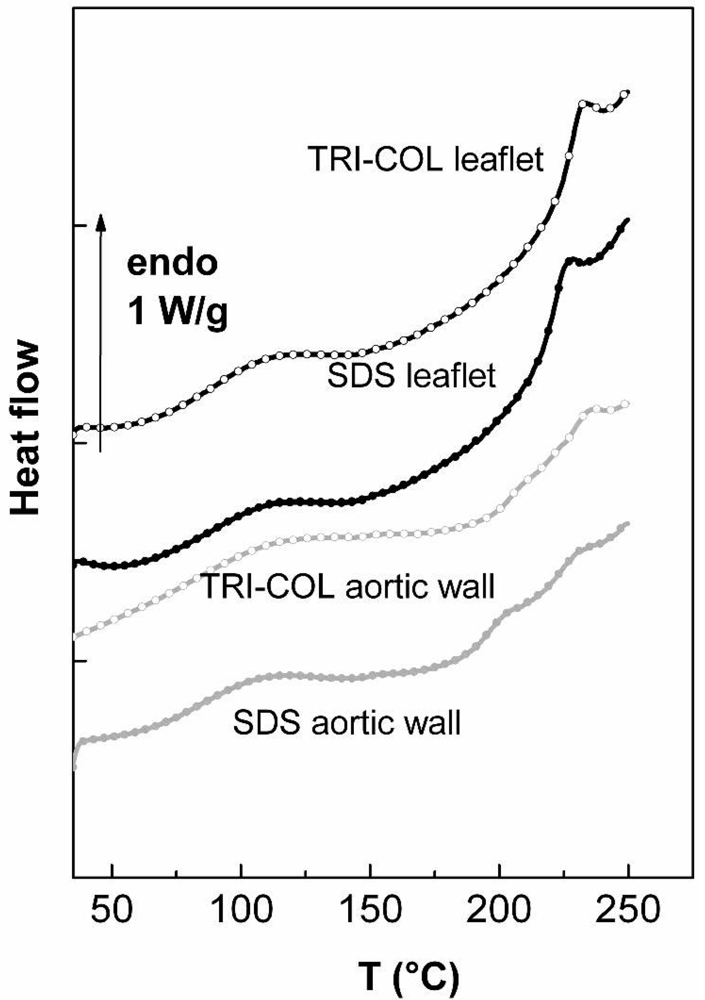
2.4.1. Valvular Leaflet
| collagen | elastin | ||
|---|---|---|---|
| ΔHd denaturation (J/g) | Td denaturation (°C) | Tgelastin (°C) | |
| TRI-COL leaflet | 10.33 ± 0.54 | 232.3 ±0.4 | Not meas. |
| TRI-COL aortic wall | 3.89 ± 0.33 | 234.2 ±0.5 | 204.7 ± 0.4 |
| SDS leaflet | 11.65 ± 0.4 | 224.9 ± 0.7 | Not meas. |
| SDS aortic wall | 3.04 ± 0.38 | 228.6 ± 0.9 | 196.7 ± 0.5 |
2.4.2. Aortic Wall
3. Experimental Section
3.1. Preparation of the Samples
3.1.1. Bovine Pericardia
- -
- Control samples are fresh tissue stored in PBS: bovine pericardium was obtained fresh from the abattoir and placed in chilled phosphate buffered saline (pH 7.4) until the time of analysis or implantation. Extraneous fat or muscle was removed and sections with heavy vasculature or attached ligaments were discarded. These samples are referred to as control or /PBS.
- -
- Fresh tissue stored in 80% ethanol: fresh bovine pericardium stored in chilled PBS was transferred to 80% ethanol buffered HEPES (10mM) solution for 3 days prior to analysis. These samples are referred as /Eth
- -
- Glutaraldehyde treated, PBS stored: fresh bovine pericardium stored in PBS was transferred to a buffered solution of 0.25% glutaraldehyde for 1 week. The tissue was transferred to chilled PBS for 3 days prior to analysis. These samples are referred as Glu/PBS.
- -
- Glutaraldehyde treated, stored in 80% ethanol: glutaraldehyde treated bovine pericardium was transferred to 80% ethanol buffered HEPES (10mM) solution for 3 days prior to analysis. These samples are referred as Glu/Eth.
- -
- Glutaraldehyde treated, stored in octanol: glutaraldehyde treated bovine pericardium was transferred to 5% octanol/40% ethanol solution for 3 days prior to analysis. These samples are referred as Glu/Oct
- -
- Glutaraldehyde treated, stored in glutaraldehyde: fresh bovine pericardium stored in PBS was transferred to a buffered solution of 0.25% glutaraldehyde for 1 week prior to analysis. These samples are referred as Glu/Glu.
3.1.2. Collagen Treated by the Plasma Jet
Treatment in the freeze-dried state
Treatment in the hydrated state
3.1.3. Porcine Valvular Leaflets and Porcine Aortic Walls
3.2. Differential Scanning Calorimetry (DSC)
4. Conclusions
Acknowledgments
References
- Lee, C.; Park, C.S.; Lee, C.H.; Kwak, J.G.; Kim, S.J.; Shim, W.S.; Song, J.Y.; Choi, E.Y.; Lee, S.Y. Durability of bioprosthetic valves in the pulmonary position: Long-term follow-up of 181 implants in patients with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2011, 142, 351–358. [Google Scholar]
- Bottio, T.; Tarzia, V.; Dal Lin, C.; Buratto, E.; Rizzoli, G.; Spina, M.; Gandaglia, A.; Naso, F.; Gerosa, G. The changing hydrodynamic performance of the decellularized intact porcine aortic root: Considerations on in-vitro testing. J. Heart Valve Dis. 2010, 4, 485–491. [Google Scholar]
- Vesely, I. Heart valve tissue engineering. Circ. Res. 2005, 97, 743–755. [Google Scholar]
- Lee, J.M.; Pereira, C.A.; Kan, L.W.K. Effect of molecular structure of poly(glycidyl ether) reagents on crosslinking and mechanical properties of bovine pericardial xenograft materials. J. Biomed. Mat. Res. 1994, 28, 981–992. [Google Scholar]
- Courtman, D.W.; Pereira, C.A.; Kashef, V.; McComb, D.; Lee, L.M.; Wilson, G.J. Development of a pericardial acellular matrix biomaterial: Biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. 1994, 28, 655–666. [Google Scholar]
- Pasquino, E.; Pascale, S.; Andreon, M.; Rinaldi, S.; Laborde, F.; Galloni, M. Bovine pericardium for heart valve bioprostheses: In vitro and in vivo characterization of new chemical treatments. J. Mater. Sci. 1994, 5, 850–854. [Google Scholar]
- Gratzer, P.F.; Lee, J.M. Altered mechanical properties in aortic elastic tissue using glutaraldehyde/solvent solutions of various dielectric constant. J. Biomed. Mater. Res. 1997, 37, 497–507. [Google Scholar]
- Hønge, J.L.; Funder, J.A.; Jensen, H.; Dohmen, P.M.; Konertz, W.F.; Hasenkam, J.M. Recellularization of decellularized mitral heart valves in juvenile pigs. J. Heart Valve Dis. 2010, 19, 584–592. [Google Scholar]
- Vesely, I.; Lozon, A. Natural preload of aortic valve leaflet components during glutaraldehyde fixation: Effects on tissue mechanics. J. Biomech. 1993, 26, 121–131. [Google Scholar]
- Colomb, G.; Shoen, F.J.; Smith, M.S.; Linden, J.; Dixon, M.; Levy, R.J. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprosthesis. Am. J. Pathol. 1987, 127, 122–130. [Google Scholar]
- Nimmi, M.E.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K.J. Chemically modified collagen: A natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar]
- Bodnar, E.; Olsen, E.G.J.; Florio, R.; Dobrin, J. Damage of porcine aortic valve tissue caused by the surfactant sodium dodecyl sulphate. J. Thorac. Cardiovasc. Surg. 1986, 34, 82–85. [Google Scholar]
- Gendler, E.; Gendler, S.; Nimni, M.E. Toxic reactions evoked by glutaraldehyde-fixed pericardium and cardiac valve tissue bioprostheses. J. Biomed. Mater. Res. 1984, 18, 727–736. [Google Scholar]
- Vesely, I. The hybrid autograft/xenograft aortic valve. J. Heart Valve Dis. 1997, 6, 292–295. [Google Scholar]
- Eybl, E.; Griesmacher, A.; Grimm, M.; Wolner, E.J. Toxic effects of aldehydes released from fixed pericardium on bovine endothelial cells. J. Biomed. Mater. Res. 1989, 23, 1355–1365. [Google Scholar]
- Lew, D.H.; Liu, P.H.T.; Orgill, D.P. Optimization of UV cross-linking density for durable and nontoxic collagen GAG dermal substitute. J. Biomed. Mater. Res. B 2007, 82, 51–56. [Google Scholar]
- Wight, T.N. Arterial Wall. In Extracellular Matrix; Comper, W.D., Ed.; Harwood Academic Publishers: Melbourne, Australia, 1996; Volume 1, pp. 175–194. [Google Scholar]
- Samouillan, V.; Dandurand-Lods, J.; Lamure, A.; Maurel, E.; Lacabanne, C.; Gerosa, G.; Venturini, A.; Casarotto, D.; Gherardini, L.; Spina, M. Thermal analysis characterization of aortic tissues for cardiac valve bioprostheses. J. Biomed. Mater. Res. 1999, 46, 531–538. [Google Scholar]
- Perry, M.P.; Stypa, B.K.; Tenn, K.K. Kumashiro, Solid-state (13)C NMR reveals effects of temperature and hydration on elastin. Biophys. J. 2002, 82, 1086–1095. [Google Scholar]
- Harrington, W.F.; von Hippel, P.H. The structure of collagen and gelatin. Adv. Protein Chem. 1969, 16, 1–138. [Google Scholar]
- Privalov, P.L.; Tiktopulo, E.I.; Tischenko, V.M. Stability and mobility of the collagen structure. J. Mol. Biol. 1979, 127, 203–216. [Google Scholar]
- Balian, G.; Bowes, J.H. The structure and properties of collagen. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press: London, UK, 1977; pp. 1–27. [Google Scholar]
- Miles, C.; Ghelashvili, M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys. J. 1999, 76, 3243–3252. [Google Scholar]
- Sionkowska, A. Thermal stability of UV-irradiated collagen in bovine lens capsules and in bovine cornea. J. Photochem. Photobiol. B 2005, 80, 87–92. [Google Scholar]
- Privalov, P.L. Stability of proteins which do not present a single co-operative system. Adv. Prot. Chem. 1982, 25, 1–104. [Google Scholar]
- Burjanadze, T.V. Thermodynamic substantiation of water-bridged collagen structure. Biopolymers 1992, 32, 941–949. [Google Scholar]
- Miles, C.; Sionkowska, A.; Hulin, S.; Sims, T.J.; Avery, N.C.; Balley, A.J. Identification of an intermediate state in the helix-coil degradation of collagen by ultraviolet light. J. Biol. Chem. 2000, 275, 33014–33020. [Google Scholar]
- Debelle, L.; Tamburro, A.M. Dissection of human tropoelastin: Exon-by-exon chemical synthesis and related conformational studies. Int. J. Biochem. Cell. Biol. 1999, 31, 261–272. [Google Scholar]
- Li, B.; Alonso, D.O.; Daggett, V. The molecular basis for the inverse temperature transition of elastin. J. Mol. Biol. 2001, 305, 581–592. [Google Scholar]
- Hoeve, C.A.J.; Flory, P.J. The elastic properties of elastin. Biopolymers 1974, 13, 677–686. [Google Scholar]
- Lillie, M.A.; Gosline, J.M. The effects of hydration on the dynamic mechanical properties of elastin. Biopolymers 1990, 29, 1147–1160. [Google Scholar]
- Cecorrulli, G.; Scandola, M.; Pezzin, G. Calorimetric investigation of some elastin-solvent systems. Biopolymers 1977, 16, 1505–1512. [Google Scholar]
- Kakivaya, S.R.; Hoeve, C.A.J. The glass point of elastin. Proc. Nat. Acad. Sci. USA 1975, 72, 3505–3507. [Google Scholar]
- Samouillan, V.; Andre, C.; Dandurand, J.; Lacabanne, C. Effect of water on the molecular mobility of elastin. Biomacromolecules 2004, 5, 958–964. [Google Scholar]
- Samouillan, V.; Lamure, A.; Maurel, E.; Dandurand, J.; Lacabanne, C.; Ballarin, F.; Spina, M. Characterisation of elastin and collagen in aortic bioprostheses. Med. Biol. Eng. Comput. 2000, 38, 226–231. [Google Scholar]
- Samouillan, V.; Dandurand, J.; Lacabanne, C.; Stella, A.; Gargiulo, M.; Degani, A.; Gandaglia, A.; Spina, M. Characterization of aneurysmal aortas by biochemical, thermal, and dielectric techniques. J. Biomed. Mater. Res. A 2010, 95, 611–619. [Google Scholar]
- Sarrette, J.P.; Cousty, S.; Merbahi, N.; Negre-Salvayre, A.; Clement, F. Observation of antibacterial effects obtained at atmospheric and reduced pressures in afterglow conditions. Eur. Phys. J. Appl. Phys. 2010, 49, 13108. [Google Scholar]
- Villeger, S.; Sarrette, J.P.; Ricard, A. Synergy between N and O atom action and substrate temperature in a sterilization process using a flowing N2-O2 microwave post-discharge. Plasma. Proc. Polym. 2005, 2, 709–714. [Google Scholar]
- Sardella, E.; Detomaso, L.; Gristina, R.; Senesi, G.; Agheli, H.; Sutherland,, D.; d'Agostino, R.; Favia, P. Nano-structured cell-adhesive and cell-repulsive plasma-deposited coatings: Chemical and topographical effects on Keratinocyte adhesion. Plasma Proc. Polym. 2008, 5, 540–551. [Google Scholar]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar]
- Morfill, G.E.; Kong, M.G.; Zimmerman, J.L. Focus on plasma medicine. N. J. Phys. 2009, 11, 115011:1–115011:9. [Google Scholar]
- Stoffels, E.; Roks, A.J.M.; Deelman, L.E. Delayed effects of cold atmospheric plasma on vascular cells. Plasma. Proc. Polym. 2008, 5, 599–605. [Google Scholar]
- Lloyd, G.; Friedman, G.; Jafri, S.; Schultz, G.; Fridman, A.; Harding, K. Gas plasma: Medical uses and development in wound care. Plasma. Proc. Polym. 2010, 7, 194–211. [Google Scholar]
- Caruso, A.B.; Dunn, M.D. Functional evaluation of collagen fiber scaffolds for ACL reconstruction: Cyclic loading in proteolytic enzyme solutions. J. Biomed. Mater. Res. 2004, 69A, 164–171. [Google Scholar]
- Chan, B.P.; So, K.F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J. Biomed. Mater. Res. 2005, 75A, 689–701. [Google Scholar]
- Kato, Y.; Uchida, K.; Kawakishi, S. Aggregation of collagen exposed to UVA in the presence of riboflavin: a plausible role of tyrosine modification. Photochem. Photobiol. 1994, 59, 343–349. [Google Scholar]
- Bertipaglia, B.; Ortolani, F.; Petrelli, L.; Gerosa, G.; Spina, M.; Pauletto, P.; Casarotto, D.; Marchini, M.; Sartore, S. Cell characterization of porcine aortic valve and decellularrized leaflets repopulated with aortic valve interstitial cells: The VESALIO project Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur. Ann. Thorac. Surg. 2003, 75, 1274–1282. [Google Scholar]
- Courtman, D.W.; Pereira, C.A.; Kashef, V.; McColomb, D.; Lee, J.M.; Wilson, G.J. Development of a pericardial acellular matrix: A biomaterial approach for coronary artery bypass and heart valve replacement. Ann. Thorac. Surg. 1995, 60, 353–358. [Google Scholar]
- Henriquez, M.; Lissi, E.; Abuin, E.; Ciferri, A. Assembly of amphilic compounds and rigid polymers. 1. Interaction of SDS with collagen. Macromolecules 1994, 27, 6834–6840. [Google Scholar]
- Kawazoye, S.; Tian, S.F.; Toda, S.; Takashima, T.; Sunaga, T.; Fujitani, N.; Higashino, H.; Matsumura, S. The mechanism of interaction of SDS with elastic fibers. J. Biochem. 1995, 117, 1254–1260. [Google Scholar]
- Bush, K.; Garvey, K.A.; Gosline, J.M.; Aaron, B.B. Effects of hydrophobic elastin ligands on the stress-strain properties of elastin fibers. Connect. Tissue Res. 1982, 9, 157–163. [Google Scholar]
- Mukherjee, D.P.; Kagan, H.M.; Jordan, R.E.; Franzblau, C. Solute effects on the mechanical properties of arterial elastin. Connect. Tissue Res. 1976, 4, 177–179. [Google Scholar]
- Merbahi, N.; Yousfi, M.; Eichwald, O. Device for emitting a plasma jet from the atmospheric pressure air at ambient temperature and pressure, and use of said device. N° of the international Publication of the patent: WO 2011/00170 A1, 1 June 2011. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samouillan, V.; Delaunay, F.; Dandurand, J.; Merbahi, N.; Gardou, J.-P.; Yousfi, M.; Gandaglia, A.; Spina, M.; Lacabanne, C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J. Funct. Biomater. 2011, 2, 230-248. https://doi.org/10.3390/jfb2030230
Samouillan V, Delaunay F, Dandurand J, Merbahi N, Gardou J-P, Yousfi M, Gandaglia A, Spina M, Lacabanne C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. Journal of Functional Biomaterials. 2011; 2(3):230-248. https://doi.org/10.3390/jfb2030230
Chicago/Turabian StyleSamouillan, Valérie, Florian Delaunay, Jany Dandurand, Nofel Merbahi, Jean-Pierre Gardou, Mohammed Yousfi, Alessandro Gandaglia, Michel Spina, and Colette Lacabanne. 2011. "The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials" Journal of Functional Biomaterials 2, no. 3: 230-248. https://doi.org/10.3390/jfb2030230
APA StyleSamouillan, V., Delaunay, F., Dandurand, J., Merbahi, N., Gardou, J.-P., Yousfi, M., Gandaglia, A., Spina, M., & Lacabanne, C. (2011). The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. Journal of Functional Biomaterials, 2(3), 230-248. https://doi.org/10.3390/jfb2030230




