Artificial Red Blood Cells as Potential Photosensitizers in Dye Laser Treatment Against Port-Wine Stains
Abstract
:1. Introduction
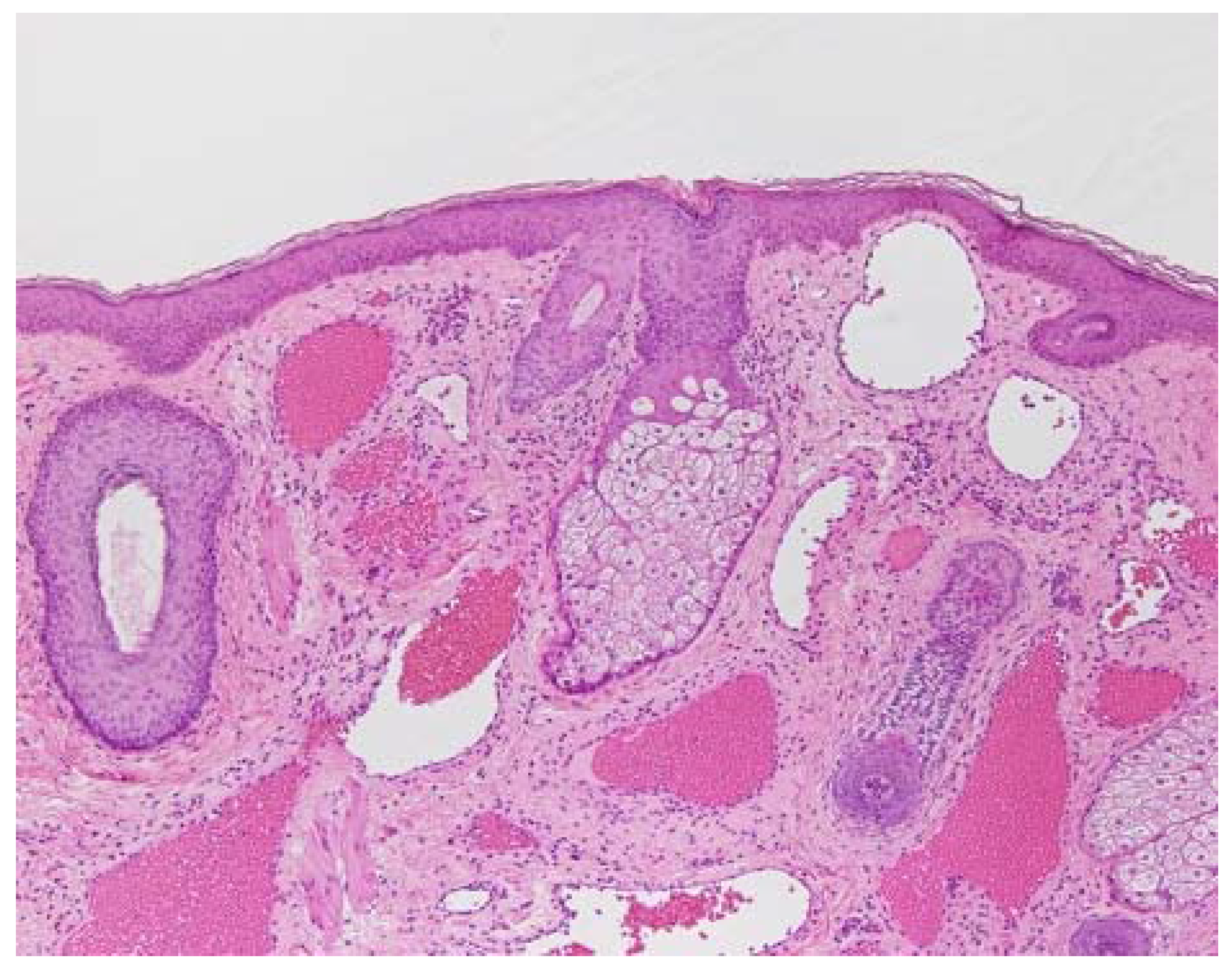
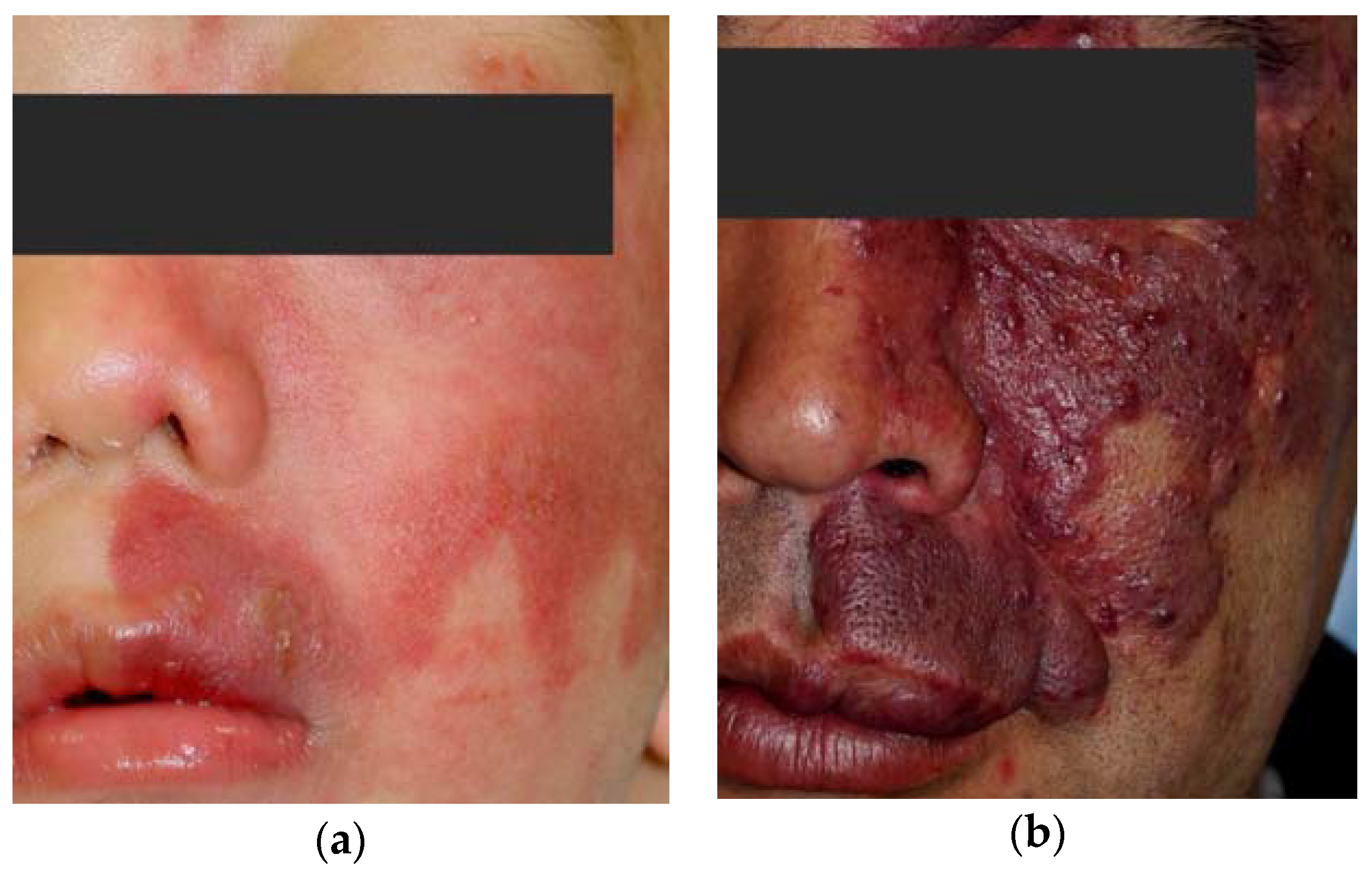
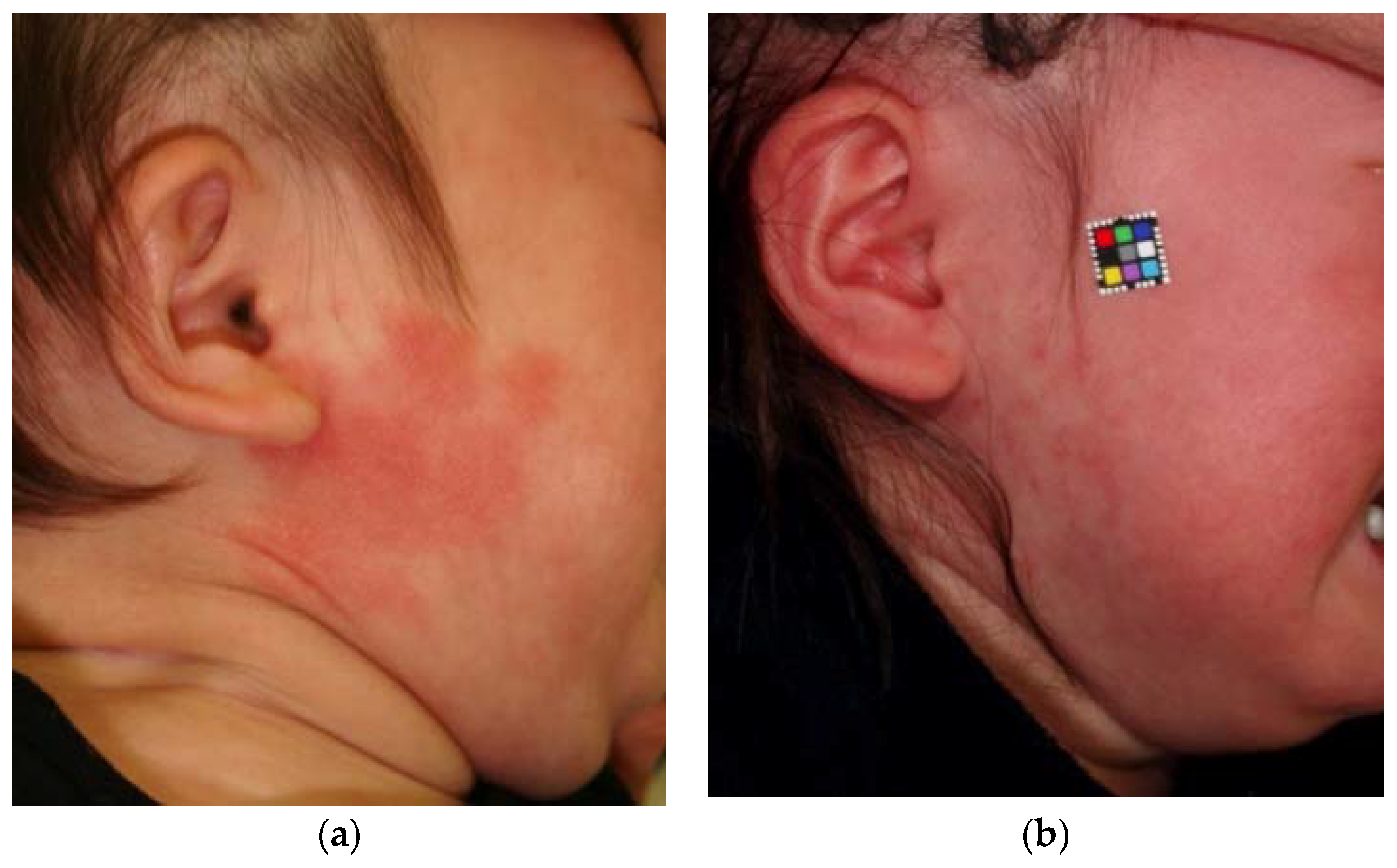
2. Pathology of Port-Wine Stains
3. PWSs Treatment with Light Therapy Equipment
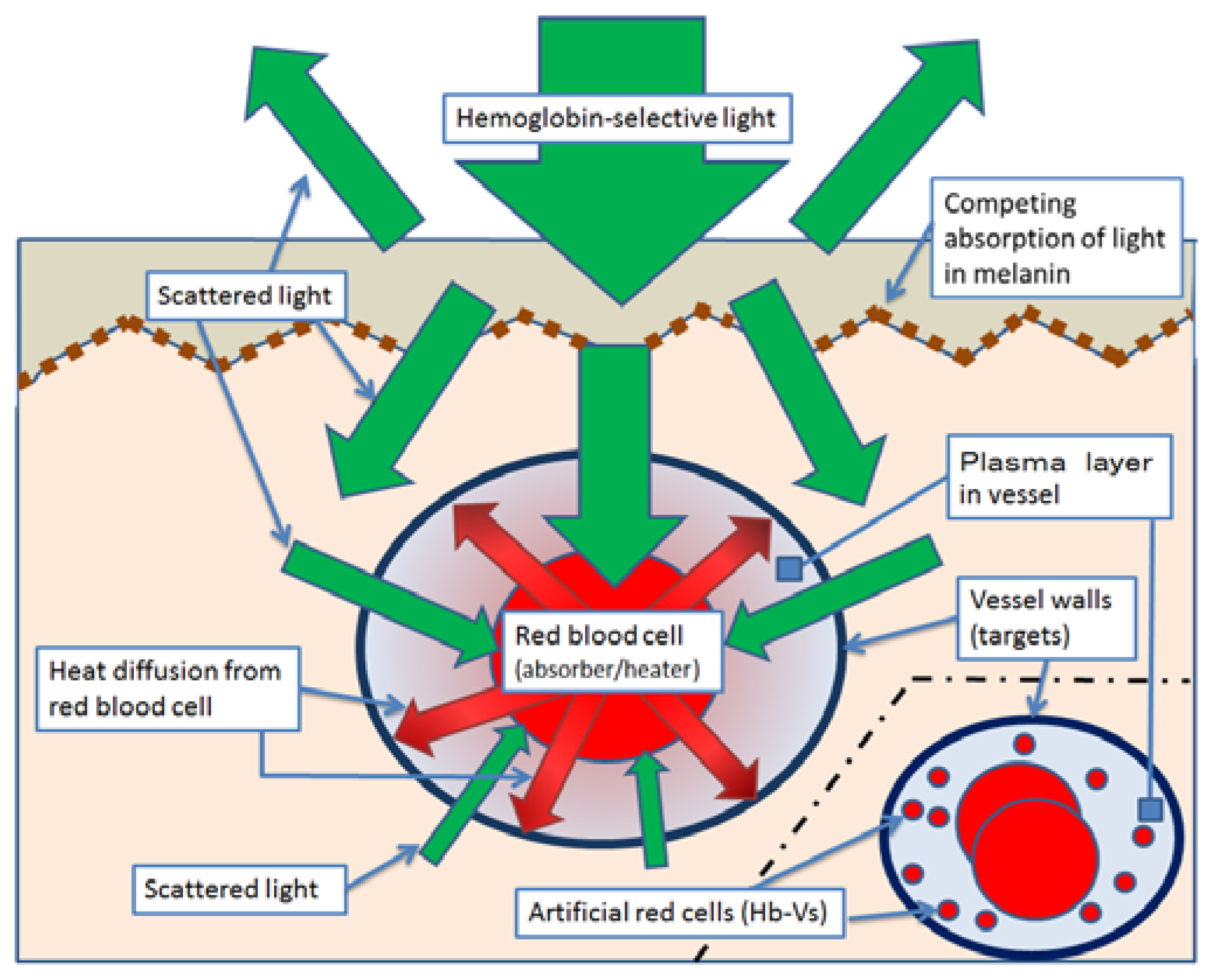
4. Effect and Limit of PWS Treatment with Light Therapy Equipment
5. Break Through the Limits of Laser Treatment Against PWS with Hb-Vs
5.1. Observation of Artificial Red Blood Cells in Microvessels and Microtubes
5.2. The Effects of Dye Laser Irradiation on Hb-Vs
5.3. Changes in Chicken Wattle Microvessels with Dye Laser Irradiation after Hb-V Intravenous Injections
6. Discussion
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rikihisa, N.; Watanabe, S.; Satoh, K.; Saito, Y.; Sakai, H. Photosensitizer effects of artificial red cells on dye laser irradiation in an animal model assuming port-wine stain treatment. Plast. Reconstr. Surg. 2017, 139, 707e–716e. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, N. A consideration about advantageous effect of the laser treatment of port-wine stains with blood substitutes including hemoglobin. Artif. Blood. 2011, 18, 110–113. (in Japanese). [Google Scholar]
- Sakai, H.; Suzuki, Y.; Kinoshita, M.; Takeoka, S.; Maeda, N.; Tsuchida, E. O2 release from Hb vesicles evaluated using an artificial, narrow O2-permeable tube: comparison with RBCs and acellular Hbs. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, J.B. Malformation. In Vascular Birthmarks Hemangioma and Malformation, 1st ed.; Mulliken, J.B., Young, A.E., Eds.; Saunders: Philadelphia, PA, USA, 1988; pp. 170–195. [Google Scholar]
- Enjolras, O.; Wassef, M.; Chapot, R. Color Atras of Vascular Tumors and Vascular Malformations, 1st ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 125–127. [Google Scholar]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.C.; et al. Vascular anomalies classification: Recommendations from the international society for the study of vascular anomalies. Pediatrics 2015, 136, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Brightman, L.A.; Geronemus, R.G.; Reddy, K.K. Laser treatment of port-wine stains. Clin. Cosmet. Investig. Dermatol. 2015, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, G.B.; Anderson, R.R.; Manstein, D.; Zenzie, H.H.; Smirnov, M.Z. Extended theory of selective photothermolysis. Lasers Surg. Med. 2001, 29, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: Precise microsurgery by elective absorption of pulsed radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Sivarajan, V.; Maclaren, W.M.; Mackay, I.R. The effect of varying pulse duration, wavelength, spot size, and fluence on the response of previously treated capillary vascular malformations to pulsed-dye laser treatment. Ann. Plast. Surg. 2006, 57, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Sakurai, H.; Takeuchi, M.; Yamaki, T.; Soejima, K.; Groff, W.F.; Nozaki, M. Treatment of resistant port-wine stains with a variable-pulse pulsed dye laser. Dermatol. Surg. 2007, 33, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Hamori, Y.; Kurokawa, M.; Noda, K.; Hattori, R.; Takeda, T. The linearly polarized light near-infrared therapy as a pre-treatment of pulsed dye laser for hemangioma simplex. J. Jpn. Soc. Plast. Reconstr. Surg. 2004, 24, 753–760. (in Japanese). [Google Scholar]
- Zhang, B.; Zhang, T.H.; Huang, Z.; Li, Q.; Yuan, K.H.; Hu, Z.Q. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn. Ther. 2014, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, E.; Sou, K.; Nakagawa, A.; Sakai, H.; Komatsu, T.; Kobayashi, K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjug. Chem. 2009, 20, 1419–1440. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sou, K.; Horinouchi, H.; Kobayashi, K.; Tsuchida, E. Haemoglobin-vesicles as artificial oxygen carriers: Present situation and future visions. J. Int. Med. 2008, 263, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Sakai, H.; Takeuchi, D.; Kagaya, Y.; Tashiro, K.; Naito, M.; Mihara, M.; Narushima, M.; Iida, T.; Koshima, I. Normothermic preservation of the rat hind limb with artificial oxygen-carrying hemoglobin vesicles. Transplant 2015, 99, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Intaglietta, M.; Tsuchida, E. Efficacy of red cell substitutes in microcirculation. Artif. Blood 1998, 6, 76–87. (in Japanese). [Google Scholar]
- Sakai, H.; Tsai, A.G.; Kerger, H.; Park, S.I.; Takeoka, S.; Nishide, H.; Tsuchida, E.; Intaglietta, M. Subcutaneous microvascular responses to hemodilution with a red cell substitute consisting of polyethylene glycol-modified vesicles encapsulating hemoglobin. J. Biomed. Mater. Res. 1998, 40, 66–78. [Google Scholar] [CrossRef]
- Fedosov, D.A.; Caswell, B.; Popel, A.S.; Karniadakis, G.E. Blood flow and cell-free layer in microvessels. Microcirculation 2010, 17, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Nakajima, T.; Ogata, H.; Kishi, K. Histological responses of cutaneous vascular lesions following photodynamic therapy with talaporfin sodium: A chicken comb model. Keio J. Med. 2009, 58, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, J.; Shao, X.; Sang, H.; Zhou, Z. The effects of 595- and 1064-nm lasers on rooster comb blood vessels using dual-wavelength and multipulse techniques. Dermatol. Surg. 2011, 37, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Autologous Blood Transfusion (Collection and Reinfusion of the Patient’s Own Red Blood Cells). Available online: http://www.transfusionguidelines.org/transfusion-handbook/6-alternatives-and-adjuncts-to-blood-transfusion/6–1-autologous-blood-transfusion-collection-and-reinfusion-of-the-patient-s-own-red-blood-cells (accessed on 1 February 2017).



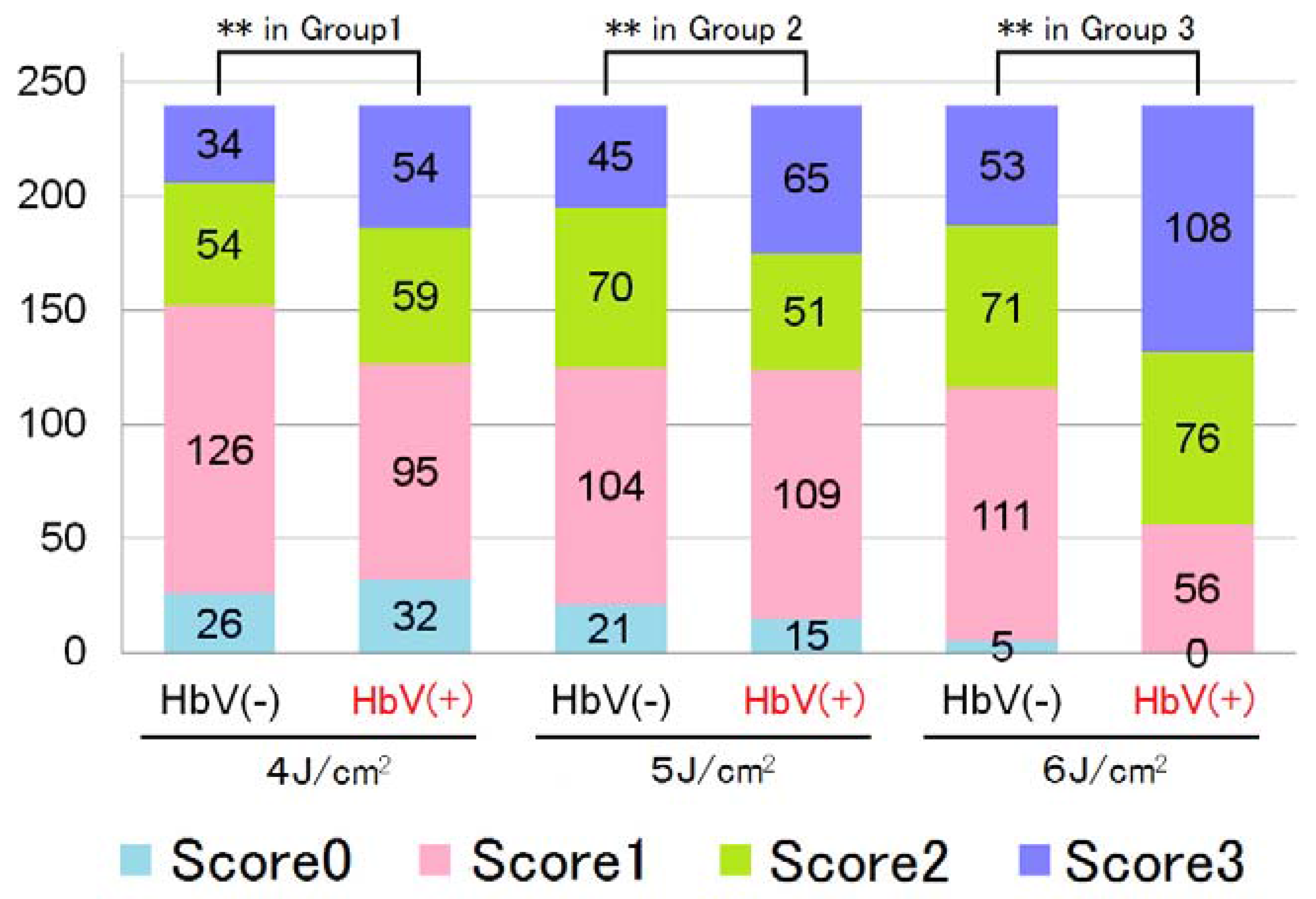
| Histological Findings Score | Observed Phenomenon |
|---|---|
| 0 | No significant change |
| 1 | Agglutination or denucleation of RBCs |
| 2 | Injury to the vascular wall |
| 3 | Perivascular hemorrhage |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rikihisa, N.; Watanabe, S.; Saito, Y.; Sakai, H. Artificial Red Blood Cells as Potential Photosensitizers in Dye Laser Treatment Against Port-Wine Stains. J. Funct. Biomater. 2017, 8, 14. https://doi.org/10.3390/jfb8020014
Rikihisa N, Watanabe S, Saito Y, Sakai H. Artificial Red Blood Cells as Potential Photosensitizers in Dye Laser Treatment Against Port-Wine Stains. Journal of Functional Biomaterials. 2017; 8(2):14. https://doi.org/10.3390/jfb8020014
Chicago/Turabian StyleRikihisa, Naoaki, Shoji Watanabe, Yoshiaki Saito, and Hiromi Sakai. 2017. "Artificial Red Blood Cells as Potential Photosensitizers in Dye Laser Treatment Against Port-Wine Stains" Journal of Functional Biomaterials 8, no. 2: 14. https://doi.org/10.3390/jfb8020014






