A Smart pH-Responsive Three Components Luminescent Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NVPD
2.3. Preparation and Characterization of Gelation
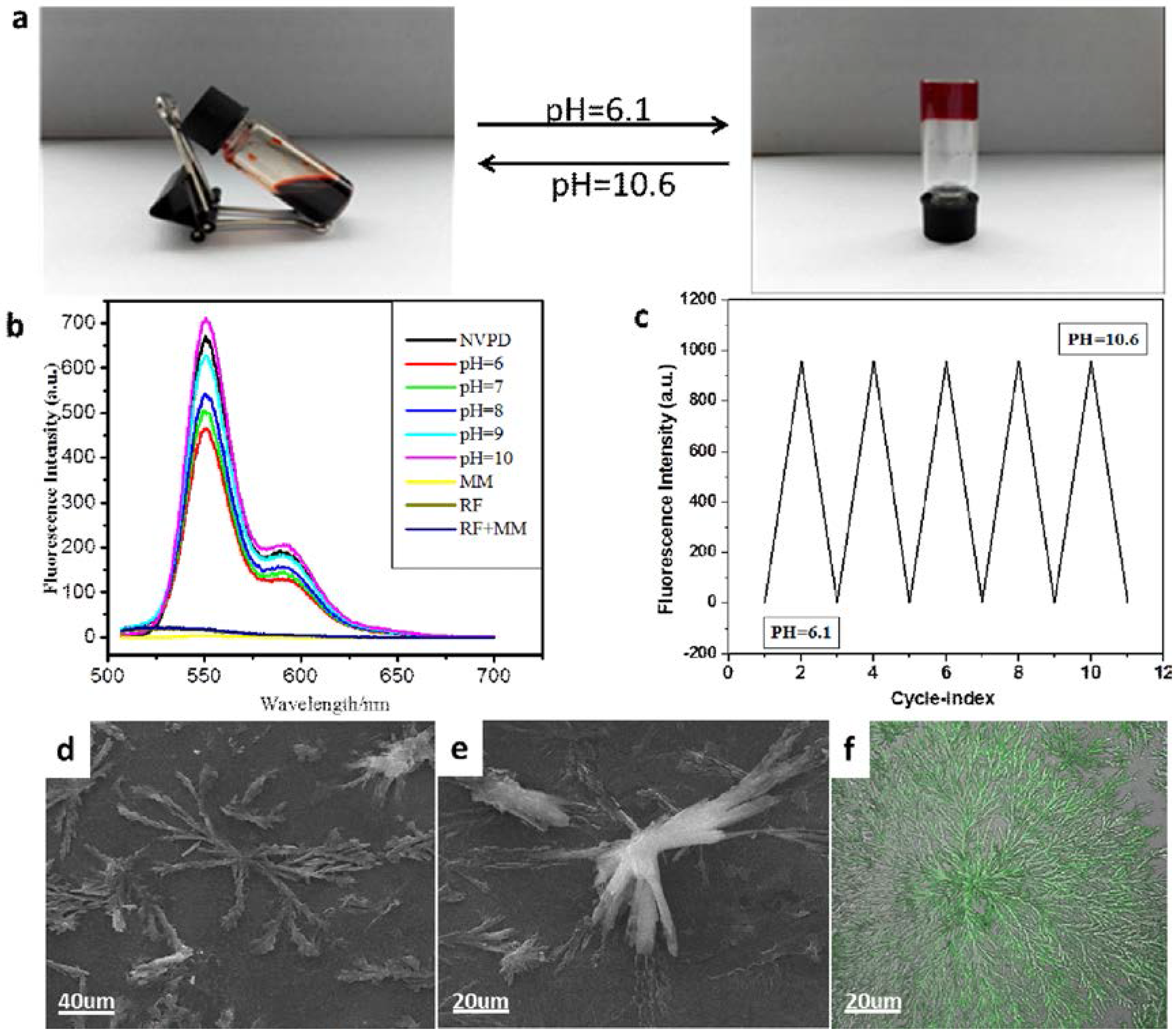
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Structure Function Relationship of Calcium Alginate Hydrogels. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Claussen, R.C.; Rabatic, B.M.; Stupp, S.I. Aqueous self-assembly of unsymmetric Peptide bolaamphiphiles into nanofibers with hydrophilic cores and surfaces. J. Am. Chem. Soc. 2003, 125, 12680–12681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Deng, M.; Wang, Y.; Liang, D.; Yan, Y.; Huang, J. Special Effect of β-Cyclodextrin on the Aggregation Behavior of Mixed Cationic/Anionic Surfactant Systems. J. Phys. Chem. B 2009, 113, 7498–7504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, Y.; Huang, J. Versatility of Cyclodextrins in Self-Assembly Systems of Amphiphiles. Adv. Colloid Interface Sci. 2011, 169, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Willerich, I.; Gröhn, F. Single-Crystalline Molecular Flasks: Chemical Transformation with Bulky Reagents in the Pores of Porous Coordination Networks. Angew. Chem. Int. Ed. 2010, 49, 8104–8108. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, D.; Haridas, V.; Gilardi, R.; Karle, I.L. Self-Assembling Aromatic-Bridged Serine-Based Cyclodepsipeptides (Serinophanes): A Demonstration of Tubular Structures Formed through Aromatic π−π Interactions. J. Am. Chem. Soc. 1998, 120, 10793–10800. [Google Scholar] [CrossRef]
- Mynar, J.L.; Yamamoto, T.; Kosaka, A.; Fukushima, T.; Ishii, N.; Aida, T. Radially Diblock Nanotube: Site-Selective Functionalization of a Tubularly Assembled Hexabenzocoronene. J. Am. Chem. Soc. 2008, 130, 1530–1531. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Besseling, N.A.; de Keizer, A.; Marcelis, A.; Drechsler, M.; Stuart, C.; Martien, A. Hierarchical self-organization in solutions containing metal ions, ligand, and diblock copolymer. Angew. Chem. Int. Ed. 2007, 46, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ding, Y.; Yang, Y.; Yan, Y.; Huang, J.; de Keizer, A.; Stuart, M.A.C. Fluorescence Enhancement by Microphase Separation-Induced Chain Extension of Eu3+ Coordination Polymers: Phenomenon and Analysis. Soft Matter 2011, 7, 2720–2724. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, L.; Drechsler, M.; Sun, Y.; Liu, Z.; Huang, J.; Tang, B.Z.; Li, Z.; Stuart, M.A.C.; Yan, Y. Spontaneous Formation of Nanosized Unilamellar Polyion Complex Vesicles with Tunable Size and Properties. J. Am. Chem. Soc. 2014, 136, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.B.; Rowan, S.J. Multistimuli, Multiresponsive Metallo-Supramolecular Polymers. J. Am. Chem. Soc. 2003, 125, 13922–13923. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.; Takeuchi, M.; Fujita, N.; Numata, M.; Shinkai, S. Post-Polymerization of Preorganized Assemblies for Creating Shape-Controlled Functional Materials. Chem. Soc. Rev. 2007, 36, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, C.J.; Nilson, B.L. A Reductive Trigger for Peptide Self-Assembly and Hydrogelation. J. Am. Chem. Soc. 2010, 132, 9526–9527. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Qiud, Y.; Loh, X.J. PLA-based thermogel for the sustained delivery of chemotherapeutics in a mouse model of hepatocellular carcinoma. RSC Adv. 2016, 6, 44506–44513. [Google Scholar] [CrossRef]
- Ye, H.; Owh, C.; Jiang, S.; Ng, C.Z.Q.; Wirawan, D.; Loh, X.J. A Thixotropic Polyglycerol Sebacate-Based Supramolecular Hydrogel as an Injectable Drug Delivery Matrix. Polymers 2016, 8, 130. [Google Scholar] [CrossRef]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chen, X.H.; Wang, W.Z.; Loh, X.J. Engineering Bioresponsive Hydrogels toward Healthcare Applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Synthesis of magnetic mesoporous silica composites via a modified Stöber approach. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Chee, P.L.; Lakshmanan, L.; Jiang, S.; Ye, H.Y.; Kai, D.; Loh, X.J. An Injectable Double-Network Hydrogel for Cell Encapsulation. Aust. J. Chem. 2016, 69, 388–393. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.J.; Li, Z.B.; Wong, M.H.; Rajamani, L.; Lingam, G.; Loh, X.J. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules 2015, 16, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Zhang, H.Q.; Yashima, E. Chiral stimuli-responsive gels: Helicity induction in poly (phenylacetylene) gels bearing a carboxyl group with chiral amines. J. Am. Chem. Soc. 2003, 125, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Functionalized Nanostructures with Application in Regenerative Medicine. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Hauser, C.A.E.; Zhang, S.G. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010, 39, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Rybtchinski, B. Adaptive Supramolecular Nanomaterials Based on Strong Noncovalent Interactions. ACS Nano 2011, 5, 6791–6818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Li, Y.W.; Xue, X.D.; Chu, P.F.; Liu, C.; Yang, K.; Jiang, Y.G.; Chen, W.Q.; Zou, G.Z.; Liang, X.J. A smart pH-switchable luminescent hydrogel. Chem. Commun. 2015, 51, 4168–4171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Ana, P.Y.; Liu, X.F. Bell-shaped sol–gel–sol conversions in pH-responsive worm-based nanostructured fluid. Soft Matter 2015, 11, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Low, Z.W.; Liow, S.S.; Karim, A.A.; Ye, H.Y.; Jin, G.R.; Li, K.; Loh, X.J. Development of Lignin Supramolecular Hydrogels with Mechanically Responsive and Self-Healing Properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Yu, J.H.; Fan, H.L.; Huang, J.; Chen, J.H. Fabrication and evaluation of reduction-sensitive supramolecular hydrogel based on cyclodextrin/polymer inclusion for injectable drug-carrier application. Soft Matter 2011, 7, 7386–7394. [Google Scholar] [CrossRef]
- Wang, A.D.; Shi, W.Y.; Huang, J.B.; Yan, Y. Adaptive Soft Molecular Self-Assemblies. Soft Matter 2016, 12, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.K.; Asthana, D.; Mukhopadhyay, P.; Summa, D.; Muccioli, L.; Zannoni, C.; Beljonne, D.; Rowan, A.E.; Malik, S. Assemblies of perylene diimide derivatives with melamine into luminescent hydrogels. Chem. Commun. 2011, 47, 11858–11860. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Fluid–Structure Interactions: Slender Structures and Axial Flow, 2nd ed.; Elsevier Academic Press: London, UK, 2004. [Google Scholar]
- Kopecek, J. Smart and genetically engineered biomaterials and drug delivery systems. Eur. J. Pharm. Sci. 2003, 20, 1–16. [Google Scholar] [CrossRef]
- Mano, J.F. Multi-shape active composites by 3D printing of digital shape memory polymers. Adv. Eng. Mater. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, M.; Todd, S.J.; Hirst, A.R.; McDonald, T.O.; Ulijn, R.V. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2013, 1, 11–39. [Google Scholar] [CrossRef]
- Jiang, L.X.; Yan, Y.; Drechsler, M.; Huang, J.B. Enzyme-Triggered Model Self-Assembly in Surfactant/Cyclodextrin Systems. Chem. Commun. 2012, 48, 7347–7349. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Tanaka, T.; Kokufuta, E. Stimuli sensitive polymers and self-regulated drug delivery systems. Nature 1991, 349, 400–401. [Google Scholar]
- Seow, Y.; Hauser, C.A.E. Short to ultrashort peptide hydrogels for biomedical uses. Mater. Today 2014, 17, 381–388. [Google Scholar] [CrossRef]
- Zhan, X.W.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and Related Diimides for Organic Electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Misek, J.; Sakai, N.; Matile, S. Supramolecular n/p-heterojunction photosystems with oriented multicolored antiparallel redox gradients (OMARG-SHJs). Chem. Soc. Rev. 2010, 39, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. ChemInform Abstract: Self-Assembly Strategies for Integrating Light Harvesting and Charge Separation in Artificial Photosynthetic Systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.J.; Tremblay, N.J.; Yeh, M.-L.; Katz, H.E. Fluorination of Metal Phthalocyanines: Single-Crystal Growth, Efficient N-Channel Organic Field-Effect Transistors, and Structure-Property Relationships. Chem. Mater. 2011, 23, 568–582. [Google Scholar] [CrossRef]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Mullen, K. Functional superhydrophobic surfaces made of Janus micropillars. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning Orbital Energetics in Arylene Diimide Semiconductors. Materials Design for Ambient Stability of n-Type Charge Transport. J. Am. Chem. Soc. 2007, 129, 12529–12534. [Google Scholar] [CrossRef] [PubMed]
- An, Z.S.; Yu, J.S.; Jones, S.C.; Barlow, S.; Yoo, S.; Domercq, B.; Prins, P.; Siebbeles, L.D.A.; Kippelen, B.; Marder, S.R. High Electron Mobility in Room-Temperature Discotic Liquid-Crystalline Perylene Diimides. Adv. Mater. 2005, 17, 2580–2583. [Google Scholar] [CrossRef]
- Wurthner, F.; Kaiser, T.E.; Saha-Moller, C.R. Interfacial Effects in Iron-Nickel Hydroxide–Platinum Nanoparticles Enhance Catalytic Oxidation. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar]
- Malinovskii, L.V.; Wenger, D.; Haener, R. Nucleic acid-guided assembly of aromatic chromophores. Chem. Soc. Rev. 2010, 39, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Kumar, N.S.S.; Krishna, A.; Rao, D.S.S.; Prasad, S.K.; Das, S. Formation of Highly Luminescent Supramolecular Architectures Possessing Columnar Order from octupolar Oxadiazole Derivatives: Hierarchical Self-assembly from Nanospheres to Fibrous Gels. Adv. Funct. Mater. 2009, 19, 2064–2073. [Google Scholar] [CrossRef]
- Hippius, C.; van Stokkum, I.H.M.; Gsanger, M.; Groeneveld, M.M.; Williams, R.M.; Wurthner, F. Sequential FRET Processes in Calix[4]arene-Linked Orange-Red-Green Perylene Bisimide Dye Zigzag Arrays. J. Phys. Chem. C 2008, 112, 2476–2486. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, D.; Schenning, A.P.H.J. Hydrogen-bonded Supramolecular π-Functional Materials. Chem. Mater. 2011, 23, 310–325. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar]
- Bullock, J.E.; Carmieli, R.; Mickley, S.M.; Vura-Weis, J.; Wasielewski, M.R. Photoinitiated Charge Transport through π-Stacked Electron Conduits in Supramolecular Ordered Assemblies of Donor−Acceptor Triads. J. Am. Chem. Soc. 2009, 131, 11919–11929. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Che, Y.; Moore, J.S. One-Dimensional Self-Assembly of Planar π-Conjugated Molecules: Adaptable Building Blocks for Organic Nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Stepanenko, V.; Dehm, V.; Prins, P.; Siebbeles, L.D.A.; Seibt, J.; Marquetand, P.; Engel, V.; Wurthner, F. Photoluminescence and Conductivity of Self-Assembled π–π Stacks of Perylene Bisimide Dyes. Chem.—Eur. J. 2007, 13, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Shirman, E.; Weissman, H.; Shimoni, E.; Wolf, S.G.; Pinkas, I.; Rybtchinski, B. Supramolecular Gel Based on a Perylene Diimide Dye: Multiple Stimuli Responsiveness, Robustness, and Photofunction. J. Am. Chem. Soc. 2009, 131, 14365–14373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Zhang, S.; Liu, Y.; Mei, J.; Chen, S.J.; Lu, P.; Qin, A.J.; Ma, Y.G.; Sun, J.Z.; Tang, B.Z. Tetra phenyl-ethenyl-modified perylene bisimide: Aggregation-induced red emission, electrochemical properties and ordered microstructures. J. Mater. Chem. 2012, 22, 7387–7394. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, L.; Cao, X.; Jiang, M.; Yi, T. Morphological transformation between three-dimensional gel network and spherical vesicles via sonication. Soft Matter 2012, 8, 4494–4498. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Wen, Y.; Liu, K.; Chen, L.; Mao, Y.; Yang, S.; Yi, T. (−)-Menthol based thixotropic hydrogel and its application as a universal antibacterial carrier. Soft Matter 2014, 10, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Manna, S.; Nandi, A.K. Temperature and pH sensitive photoluminescence of riboflavin-methyl cellulose hydrogel: Towards AND molecular logic gate behaviour. Soft Matter 2009, 5, 3992–3996. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting Metal Nanowires within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Ery, C.V.; Paternostre, M.; Robert, B.; Gulik-Krzywicki, T.; Narayanan, T.; Dedieu, J.C.; Keller, G.; Torres, M.L.; Cherif-Cheikh, R.; Calvom, P.; et al. Molecular Biomimetics and Materials Design. Proc. Natl. Acad. Sci. USA 2003, 100, 10258–10262. [Google Scholar]
- Xing, B.G.; Yu, C.W.; Chow, K.H.; Ho, P.L.; Fu, D.G.; Xu, B. Hydrophobic Interaction and Hydrogen Bonding Cooperatively Confer a Vancomycin Hydrogel: A Potential Candidate for Biomaterials. J. Am. Chem. Soc. 2002, 124, 14846–14847. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, W.; Cheng, L.; Huang, P.; Peng, Y.; Wu, Y.; Li, X.; Li, X.; Fan, X. A Smart pH-Responsive Three Components Luminescent Hydrogel. J. Funct. Biomater. 2016, 7, 25. https://doi.org/10.3390/jfb7030025
Li Y, Liu W, Cheng L, Huang P, Peng Y, Wu Y, Li X, Li X, Fan X. A Smart pH-Responsive Three Components Luminescent Hydrogel. Journal of Functional Biomaterials. 2016; 7(3):25. https://doi.org/10.3390/jfb7030025
Chicago/Turabian StyleLi, Yibao, Wei Liu, Linxiu Cheng, Ping Huang, Yu Peng, Yongquan Wu, Xun Li, Xiaokang Li, and Xiaolin Fan. 2016. "A Smart pH-Responsive Three Components Luminescent Hydrogel" Journal of Functional Biomaterials 7, no. 3: 25. https://doi.org/10.3390/jfb7030025




